Abstract
Background
It is estimated that 1 million persons in Germany suffer from hepatic cirrhosis, which is the final stage of chronic inflammation of the liver. Cirrhosis has multiple causes, all of which lead to structural changes of the liver and to portal hypertension. The main complications of cirrhosis arise in turn: These include bleeding from collateral veins, ascites, hepatocellular carcinoma, encephalopathy, and infection leading to organ failure.
Methods
We present the treatment of the main complications of liver cirrhosis with reference to the relevant literature (phase II and III trials, meta-analyses, and reviews).
Results
Endoscopic treatment (ligation) is used for the primary and secondary prophylaxis of variceal bleeding. Drugs to lower portal pressure (e.g., beta-blockers) are an established means of preventing initial or recurrent variceal bleeding over the long term. Vasoconstrictors such as terlipressin are mainly used to treat acute hemorrhage and type 1 hepatorenal syndrome. The main treatment of ascites is with spironolactone, in combination with a loop diuretic where indicated. A shunt (TIPS) is used to treat severe or repeat variceal hemorrhage or refractory ascites. Antibiotics play a well-established role in the treatment of acute hemorrhage, in the treatment and prevention of spontaneous bacterial peritonitis, and in the treatment of encephalopathy. The treatment of hepatocellular carcinoma depends on its extent of spread and on the degree of decompensation of cirrhosis.
Conclusion
For most of the main complications of liver cirrhosis, there are treatments that have been well-tested in randomized trials. Liver transplantation should also be considered in every case.
Cirrhosis of the liver is characterized by a nodular state of the hepatic parenchyma with the formation of fibrous septa, abnormal cell activation, infiltration of inflammatory cells, and changes of the vascular bed. The main causes of cirrhosis are listed in Table 1. In many cases, more than one of these causes operate in parallel. In Germany, the most common single cause is alcoholism (50% to 60% of all patients) (1, e1, e2).
Table 1. The causes, treatment, and prevention of liver cirrhosis.
| Cause | Treatment and prevention |
| Hepatitis C | Interferon, ribavirin, |
| protease inhibitors | |
| Hepatitis B | (Interferon), |
| nucleot(s)ide analogues | |
| Alcoholism | Abstinence |
| Hemochromatosis | Phlebotomy |
| Wilson’s disease | D-penicillamine, trientine |
| Primary biliary cirrhosis | Ursodeoxycholic acid |
| Secondary biliary cirrhosis | Timely biliary drainage |
| Autoimmune hepatitis | Prednisone, budenoside, azathioprine |
| Budd-Chiari syndrome | TIPS |
| (transjugular intrahepatic portal-systemic [stent-]shunt) |
As the structure of the liver is altered, its function becomes progressively impaired as well, and portal hypertension develops. This process is associated with vascular dysfunction and the secretion of vasoconstrictive hormones. The timely cessation of exposure to substances that harm the liver can prevent cirrhosis or halt its progression (Table 1).
If the process of progressive inflammation and scarring of the liver is not interrupted, the ensuing complications can include
gastrointestinal bleeding,
ascites,
hepatic encephalopathy, and
hepatocellular carcinoma.
The treatment of these complications will be discussed in the following sections. In addition to the treatments under discussion here, the indication for liver transplantation should always be considered as well.
Bleeding
Gastrointestinal bleeding is a dreaded complication of cirrhosis. More than 80% of hemorrhages due to cirrhosis arise from esophageal varices (1, e1, e3); less common sources include extra-esophageal collateral vessels and diffuse bleeding from the gastric mucosa.
Collateral vessels, including those in the esophageal wall, form when the portal pressure is 10 mmHg or higher. As the vessels in the distal third of the esophagus and at the gastro-esophageal junction are covered only by a thin epithelial layer, this is the most common site of bleeding. Variceal bleeding carries a high mortality (20% to 30%) (1, 2, e1).
This problem is treated in three phases (2):
prevention of the first hemorrhage,
treatment of acute hemorrhage,
and prevention of recurrent hemorrhage.
Prevention of the first hemorrhage
Evidence on the efficacy of primary prevention is available from a number of randomized controlled trials (RCTs). There are two main approaches to primary prevention:
the administration of a nonselective beta-blocker to lower portal pressure via a reduction of cardiac output and increase of splanchnic vascular resistance, and
the endoscopic ligation of varices.
Patients with small varices and endoscopic risk factors, or with decompensated (Child C) cirrhosis, should be treated with a nonselective beta-blocker (propranolol or perhaps carvedilol) unless these are contraindicated for other medical reasons (2, 3). All patients with large esophageal varices (greater than 5 mm in diameter) should be treated either with beta-blockers or with endoscopic ligation. These treatments lower the risk of variceal hemorrhage by about half, from 40% to 20% in two years (4). Isolated varices of the gastric fundus can be treated with the injection of tissue sealants (e4). The choice of treatment depends on local expertise, the patient’s preferences and clinical characteristics, and a careful evaluation of the potential side effects and contraindications.
Acute variceal hemorrhage
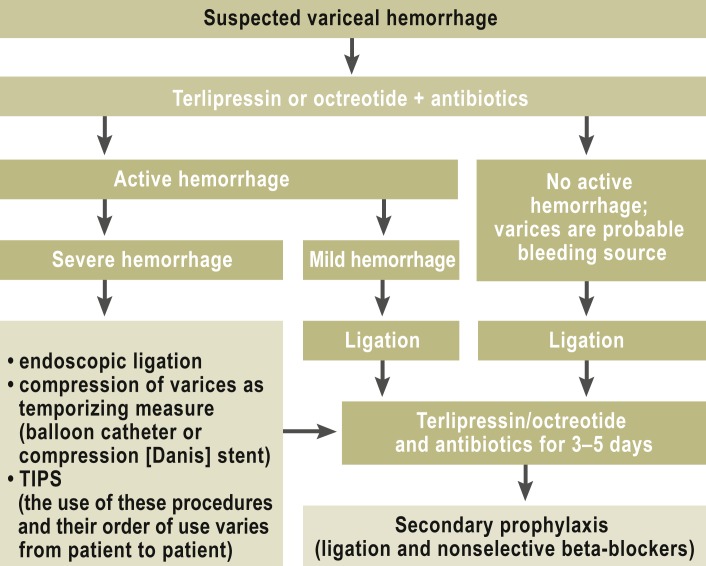
Acute variceal hemorrhage is treated according to the algorithm shown in Figure 1. The findings of at least one controlled trial (5) indicate that the insertion of a transjugular, intrahepatic portal-systemic shunt (TIPS) within 72 hours is justified in patients with decompensated cirrhosis and acute hemorrhage.
Figure 1.
Algorithm for the treatment of acute variceal hemorrhage
(TIPS = transjugular intrahepatic portal-systemic shunt)
Early treatment with vasoactive drugs (e5) and antibiotic treatment/prophylaxis (2, 6) are now considered standard treatment. Their purpose is to stop the bleeding before endoscopy, if possible, and to prevent infection, which would significantly worsen the prognosis (e6).
Prevention of recurrent hemorrhage
The risk of recurrent hemorrhage in the first year after the successful treatment of an acute hemorrhage from esophageal varices is 60% to 80% (4) if a TIPS is not inserted at the time of the initial hemorrhage. This risk can be lowered by pharmacotherapy (propranolol), endoscopic treatment (repeated ligation), or shunting (surgical shunt procedure or TIPS). Repeated ligation combined with the regular administration of a nonselective beta-blocker is currently considered standard treatment for the prevention of recurrent hemorrhage (2, 7); it lowers the risk from 60%–80% to 20%–30%. Controlled trials have shown an equivalent benefit from medical recurrence prevention (with beta-blockers and nitrates) and endoscopic recurrence prevention (8).
TIPS and open shunting operations are the most effective form of recurrence prophylaxis but carry the risk of hepatic encephalopathy and worsening of liver function. Shunting should always be considered after a severe, recurrent hemorrhage, with careful attention to its contraindications (Box 1). The rate of recurrent hemorrhage in the first two years after TIPS insertion is 15% to 20% (9). The risk of stenosis in the TIPS outflow tract is over 50%, but this can be lowered by the insertion of a coated stent, which may also prolong patient survival (e7).
Box 1. Contraindications to TIPS insertion.
Chronic, recurrent hepatic encephalopathy above grade I
Serum bilirubin concentration above 3–5 mg/dL (exception: acute decompensation in active variceal bleeding from the esophagus or gastric fundus)
Most forms of portal vein thrombosis
Congestive heart failure (NYHA >II, ejection fraction <40%)
Pulmonary hypertension (mPAP >45 mm Hg)
Multifocal hepatocellular carcinoma
Cystic liver
Uncontrollable infection/sepsis
TIPS, transjugular intrahepatic portal-sytemic (stent-)shunt; NYHA, New York Heart Association; PAP, pulmonary arterial pressure
Ascites and associated disturbances
Ascites
The one-year mortality of persons with liver cirrhosis and ascites is about 40% (e8).
Ascites is treated in stepwise fashion. Patients with ascites tend to retain sodium and fluids, so sodium and fluid restriction is advised. The restriction of sodium intake to less than 5 g of NaCl per day is recommended only for persons whose ascites is difficult to mobilize; for those whose ascites is easier to treat, no restriction is recommended other than refraining from putting additional salt on food. Strict fluid restriction to 1.5 L or less per day is recommended for persons with hyponatremia (serum sodium concentration below 125 mmol/L) (10).
The administration of the aldosterone antagonist spironolactone (initial dose, 100 mg/day; maximal dose, 400 mg/day), which inhibits sodium resorption in the distal tubule, is standard, as it is a causally directed treatment of the renal consequences of hyperreninemia. If the response to treatment is inadequate (target: weight loss by around 1 kg per day but not more in patients with peripheral edema), a loop diuretic can be given as well (e.g., furosemide in an initial dose of 20–40 mg/day and a maximal dose of 160 mg/day) (10). Diuretic therapy may lead to complications such as electrolyte disturbances or a rise in renal retention parameters; alternatively, it may fail to mobilize the ascites. Such cases are designated as refractory ascites.
Possible treatments for refractory ascites include repeated removal of large quantities of ascitic fluid by paracentesis and the insertion of a TIPS. The removal of more than 5 L of ascites by paracentesis may be followed by vasoconstrictive counter-regulation and worsening of renal function; these can be prevented, or at least alleviated, by albumin administration. Whenever more than 5 L of ascites are removed, 8 g of albumin should be given per liter of ascitic fluid removed (e9).
TIPS insertion lowers the portal-systemic pressure gradient by about half, decreases the activation of the renin-angiotensin-aldosterone system, and thereby leads to increased sodium excretion by the kidneys. In many cases, the kidneys then respond better to diuretics as well. Multiple clinical trials have demonstrated the superiority of TIPS insertion to regular paracentesis for ascites (11, 12). The timely insertion of a TIPS may also prolong patient survival (12, 13). Before a TIPS is inserted, meticulous attention should always be paid to the potential contraindications (Box 1). Patients should be informed of the risk of hepatic encephalopathy.
Hepatorenal syndrome
Hepatorenal syndrome (HRS) is a state of potentially reversible renal dysfunction without morphological correlate (criteria for HRS: Box 2). Of its two types (type I and type II), type I—acute HRS—has a less favorable prognosis. It is associated with rapid worsening of hepatic function and with high mortality (10, e10).
Box 2. Defining criteria for hepatorenal syndrome (HRS) (9).
All of the following diagnostic criteria for type I/II HRS must be fulfilled:
Liver cirrhosis with ascites
Serum creatinine concentration >1.5 mg/dL (>133 µmol/L), type I: >2.5 mg/dL
No lowering of the serum creatinine concentration after at least two days without diuretic treatment and after volume expansion with albumin (recommended dose: 1 g per kg body weight per day, up to a maximum of 100 g/d)
No evidence of shock
No treatment with nephrotoxic drugs
-
No renal parenchymal changes:
no proteinuria >500 mg/day,
no microhematuria (>50 RBC),
normal configuration of kidneys on ultrasonographic examination
The only curative treatment is liver transplantation. All other treatments are only temporizing measures before transplantation.
Multiple randomized trials have documented the therapeutic benefit of the vasoconstrictor terlipressin (given as a 1–2 mg bolus every six hours, or as an infusion of 3 mg over 24 hours) (e11) in combination with albumin (20–100 g/day). A meta-analysis of these trials revealed that patients treated with terlipressin and albumin have a somewhat higher initial survival rate than those treated with albumin alone (15-day mortality: relative risk [RR] = 0.81; 95% confidence interval [95% CI] = 0.68–0.97) (14). Overall survival, however, was not significantly worse in the placebo groups (14).
After a positive response to terlipressin, the insertion of a TIPS can be considered for selected patients (bilirubin concentration <3–5 mg/dL) (e12).
Hyponatremia
Marked hyponatremia (sodium concentration <125 mmol/L) in the setting of cirrhosis is generally dilutional and thus should not be treated with normal saline. Rather, the patient’s oral fluid intake should be restricted (10).
Several trials of V2-receptor antagonists have been conducted recently (15) (vaptans: inhibition of the effect of antidiuretic hormone [ADH] at the collecting tubule). These drugs raise the serum sodium concentration and also mildly improve the response of ascites to diuretics, but they do not prolong survival. The vaptans have therefore not been approved to date for the treatment of hyponatremia in cirrhosis.
Spontaneous bacterial peritonitis
Spontaneous bacterial peritonitis (SBP) is an infection of ascitic fluid without any determinable cause, defined as the demonstration of more than 250 segmented granulocytes per µL of fluid and/or bacteria in the ascitic fluid. SBP must be treated rapidly with antibiotics in all cases.
Uncomplicated cases of SBP acquired in the outpatient setting can be treated with an oral quinolone (10), but consideration must be given to the currently increasing antibiotic resistance of Gram-negative bacteria and the increasing prevalence of Gram-positive bacteria. Nosocomially acquired SBP should be treated with intravenous third-generation cephalosporins; here, too, consideration must be given to possible drug resistance, e.g., ESBL [extended-spectrum beta-lactamase]) production. Alternatively, penicillins with beta-lactamase inhibitors can be given (10).
Empirical antibiotic therapy should be continued for at least five days. The cell count in the ascitic fluid should fall by >25% in two to three days. If not, the antibiotic should be adjusted in accordance with the local pathogen spectrum.
In complicated cases of SBP (i.e., those accompanied by hepatic encephalopathy, shock, ileus, gastrointestinal bleeding, or a bilirubin concentration above 3 mg/dL), the antibiotic should be given intravenously; if a high-risk constellation is present (high bilirubin, poor renal function), albumin should also be given (1.5 g/kg body weight on day 1, 1 g/kg body weight on day 3). It is unclear whether albumin administration would be beneficial for all patients with SBP (10).
The successful treatment of SBP should be followed by a period of antibiotic treatment for secondary prophylaxis. A quinolone or trimethoprim-sulfamethoxazole can be given for this purpose (10). Consideration should be given to increasing quinolone resistance and to the local resistance situation.
Hepatocellular carcinoma
Liver cirrhosis is considered a precancerous condition (e13). The incidence of hepatocellular carcinoma in patients with cirrhosis depends on the underlying etiology; in patients with HBV-associated cirrhosis (HBV = hepatitis B virus), the incidence is 3%–8 % per year (e14). It is recommended that patients with documented hepatic cirrhosis should undergo screening for hepatocellular carcinoma by clinical examination, laboratory testing, and ultrasonography once every six months. Measurement of the tumor marker alpha-1-fetoprotein is recommended by some authors, but not by others (16, 17). For patients with hepatic cirrhosis who have foci exceeding 1 cm in size, the diagnosis of hepatocellular carcinoma (HCC) can be considered to be established if dynamic computed tomography or magnetic resonance imaging with contrast medium reveals the typical findings (contrast enhancement in the early arterial phase and washout in the venous phase); in such cases, further confirmation by biopsy is unnecessary. Biopsy is always required, however, when the foci are less than 1 cm in size and when the imaging studies yield atypical findings; it is also always required if a patient without cirrhosis is suspected of having HCC (16, 17).
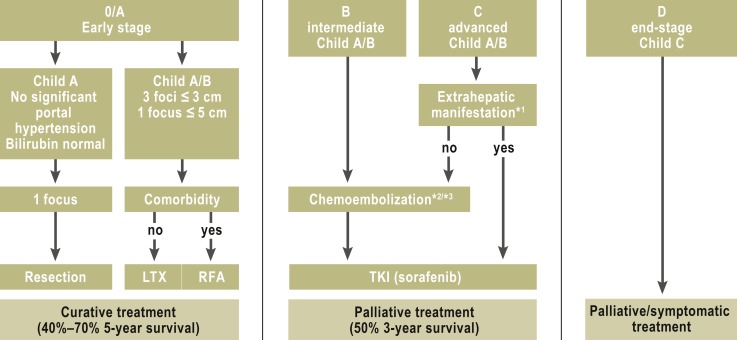
The treatment depends on the stage of disease according to the Barcelona Clinic Liver Cancer (BCLC) staging system, which takes account of the spread of tumor as well as clinical features including the presence or absence of portal hypertension, the degree of hepatic decompensation (Child-Pugh stage), and the patient’s general state of health (fFgure 2) (16, 17).
Figure 2.
The stage-based treatment of hepatocelluar carcinoma (modified Barcelona Clinic Liver Cancer [BCLC] system)
*1Extrahepatic manifestation: N1, M1, and vascular invasion
*2Transarterial Chemoembolization (TACE) is sometimes used as bridging or downstaging in preparation for liver transplantation.
*3If TACE fails or is contraindicated, selective internal radiotherapy (SIRT) can be considered.
LTX, liver transplantation; RFA, radiofrequency ablation; TKI, tyrosine kinase inhibitor (modified from [16])
Hepatocellular carcinoma is resected only in patients with early disease (BCLC stage 0 and A without significant portal hypertension: pressure gradient <10 mm Hg, no esophageal varices, platelet count at least 100 000/mL) and those with a normal serum bilirubin concentration who have a single, small tumor. Patients with stage A or B HCC who meet the Milan criteria (one focus ≤5 cm or at most three foci ≤3 cm) are candidates for liver transplantation (5-year survival up to 70%). An extension of the Milan criteria is currently under consideration. Waiting times for liver transplantation are now usually longer than six months; during this period, patients with HCC can be treated with transarterial chemoembolization (TACE) or radiofrequency ablation (RFA) (17). Such temporizing measures should not, however, be performed with the object of making patients who initially are not satisfactory candidates for transplantation suitable according to the Milan criteria (17).
Curative treatments for HCC include surgery and local ablative techniques such as radiofrequency ablation (BCLC stage A). The recurrence rate after five years, however, can be as high as 70% (e15). Transarterial techniques such as chemoembolization (TACE) are used for BCLC stage B disease; selective internal radiotherapy (SIRT) is used in this stage of disease as well, although its benefit in the treatment of HCC has yet to be documented in controlled trials (16, 17, e16).
Systemic treatment should be considered for patients in BCLC stage C and in cases of tumor progression after local ablative treatment (18). The approval study for the tyrosine kinase inhibitor sorafenib revealed a prolongation of survival in the treatment group from 8 to 11 months (18). In advanced disease (BCLD stage D with Child C cirrhosis and poor general condition), symptomatic relief is the only option; specific treatment for HCC is rarely of any benefit in such cases (Figure 2).
Encephalopathy
Hepatic encephalopathy (HE) is a common complication of cirrhosis. It manifests itself in a broad spectrum of neuropsychiatric disturbances (Table 2), including impairment of
Table 2. The stages of hepatic encephalopathy (HE).
| HE stage | Consciousness | Cognitive function and behavior | Neurologicaldeficits |
| Stage 0 | Normal | Normal | Normal neurological examination, abnormal psychometric test findings |
| Stage 1 | Mild clouding | Short attention span, errors in addition and subtraction | Mild shaking, no tremor |
| Stage 2 | Lethargy | Disorientation, inappropriate behavior | Marked shaking, slurred speech |
| Stage 3 | Somnolent but arousable | Marked disorientation, bizarre behavior | Muscle rigidity, clonus, hyperreflexia |
| Stage 4 | Coma | Coma | Decerebrate posturing |
 | |||
consciousness,
memory and cognition,
motor skills,
and personality.
HE markedly worsens the quality of life of many patients with liver cirrhosis. Patients with HE have a poor prognosis (19). Encephalopathy is often triggered by an infection (spontaneous bacterial peritonitis or urinary tract infection), gastrointestinal bleeding, electrolyte disturbances, portal venous thrombosis, or worsening of liver function.
Hepatic encephalopathy arises as the result of portal-systemic shunting with impaired hepatic removal of neurotoxic substances that are present in the intestinal circulation from portal venous blood; these substances, together with ammonia, cause neurochemical changes. The pathophysiological mechanisms of HE include astrocyte swelling, increased formation of reactive oxygen and nitrogen radicals leading to changes in intracellular proteins and RNA (20), and inflammatory processes in the central nervous system (e17). The neurotoxins and inflammatory mediators involved in these processes are still incompletely understood, as is the role of the intestinal bacterial flora as the source of toxins. Ammonia is the main surrogate marker of HE in the bloodstream, but the blood ammonia concentration is poorly correlated with the degree of psychomotor dysfunction.
HE is subdivided into an acute or episodic type with impaired consciousness, which may range all the way to coma (acute HE); a persistently manifest type (chronic HE); and a type with minimal manifestations (subclinical HE) (e18). Various grading scales have been proposed for the severity of HE (20, e17). The clinical examination can be supplemented by psychometric tests such as the number connection test or the line-tracing test (21, e19, e20). The objective tests that cannot be influenced by a learning effect range from a plain EEG (with characteristic triphasic waves) to measurement of changes in the critical flicker frequency and to the demonstration of delayed sensorimotor evoked potentials (e19, e20).
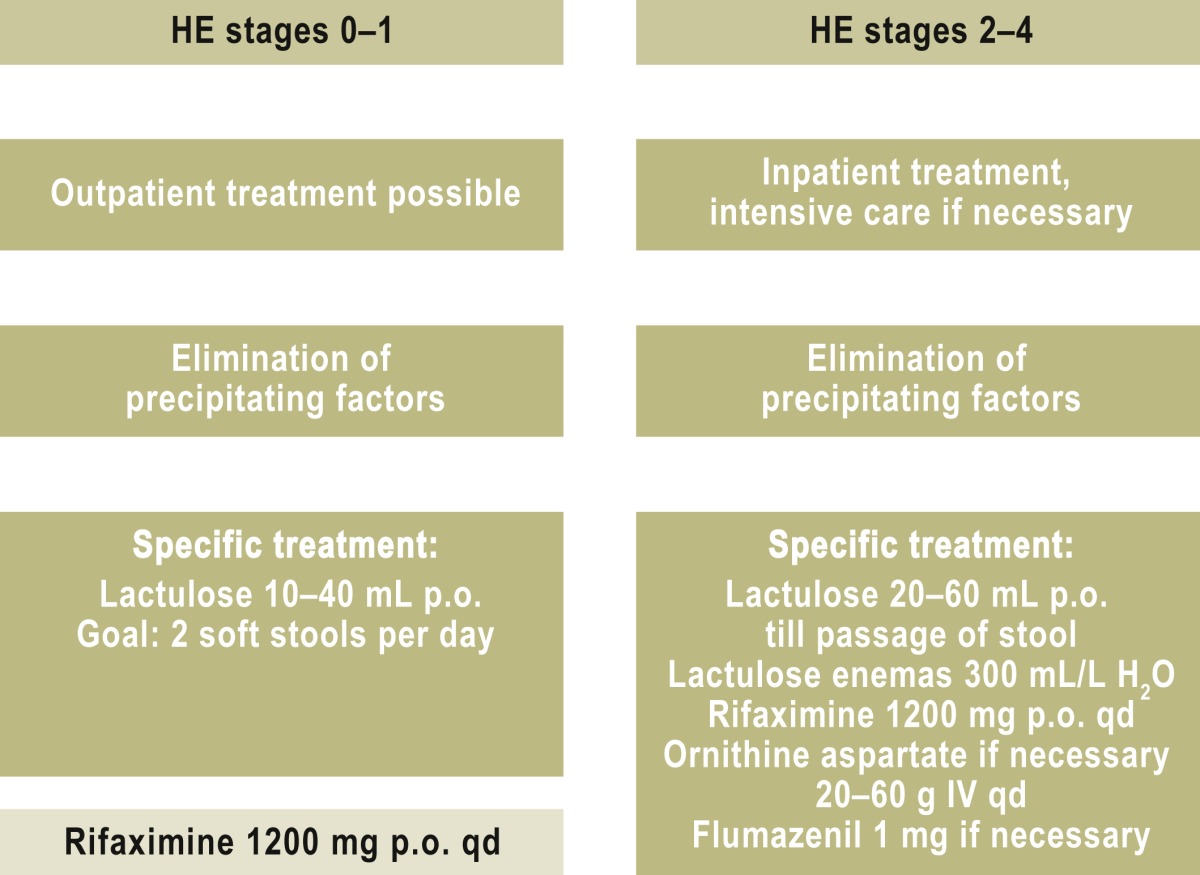
The treatment of hepatic encephalopathy (Table 2) can be divided into the treatment of acute episodes of coma and the treatment and/or prevention of chronic, recurrent encephalopathic states. The goal of treatment is to eliminate precipitating factors (sepsis, gastrointestinal bleeding, electrolyte disturbances, renal insufficiency, constipation) while lowering the concentration of neurotoxins in the bowel through the administration of non-resorbable disaccharides and antibiotics. Although long experience supports the use of these treatments, a Cochrane analysis concluded in 2004 that the available data were of inadequate quality for present-day evidence-based medicine (22). In the meantime, it has in fact been demonstrated that lactulose and the non-resorbable antibiotic rifaximin can prevent recurrent HE episodes (23, 24). Non-resorbable disaccharides, such as lactulose, change the intestinal milieu and thereby alter the colonic bacterial flora.
Moreover, orally administered non-resorbable antibiotics reduce the formation of neurotoxic substances in the bowel. Rifaximin is only minimally resorbed and has a more favorable side-effect profile than other poorly resorbed antibiotics. Further controlled trials have revealed that rifaximin improves psychomotor performance and health-related quality of life in patients with minimal HE (e21, e22). Rifaximin is currently given over the long term for HE prophylaxis (21, e23), but the optimal duration of treatment is unknown. Approval for use of the drug for this indication has recently been granted in Germany.
Dietary protein restriction is obsolete, as it is now recognized that this leads to protein degradation and loss of muscle mass, thereby worsening the nutritional and general condition of patients with cirrhosis. As for other dietary factors, a recent Cochrane analysis came to the conclusion that pre- and probiotic agents do, in fact, lower the serum ammonia concentration, but there is no evidence that they bring about any clinically relevant improvement (25).
Hepatic myelopathy is a very rare but clinically significant variant of HE. Affected patients have practically unaltered consciousness but develop spastic paraparesis, hyperreflexia, and positive Babinski signs. This complication does not respond to the usual conservative treatments for HE and constitutes an urgent indication for liver transplantation (e24).
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Prof. Sauerbruch and Dr. Appenrodt have received lecture honoraria and reimbursement of congress participation fees and travel expenses from the Falk Foundation.
PD Dr. Schmitz and Prof. Spengler state that no conflict of interest exists.
References
- 1.Schepke M, Kleber G, Nürnberg D, et al. German Study Group for the Primary Prophylaxis of Variceal Bleeding. Ligation versus propranolol for the primary prophylaxis of variceal bleeding in cirrhosis. Hepatology. 2004;40:65–72. doi: 10.1002/hep.20284. [DOI] [PubMed] [Google Scholar]
- 2.de Franchis R, Baveno V. Faculty: Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Heptol. 2010;53:762–768. doi: 10.1016/j.jhep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Merkel C, Marin R, Angeli P, et al. Gruppo Triveneto per l’ipertensione Portale. A placebo-controlled clinical trial of nadolol in the prophylaxis of growth of small esophageal varices in cirrhosis. Gastroenterology. 2004;127:476–484. doi: 10.1053/j.gastro.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 4.D’Amico G, Criscuoli V, Fili D, Mocciaro F, Pagliaro L. Meta-analysis of trials for variceal bleeding. Hepatology. 2002;36:1024–1025. doi: 10.1053/jhep.2002.34737. 4 Pt 1 1023 4; author's reply. [DOI] [PubMed] [Google Scholar]
- 5.García-Pagán JC, Caca K, Bureau C, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370–2379. doi: 10.1056/NEJMoa0910102. [DOI] [PubMed] [Google Scholar]
- 6.Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila F, et al. Meta-analysis: antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding - an updated Cochrane review. Aliment Pharmacol Ther. 2011;34:509–518. doi: 10.1111/j.1365-2036.2011.04746.x. [DOI] [PubMed] [Google Scholar]
- 7.Funakoshi N, Ségalas-Largey F, Duny Y, et al. Benefit of combination ß-blocker and endoscopic treatment to prevent variceal rebleeding: a meta-analysis. World J Gastroenterol. 2010;16:5982–5992. doi: 10.3748/wjg.v16.i47.5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding SH, Liu J, Wang JP. Efficacy of beta-adrenergic blocker plus 5-isosorbide mononitrate and endoscopic band ligation for prophylaxis of esophageal variceal rebleeding: a meta-analysis. World J Gastroenterol. 2009;15:2151–2155. doi: 10.3748/wjg.15.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng M, Chen Y, Bai J, et al. Transjugular intrahepatic portosystemic shunt versus endoscopic therapy in the secondary prophylaxis of variceal rebleeding in cirrhotic patients: meta-analysis update. J Clin Gastroenterol. 2008;42:507–516. doi: 10.1097/MCG.0b013e31815576e6. [DOI] [PubMed] [Google Scholar]
- 10.Gerbes AL, Gülberg V, Sauerbruch T, et al. German S 3-guideline “ascites, spontaneous bacterial peritonitis, hepatorenal syndrome”. Z Gastroenterol. 2011;49:749–779. doi: 10.1055/s-0031-1273405. [DOI] [PubMed] [Google Scholar]
- 11.Rössle M, Ochs A, Gülberg V, et al. A comparison of paracentesis and transjugular intrahepatic portosystemic shunting in patients with ascites. N Engl J Med. 2000;342:1701–1707. doi: 10.1056/NEJM200006083422303. [DOI] [PubMed] [Google Scholar]
- 12.Salerno F, Merli M, Riggio O, et al. Cazzaniga M, Valeriano V, Pozzi M, Nicolini A, Salvatori F. Randomized controlled study of TIPS versus paracentesis plus albumin in cirrhosis with severe ascites. Hepatology. 2004;40:629–635. doi: 10.1002/hep.20364. [DOI] [PubMed] [Google Scholar]
- 13.Salerno F, Cammà C, Enea M, Rössle M, Wong F. Transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis of individual patient data. Gastroenterology. 2007;133:825–834. doi: 10.1053/j.gastro.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 14.Gluud LL, Christensen K, Christensen E, Krag A. Systematic review of randomized trials on vasoconstrictor drugs for hepatorenal syndrome. Hepatology. 2010;51:576–584. doi: 10.1002/hep.23286. [DOI] [PubMed] [Google Scholar]
- 15.Ginès P, Wong F, Watson H, Milutinovic S, del Arbol LR, Olteanu D. HypoCAT Study Investigators. Effects of satavaptan, a selective vasopressin V(2) receptor antagonist, on ascites and serum sodium in cirrhosis with hyponatremia: a randomized trial. Hepatology. 2008;48:204–213. doi: 10.1002/hep.22293. [DOI] [PubMed] [Google Scholar]
- 16.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC Clinical Practice Guidelines Management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 19.Cholongitas E, Marelli L, Shusang V, et al. A systematic review of the performance of the model for end-stage liver disease (MELD) in the setting of liver transplantation. Liver Transpl. 2006;12:1049–1061. doi: 10.1002/lt.20824. [DOI] [PubMed] [Google Scholar]
- 20.Häussinger D, Schliess F. Pathogenetic mechanisms of hepatic encephalopathy. Gut. 2008;57:1156–1165. doi: 10.1136/gut.2007.122176. [DOI] [PubMed] [Google Scholar]
- 21.Zhan T, Stremmel W. The diagnosis and treatment of minimal hepatic encephalopathy. Dtsch Arztebl Int. 2012;109(10):180–187. doi: 10.3238/arztebl.2012.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Als-Nielsen B, Gluud LL, Gluud C. Non-absorbable disaccharides for hepatic encephalopathy: systematic review of randomised trials. BMJ. 2004;328 doi: 10.1136/bmj.38048.506134.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma BC, Sharma P, Agrawal A, Sarin SK. Secondary prophylaxis of hepatic encephalopathy: an open-label randomized controlled trial of lactulose versus placebo. Gastroenterology. 2009;137:885–891. doi: 10.1053/j.gastro.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 24.Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071–1081. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- 25.McGee RG, Bakens A, Wiley K, Riordan SM, Webster AC. Probiotics for patients with hepatic encephalopathy. Cochrane Database Syst Rev. 2011;11 doi: 10.1002/14651858.CD008716.pub2. CD008716. [DOI] [PubMed] [Google Scholar]
- e1.Sauerbruch T, Wotzka R, Köpcke W, et al. Prophylactic sclerotherapy before the first episode of variceal hemorrhage in patients with cirrhosis. N Engl J Med. 1988;319:8–15. doi: 10.1056/NEJM198807073190102. [DOI] [PubMed] [Google Scholar]
- e2.Niederau C. Epidemiologie der Leberzirrhose. Hepatitis & More. 2011;1:11–14. [Google Scholar]
- e3.Hearnshaw SA, Logan RF, Lowe D, Travis SP, Murphy MF, Palmer KR. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut. 2011;60:1327–1335. doi: 10.1136/gut.2010.228437. [DOI] [PubMed] [Google Scholar]
- e4.Mishra SR, Sharma BC, Kumar A, Sarin SK. Primary prophylaxis of gastric variceal bleeding comparing cyanoacrylate injection and beta-blockers: a randomized controlled trial. J Hepatol. 2011;54:1161–1167. doi: 10.1016/j.jhep.2010.09.031. [DOI] [PubMed] [Google Scholar]
- e5.Augustin S, Altamirano J, González A, et al. Effectiveness of combined pharmacologic and ligation therapy in high-risk patients with acute esophageal variceal bleeding. Am J Gastroenterol. 2011;106:1787–1795. doi: 10.1038/ajg.2011.173. [DOI] [PubMed] [Google Scholar]
- e6.Arvaniti V, D’Amico G, Fede G, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–1256. doi: 10.1053/j.gastro.2010.06.019. 1256.e1-5. [DOI] [PubMed] [Google Scholar]
- e7.Angermayr B, Cejna M, Koenig F, et al. Vienna TIPS Study Group Survival in patients undergoing transjugular intrahepatic portosystemic shunt: ePTFE-covered stentgrafts versus bare stents. Hepatology. 2003;38:1043–1050. doi: 10.1053/jhep.2003.50423. [DOI] [PubMed] [Google Scholar]
- e8.Gines P, Quintero E, Arroyo V, et al. Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987;7:122–128. doi: 10.1002/hep.1840070124. [DOI] [PubMed] [Google Scholar]
- e9.Sola-Vera J, Miñana J, Ricart E. Randomized trial comparing albumin and saline in the prevention of paracentesis-induced circulatory dysfunction in cirrhotic patients with ascites. Hepatology. 2003;37:1147–1153. doi: 10.1053/jhep.2003.50169. [DOI] [PubMed] [Google Scholar]
- e10.Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310–1318. doi: 10.1136/gut.2006.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e11.Gerbes AL, Huber E, Gülberg V. Terlipressin for hepatorenal syndrome: continuous infusion as an alternative to iv. bolus administration. Gastroenterology. 2009;137:1179–1181. doi: 10.1053/j.gastro.2009.03.064. 1179; author reply. [DOI] [PubMed] [Google Scholar]
- e12.Brensing KA, Textor J, Perz J, et al. Long term outcome after transjugular intrahepatic portosystemic stent-shunt in non-transplant cirrhotics with hepatorenal syndrome: a phase II study. Gut. 2000;47:288–295. doi: 10.1136/gut.47.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e13.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- e14.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- e15.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- e16.Sangro B, Carpanese L, Cianni R, et al. European Network on Radioembolization with Yttrium-90 Resin Microspheres (ENRY). Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868–878. doi: 10.1002/hep.24451. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- e17.Butterworth RF. Hepatic encephalopathy: a central neuroinflammatory disorder? Hepatology. 2011;53:1372–1376. doi: 10.1002/hep.24228. [DOI] [PubMed] [Google Scholar]
- e18.Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy-definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–721. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- e19.Bajaj JS. Review article: the modern management of hepatic encephalopathy. Aliment Pharmacol Ther. 2010;31:537–547. doi: 10.1111/j.1365-2036.2009.04211.x. [DOI] [PubMed] [Google Scholar]
- e20.Prakash R, Mullen KD. Mechanisms, diagnosis and management of hepatic encephalopathy. Nat Rev Gastroenterol Hepatol. 2010;7:515–525. doi: 10.1038/nrgastro.2010.116. [DOI] [PubMed] [Google Scholar]
- e21.Sidhu SS, Goyal O, Mishra BP, Sood A, Chhina RS, Soni RK. Rifaximin improves psychometric performance and health-related quality of life in patients with minimal hepatic encephalopathy (the RIME Trial) Am J Gastroenterol. 2011;106:307–316. doi: 10.1038/ajg.2010.455. [DOI] [PubMed] [Google Scholar]
- e22.Bajaj JS, Heuman DM, Wade JB, et al. Rifaximin improves driving simulator performance in a randomized trial of patients with minimal hepatic encephalopathy. Gastroenterology. 2011;140:478–487. doi: 10.1053/j.gastro.2010.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e23.Neff GW, Jones M, Broda T, et al. Durability of rifaximin response in hepatic encephalopathy. J Clin Gastroenterol. 2012;46:168–171. doi: 10.1097/MCG.0b013e318231faae. [DOI] [PubMed] [Google Scholar]
- e24.Caldwell C, Werdiger N, Jakab S, et al. Use of model for end-stage liver disease exception points for early liver transplantation and successful reversal of hepatic myelopathy with a review of the literature. Liver Transpl. 2010;16 M:818–826. doi: 10.1002/lt.22077. [DOI] [PubMed] [Google Scholar]