Abstract
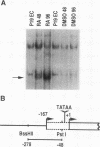
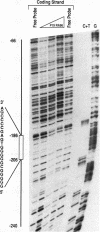
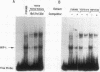
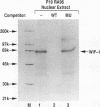
The Wnt-1 proto-oncogene is essential for proper development of the midbrain and is expressed in a spatially and temporally restricted manner during central nervous system development in mice. In vitro, the gene is specifically transcribed during the retinoic acid (RA)-induced neuroectodermal differentiation of the P19 line of embryonal carcinoma cells. The P19 cells differentiate into neurons, astrocytes, and fibroblast-like cells when treated with RA. Treatment of the cells with dimethyl sulfoxide leads to differentiation along mesodermal lineages, including skeletal and cardiac muscle. We have used the P19 cell line to study the Wnt-1 promoter and identify and characterize the transcription factor(s) that regulates the differentiation-specific transcription of Wnt-1 in RA-treated P19 cultures. Transient-transfection assays have revealed that a 230-bp region comprising positions -278 to -47 of the 5' upstream Wnt-1 sequence was sufficient to direct RA-specific transcription. This promoter fragment was shown to contain a binding site for a nuclear factor that was not detected in undifferentiated P19 stem cells or their dimethyl sulfoxide-treated derivatives but was induced in differentiating RA-treated cells. This factor was termed Wnt-1-inducing factor-1 (WiF-1). DNase I footprinting analysis has identified the G/C-rich WiF-1 binding site, and UV cross-linking studies have shown that WiF-1 is a protein with an M(r) of 65,000. WiF-1 binding activity was also detected in postpubertal mouse testis, the only tissue that expresses Wnt-1 in adults. Site-directed mutations that inhibited WiF-1 binding to the Wnt-1 promoter concomitantly abolished the activity of the promoter in RA-treated P19 cells. The active WiF-1 protein was purified by DNA affinity chromatography. Our data suggest that WiF-1 is a novel G/C box-binding transcription factor and support a physiological role for WiF-1 in the developmentally regulated expression of Wnt-1.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briggs M. R., Kadonaga J. T., Bell S. P., Tjian R. Purification and biochemical characterization of the promoter-specific transcription factor, Sp1. Science. 1986 Oct 3;234(4772):47–52. doi: 10.1126/science.3529394. [DOI] [PubMed] [Google Scholar]
- Bunick D., Johnson P. A., Johnson T. R., Hecht N. B. Transcription of the testis-specific mouse protamine 2 gene in a homologous in vitro transcription system. Proc Natl Acad Sci U S A. 1990 Feb;87(3):891–895. doi: 10.1073/pnas.87.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P., Zerial M., Lemaire P., Almendral J., Bravo R., Charnay P. A gene encoding a protein with zinc fingers is activated during G0/G1 transition in cultured cells. EMBO J. 1988 Jan;7(1):29–35. doi: 10.1002/j.1460-2075.1988.tb02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby S. D., Puetz J. J., Simburger K. S., Fahrner T. J., Milbrandt J. The early response gene NGFI-C encodes a zinc finger transcriptional activator and is a member of the GCGGGGGCG (GSG) element-binding protein family. Mol Cell Biol. 1991 Aug;11(8):3835–3841. doi: 10.1128/mcb.11.8.3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darland T., Samuels M., Edwards S. A., Sukhatme V. P., Adamson E. D. Regulation of Egr-1 (Zfp-6) and c-fos expression in differentiating embryonal carcinoma cells. Oncogene. 1991 Aug;6(8):1367–1376. [PubMed] [Google Scholar]
- Davis C. A., Joyner A. L. Expression patterns of the homeo box-containing genes En-1 and En-2 and the proto-oncogene int-1 diverge during mouse development. Genes Dev. 1988 Dec;2(12B):1736–1744. doi: 10.1101/gad.2.12b.1736. [DOI] [PubMed] [Google Scholar]
- DiNardo S., Sher E., Heemskerk-Jongens J., Kassis J. A., O'Farrell P. H. Two-tiered regulation of spatially patterned engrailed gene expression during Drosophila embryogenesis. Nature. 1988 Apr 14;332(6165):604–609. doi: 10.1038/332604a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. K., Harris J. F., McBurney M. W. Induced muscle differentiation in an embryonal carcinoma cell line. Mol Cell Biol. 1983 Dec;3(12):2280–2286. doi: 10.1128/mcb.3.12.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. K., Shackleford G. M., Brown A. M., Sanders G. S., Varmus H. E. Nucleotide sequence and expression in vitro of cDNA derived from mRNA of int-1, a provirally activated mouse mammary oncogene. Mol Cell Biol. 1985 Dec;5(12):3337–3344. doi: 10.1128/mcb.5.12.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin B. J., McMahon J. A., McMahon A. P. Expression of multiple novel Wnt-1/int-1-related genes during fetal and adult mouse development. Genes Dev. 1990 Dec;4(12B):2319–2332. doi: 10.1101/gad.4.12b.2319. [DOI] [PubMed] [Google Scholar]
- Huang H. C., Sundseth R., Hansen U. Transcription factor LSF binds two variant bipartite sites within the SV40 late promoter. Genes Dev. 1990 Feb;4(2):287–298. doi: 10.1101/gad.4.2.287. [DOI] [PubMed] [Google Scholar]
- Jackson S. P., Tjian R. Purification and analysis of RNA polymerase II transcription factors by using wheat germ agglutinin affinity chromatography. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1781–1785. doi: 10.1073/pnas.86.6.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobovits A., Shackleford G. M., Varmus H. E., Martin G. R. Two proto-oncogenes implicated in mammary carcinogenesis, int-1 and int-2, are independently regulated during mouse development. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7806–7810. doi: 10.1073/pnas.83.20.7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Villeneuve E. M., McBurney M. W., Rogers K. A., Kalnins V. I. Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. J Cell Biol. 1982 Aug;94(2):253–262. doi: 10.1083/jcb.94.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Yamamoto K. R., Tjian R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985 Sep;42(2):559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T. Purification of sequence-specific binding proteins by DNA affinity chromatography. Methods Enzymol. 1991;208:10–23. doi: 10.1016/0076-6879(91)08004-2. [DOI] [PubMed] [Google Scholar]
- Kageyama R., Merlino G. T., Pastan I. Nuclear factor ETF specifically stimulates transcription from promoters without a TATA box. J Biol Chem. 1989 Sep 15;264(26):15508–15514. [PubMed] [Google Scholar]
- Kageyama R., Pastan I. Molecular cloning and characterization of a human DNA binding factor that represses transcription. Cell. 1989 Dec 1;59(5):815–825. doi: 10.1016/0092-8674(89)90605-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanoix J., Belhumeur P., Lussier M., Royal A., Bravo R., Skup D. Regulated expression of Krox-24 and other serum-responsive genes during differentiation of P19 embryonal carcinoma cells. Cell Growth Differ. 1991 Aug;2(8):391–399. [PubMed] [Google Scholar]
- Lemaire P., Vesque C., Schmitt J., Stunnenberg H., Frank R., Charnay P. The serum-inducible mouse gene Krox-24 encodes a sequence-specific transcriptional activator. Mol Cell Biol. 1990 Jul;10(7):3456–3467. doi: 10.1128/mcb.10.7.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney M. W., Jones-Villeneuve E. M., Edwards M. K., Anderson P. J. Control of muscle and neuronal differentiation in a cultured embryonal carcinoma cell line. Nature. 1982 Sep 9;299(5879):165–167. doi: 10.1038/299165a0. [DOI] [PubMed] [Google Scholar]
- McBurney M. W., Reuhl K. R., Ally A. I., Nasipuri S., Bell J. C., Craig J. Differentiation and maturation of embryonal carcinoma-derived neurons in cell culture. J Neurosci. 1988 Mar;8(3):1063–1073. doi: 10.1523/JNEUROSCI.08-03-01063.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon A. P., Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990 Sep 21;62(6):1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- McMahon A. P., Joyner A. L., Bradley A., McMahon J. A. The midbrain-hindbrain phenotype of Wnt-1-/Wnt-1- mice results from stepwise deletion of engrailed-expressing cells by 9.5 days postcoitum. Cell. 1992 May 15;69(4):581–595. doi: 10.1016/0092-8674(92)90222-x. [DOI] [PubMed] [Google Scholar]
- McMahon A. P., Moon R. T. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell. 1989 Sep 22;58(6):1075–1084. doi: 10.1016/0092-8674(89)90506-0. [DOI] [PubMed] [Google Scholar]
- Molven A., Njølstad P. R., Fjose A. Genomic structure and restricted neural expression of the zebrafish wnt-1 (int-1) gene. EMBO J. 1991 Apr;10(4):799–807. doi: 10.1002/j.1460-2075.1991.tb08012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. F., Madden S. L., Tournay O. E., Cook D. M., Sukhatme V. P., Rauscher F. J., 3rd Characterization of the zinc finger protein encoded by the WT1 Wilms' tumor locus. Oncogene. 1991 Dec;6(12):2339–2348. [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- NEBEL B. R., AMAROSE A. P., HACKET E. M. Calendar of gametogenic development in the prepuberal male mouse. Science. 1961 Sep 22;134(3482):832–833. doi: 10.1126/science.134.3482.832. [DOI] [PubMed] [Google Scholar]
- Noordermeer J., Meijlink F., Verrijzer P., Rijsewijk F., Destrée O. Isolation of the Xenopus homolog of int-1/wingless and expression during neurula stages of early development. Nucleic Acids Res. 1989 Jan 11;17(1):11–18. doi: 10.1093/nar/17.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R., Theunissen H., Wagenaar E., Rijsewijk F., Gennissen A., Otte A., Schuuring E., van Ooyen A. The Wnt-1 (int-1) oncogene promoter and its mechanism of activation by insertion of proviral DNA of the mouse mammary tumor virus. Mol Cell Biol. 1990 Aug;10(8):4170–4179. doi: 10.1128/mcb.10.8.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982 Nov;31(1):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E. Wnt genes. Cell. 1992 Jun 26;69(7):1073–1087. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- Patwardhan S., Gashler A., Siegel M. G., Chang L. C., Joseph L. J., Shows T. B., Le Beau M. M., Sukhatme V. P. EGR3, a novel member of the Egr family of genes encoding immediate-early transcription factors. Oncogene. 1991 Jun;6(6):917–928. [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Morris J. F., Tournay O. E., Cook D. M., Curran T. Binding of the Wilms' tumor locus zinc finger protein to the EGR-1 consensus sequence. Science. 1990 Nov 30;250(4985):1259–1262. doi: 10.1126/science.2244209. [DOI] [PubMed] [Google Scholar]
- Rijsewijk F., Schuermann M., Wagenaar E., Parren P., Weigel D., Nusse R. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell. 1987 Aug 14;50(4):649–657. doi: 10.1016/0092-8674(87)90038-9. [DOI] [PubMed] [Google Scholar]
- Roelink H., Nusse R. Expression of two members of the Wnt family during mouse development--restricted temporal and spatial patterns in the developing neural tube. Genes Dev. 1991 Mar;5(3):381–388. doi: 10.1101/gad.5.3.381. [DOI] [PubMed] [Google Scholar]
- Roelink H., Wagenaar E., Lopes da Silva S., Nusse R. Wnt-3, a gene activated by proviral insertion in mouse mammary tumors, is homologous to int-1/Wnt-1 and is normally expressed in mouse embryos and adult brain. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4519–4523. doi: 10.1073/pnas.87.12.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuuring E., van Deemter L., Roelink H., Nusse R. Transient expression of the proto-oncogene int-1 during differentiation of P19 embryonal carcinoma cells. Mol Cell Biol. 1989 Mar;9(3):1357–1361. doi: 10.1128/mcb.9.3.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleford G. M., Varmus H. E. Expression of the proto-oncogene int-1 is restricted to postmeiotic male germ cells and the neural tube of mid-gestational embryos. Cell. 1987 Jul 3;50(1):89–95. doi: 10.1016/0092-8674(87)90665-9. [DOI] [PubMed] [Google Scholar]
- Simmons D. M., Voss J. W., Ingraham H. A., Holloway J. M., Broide R. S., Rosenfeld M. G., Swanson L. W. Pituitary cell phenotypes involve cell-specific Pit-1 mRNA translation and synergistic interactions with other classes of transcription factors. Genes Dev. 1990 May;4(5):695–711. doi: 10.1101/gad.4.5.695. [DOI] [PubMed] [Google Scholar]
- Sokol S., Christian J. L., Moon R. T., Melton D. A. Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell. 1991 Nov 15;67(4):741–752. doi: 10.1016/0092-8674(91)90069-b. [DOI] [PubMed] [Google Scholar]
- St-Arnaud R., Craig J., McBurney M. W., Papkoff J. The int-1 proto-oncogene is transcriptionally activated during neuroectodermal differentiation of P19 mouse embryonal carcinoma cells. Oncogene. 1989 Sep;4(9):1077–1080. [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature. 1990 Aug 30;346(6287):847–850. doi: 10.1038/346847a0. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Musci T. S., Neumann P. E., Capecchi M. R. Swaying is a mutant allele of the proto-oncogene Wnt-1. Cell. 1991 Nov 29;67(5):969–976. doi: 10.1016/0092-8674(91)90369-a. [DOI] [PubMed] [Google Scholar]
- Uzvölgyi E., Kiss I., Pitt A., Arsenian S., Ingvarsson S., Udvardy A., Hamada M., Klein G., Sümegi J. Drosophila homolog of the murine Int-1 protooncogene. Proc Natl Acad Sci U S A. 1988 May;85(9):3034–3038. doi: 10.1073/pnas.85.9.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D. G., Bailes J. A., McMahon A. P. Expression of the proto-oncogene int-1 is restricted to specific neural cells in the developing mouse embryo. Cell. 1987 Jul 3;50(1):79–88. doi: 10.1016/0092-8674(87)90664-7. [DOI] [PubMed] [Google Scholar]
- Williams T., Tjian R. Analysis of the DNA-binding and activation properties of the human transcription factor AP-2. Genes Dev. 1991 Apr;5(4):670–682. doi: 10.1101/gad.5.4.670. [DOI] [PubMed] [Google Scholar]
- Wu C., Wilson S., Walker B., Dawid I., Paisley T., Zimarino V., Ueda H. Purification and properties of Drosophila heat shock activator protein. Science. 1987 Nov 27;238(4831):1247–1253. doi: 10.1126/science.3685975. [DOI] [PubMed] [Google Scholar]
- van Ooyen A., Kwee V., Nusse R. The nucleotide sequence of the human int-1 mammary oncogene; evolutionary conservation of coding and non-coding sequences. EMBO J. 1985 Nov;4(11):2905–2909. doi: 10.1002/j.1460-2075.1985.tb04021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]