Abstract
Despite the dominant role of the hormone oxytocin (OT) in social behavior, little is known about the role of OT in the perception of social relationships. Furthermore, it is unclear whether there are sex differences in the way that OT affects social perception. Here, we employed a double-blind, placebo-controlled crossover design to investigate the effect of OT on accurate social perception. Following treatment, 62 participants completed the Interpersonal Perception Task, a method of assessing the accuracy of social judgments that requires identification of the relationship between people interacting in real life video clips divided into three categories: kinship, intimacy and competition. The findings suggest that OT had a general effect on improving accurate perception of social interactions. Furthermore, we show that OT also involves sex-specific characteristics. An interaction between treatment, task category and sex indicated that OT had a selective effect on improving kinship recognition in women, but not in men, whereas men's performance was improved following OT only for competition recognition. It is concluded that the gender-specific findings reported here may point to some biosocial differences in the effect of OT which may be expressed in women's tendency for communal and familial social behavior as opposed to men's tendency for competitive social behavior.
Keywords: oxytocin, social perception, sex differences, interpersonal behavior
INTRODUCTION
The effects of the neuropeptide oxytocin (OT) on adaptive social behavior have been the topic of increasing investigation in recent years, both in animals as well as in humans. The administration of OT in humans has been associated with a variety of complex social behaviors, including the facilitation of processing non-verbal social stimuli (Guastella et al., 2008; Rimmele et al., 2009; Fischer-Shofty et al., 2010) and social behaviors such as empathy (Domes et al., 2007), generosity (Zak et al., 2007) and eye gaze detection (Guastella et al., 2008). In the current study, we investigated whether the administration of OT affects social perception, or the ability to use non-verbal cues to interpret social interactions and relationships. Social perception predicts levels of sociability and social competence (Costanzo and Archer, 1989) and deficits in this ability are reported in various neuropsychiatric disorders (Pinkham et al., 2003), indicating its importance in everyday social behavior.
Although sex differences in social behavior are well established (Hofer et al., 2006, 2007; Schulte-Ruther et al., 2008; Derntl et al., 2010), to date little is known regarding the differential effects of the administration of OT on women and men's social behavior. Although few studies have examined sex differences in social behavior associated with OT, so far, studies that compared women and men in their designs have failed to find sex differences in the effects of OT (Alvares et al., 2010; Marsh et al., 2010; Rockliff et al., 2011).
Evolutionary psychology suggests that human mating strategies have shaped the minds and bodies of men and women differently (Buss and Schmitt, 1993; Geary, 1998). Whereas women focus more on maintaining supportive social networks for the protection of their offspring (Taylor et al., 2000; Silk, 2007), men invest their resources in inter-group aggression, which may enhance mating opportunities (Van Vugt et al., 2007). In line with this approach, Taylor et al. argued that while the physiological endocrine response to stress is similar in both sexes, the familiar fight-or-flight human response to stress may particularly characterize male behavior. Thus, while women enact the fight-or-flight reaction, they are more likely to react to stress through social communication or tend-and-befriend behaviors. Moreover, as Taylor et al. suggested, OT might be involved in the biological basis for this feminine mechanism.
Indeed, due to its imperative role in maternal functions such as labor, breastfeeding and the onset of maternal bonding, OT is traditionally viewed as a feminine hormone (see Lim and Young, 2006 for a review). It has been shown that administration of OT accelerates offspring recognition in females (Bielsky and Young, 2004), demonstrating its role in maternal behavior. In human males, intranasal administration of OT has been associated with increased trust (Kosfeld et al., 2005), but also with aggressive responses and derogation of out-group members (De Dreu et al., 2011), suggesting that OT may have a differential effect on social perception depending on the social context and depending on individual differences.
Given the sex differences in social behavior and the possible differences between men and women in their reaction to OT, the present study compared the performance of men and women following the intranasal administration of OT. To assess social perception, we used the Interpersonal Perception Task (IPT; Costanzo and Archer, 1989), which consists of various videotaped social interactions. For each interaction, the participants were required to make judgments about different aspects of the relationships presented, specifically in terms of kinship, intimacy and competition. The IPT is unique in that the scenes present unrehearsed, spontaneous behaviors that are more representative of real-life social situations than those employed in other social cognition tools. The responses of participants rely on diverse multimodal non-verbal cues, such as gestures, motions, proximity between the characters, their facial expressions and emotional prosody. The first goal of the present study was to examine the effect of the administration of OT on the accurate perception of social interactions.
Given the paucity of reports of sex difference in the effects of OT in humans, the second goal of this study was to examine the effects of OT on complex social perceptions in both sexes, thereby enabling a clearer understanding of the differential effects of this hormone on interpersonal perception among men and women. In line with the role of OT in maternal behavior and offspring recognition in females, it was speculated that OT would particularly facilitate kinship recognition in women, but not in men. Furthermore, given men's tendency for inter-group aggression and competition (Taylor et al., 2000; Van Vugt, 2009), it was speculated that OT would facilitate the perception of competition in men. Finally, it was predicted that OT would enhance understanding of intimate relationships in both men and women.
METHODS
Participants
Participants were 62 healthy participants (39 women and 23 men), ranging in age from 20 to 37 years (mean = 26.54, s.d. = 3.56). Recruitment was carried out using advertisements posted across campus at the University of Haifa, and a financial reward was offered for participation. All participants were interviewed by a senior psychiatrist. Exclusion criteria were acute, unstable, significant, or untreated medical illnesses (including arrhythmia, psychiatric conditions and head injury); a history of alcohol or drug abuse; mental retardation (IQ <75); and disturbances in visuomotor coordination. Since there are indications of interactions between OT and levels of estrogen among women (Razzoli et al., 2003), the women sample consisted of three groups: 15 women that were using contraceptive pills, 14 women in various stages of their menstrual cycle and 10 women with a regular 28-day cycle, who were during the follicular phase of their menstrual cycle (approximately Days 6 through 13).
After giving their oral and written consent, the participants were instructed to avoid using psychotropic substances (e.g. caffeine and nicotine) for at least 12 h prior to the experiment. The study protocol was approved by the Helsinki committee of Shalvata Mental Health Center, as well as by the Israeli Ministry of Health.
Treatment administration
A double-blind within-subject crossover design was used with participants randomly assigned into groups for the first administration of either OT or placebo. Participants received a single intranasal dose of OT (24 IU, Syntocinon-Spray, Novartis) or placebo 45 min prior to performing the behavioral task. Those initially receiving OT were administered 24 IU of OT via three puffs in each nostril (each puff contains 4 IU). On the second session of the experiment, 7 days later, participants underwent the same procedure with the other substance (i.e. placebo or OT). The placebo contained all inactive ingredients except for the neuropeptide. Dosage and waiting time correspond to those previously used in experiments designed to investigate the effect of intranasal administration of OT on behavior in humans (Kirsch et al., 2005; Kosfeld et al., 2005; Domes et al., 2007, 2010; Guastella et al., 2008).
The Interpersonal Perception Task
The Interpersonal Perception Task (IPT) (Costanzo and Archer, 1989) is a standardized method of assessing the accuracy of social judgments about interpersonal situations viewed on videotape. The IPT was designed to assess the perception of non-verbal aspects of social exchange, including facial expressions and body language, as well as the social meaning of statements. Thus, participants need to ‘read between the lines’ of verbal dialogue and interpret non-verbal cues in order to accurately make interpersonal judgments. Participants are required to view brief, real-life spontaneous scenes, each of which is preceded by a question that requires the subject to make a judgment about the relationship between the individuals involved in the interaction.
Three categories of relationship were used as response options in the present study: kinship, competition and intimacy. In the kinship condition, participants had to recognize close familial relationships. In the competition condition, participants had to identify which one of two interacting characters won the competition. In the intimacy condition, participants had to accurately identify a romantic relationship between couples. Scene selection was based on accuracy rates for each of the scenes (Costanzo and Archer, 1989) and category selection was based on prior research regarding the role of OT in intimate and familial relationships. Validation studies demonstrate that performance on the IPT correlates with self- and peer-ratings of sociability and social competence. Test–retest reliability for the IPT over a 5-week period was found to be 0.70 (Costanzo and Archer, 1989).
The Depression Adjective Check Lists
An adapted version of the Depression Adjective Check Lists (DACLs; Lubin, 1965) was used to evaluate general mood changes following administration of the OT or placebo. The DACL is a self-report instrument comprised of a 32-item adjective checklist, describing various mood states. The participant is requested to choose from the list the words that best describe his current mood. It is useful in the measurement of transient mood and the immediate effects of environmental (internal and external) influences, as well as in the daily monitoring of mood. In order to rule out the possibility that OT had a general effect on mood which could have influenced participants’ performance on the task, we examined DACL ratings immediately before task performance. For each participant, the number of positive and negative adjectives was calculated in each session (OT and placebo).
RESULTS
Mood assessment
A two-way repeated measures ANOVA indicated no drug effect [F(1,61) = 0.073, P = 0.788] or interaction of valence (positive, negative) by treatment [F(1,61) = 1.152, P = 0.287] for moods ratings. There was a general valence effect [F(1,61) = 14.906, P = 0.0001], demonstrating that participants were generally in a good mood; however, their mood was not affected by the administration of OT.
Social perception
In order to examine the interaction between treatment, relationship category and sex, a three-way repeated measures ANOVA was performed between the type of treatment administered (OT/placebo) and relationship category (kinship/competition/intimacy) as within-subjects factors and sex as a between-subjects factor. A significant treatment effect [F(1,60) = 10.041, P < 0.01] was found, indicating a general drug effect for OT on participants’ performance. In addition, a significant relationship category effect was evident [F(2,59) = 10.537, P < 0.001]. Further analysis using paired samples t-tests indicated that the accuracy levels for intimacy [39.52 (30.21)] were significantly lower than those for kinship [57.26 (33.69)] or competition [56.45 (29.31)], with no significant difference found in accuracy levels between the latter two categories. No significant interaction between treatment and relationship category was found [F(2,59) = 0.646, ns].
The analysis of overall sex differences showed that there was no significant effect [F(1,60) = 0.222, ns], demonstrating that in general, performance on the task by men and women was similar. Moreover, there was no interaction between sex and treatment [F(1,60) = 1.245, ns] or between sex and relationship category [F(2,59) = 1.827, ns].
Importantly, the three-way interaction between treatment type, relationship category and sex was significant [F(2,59) = 3.625, P = 0.033], indicating a differential effect of OT on the ability to perceive the different relationship categories in both sexes.
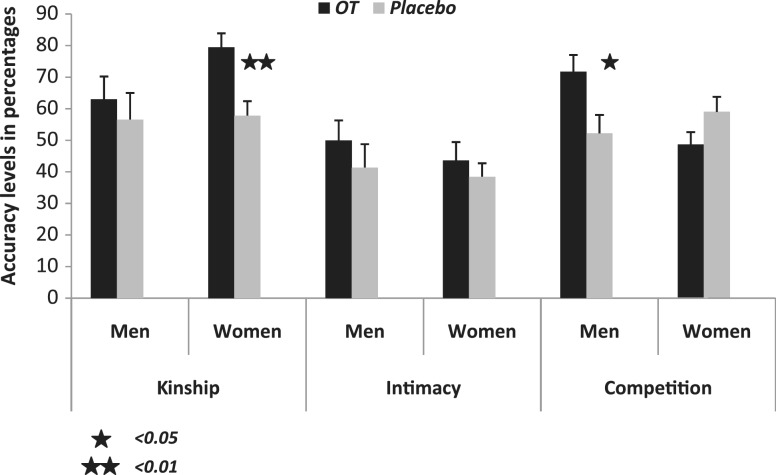
In order to further explore the source of the three-way interaction, separate follow-up t-tests were performed for each sex. This analysis revealed a significant difference between the OT and the placebo conditions in women only for the recognition of the kinship category [t(38) = 3.791, P = 0.001], but not for that of intimate category [t(38) = 0.726, ns] or competitive category [t(38) = −1.6, ns]. Thus, OT treatment significantly improved women's accuracy level only in the kinship category. On the other hand, a significant difference between the OT and the placebo conditions was found in men only for the recognition of competitive category [t(22) = 2.4, P = 0.025], but not for that of kinship category [t(22) = 0.62, ns] or intimate category [t(22) = 0.85, ns]. Thus, OT treatment significantly improved men's accuracy level only in the competition category. It is important to note here that although OT did not have a significant effect on recognizing intimate relationship, as shown in Figure 1, the accuracy rates in this condition were 5% higher following the administration of OT as compared to placebo among women and 9% among men.
Fig. 1.
Repeated measures ANOVA revealed a significant treatment effect and significant treatment by condition by gender effect. Follow-up t-tests revealed a significant treatment effect only for kinship relationships in women and only for competitive relationships in men.
In order to rule out the possibility that the participants' performance was affected by the order in which they received the two treatments, we re-analyzed the data with treatment type and relationship category as within-subjects factors and sex as a between-subjects factor, while adding group (OT in the first session/OT in the second session) as a second between-subjects factor. This analysis revealed no significant group effect [F(1,58) = 0.138, ns] or any significant interaction with either of the factors, confirming that the order in which the treatments were given had no influence on the performance of the participants.
In order to rule out the possibility that the women's performance was affected by the use of contraceptives or menstrual cycle, we re-analyzed the data with treatment type and relationship category as within-subjects factors and pill group (male/female using contraceptives/female not taking contraceptives on their follicular phase/female not taking contraceptives on various phases) as a between-subjects factor. This analysis revealed that taking contraceptives did not interact with either OT [F(3,58) = 0.135, ns], task category [F(6,112) = 0.93, ns] or with OT and task category [F(6,112) = 0.837, ns].
DISCUSSION
The main findings of the current study indicate that a single dose of OT, administered intranasally, may facilitate the perception of interpersonal relationships. These findings are in agreement with previous studies demonstrating that the administration of OT improves empathic accuracy (Bartz et al., 2010), complex emotion recognition (Domes et al., 2007), emotional empathy (Barraza and Zak, 2009; Rodrigues et al., 2009; Hurlemann et al., 2010) and emotion perception (Domes et al., 2010; Marsh et al., 2010; Schulze et al., 2011). The task used here appears to involve in addition to basic emotion recognition and empathy the ability to integrate several different non-verbal cues involved in social interactions (gestures, motion, facial expression, prosody) and draw conclusions regarding the relationships between social agents. Indeed, it has been demonstrated that individuals with frontal lesions, who show difficulty in integrating different social cues, exhibit impaired performance in this task (Mah et al., 2004).
In addition to the main effect of OT on understanding social relationship, this study is the first to show a gender-specific behavioral effect of OT: while women showed increased ability to recognize familial relationships, men demonstrated improved identification of competitive relationships following the administration of OT. Interestingly, OT had only mild non-significant effect for the recognition of intimate relationships, in both women and men. This surprising finding might reflect the difficulty of this particular relationship category, as accuracy levels for both men and women were significantly lower than the other categories. Similarly to our results, intimacy recognition in this task was previously shown to be the most difficult of all categories for both sexes (Archer et al., 1993).
The sex differences in the oxytocinergic system of humans reported here are consistent with the recent report of Domes et al. (2010), which showed a different neural activation pattern of OT in women, as opposed to the previously reported activation found in men. In their study, women who received OT exhibited increased amygdalar activation in response to fearful facial expressions, while men typically displayed the opposite pattern of decreased activation of the amygdala under the same conditions. As in the present study, Domes et al.'s results suggest a differential effect of OT on both sexes in the context of processing social behavior. Likewise, Gordon et al. (2010) recently reported a differential behavioral effect of OT in mothers and fathers. In their study, they report that maternal OT levels correlate with affectionate behavior toward the offspring, as expressed in vocalizations and facial expressions, while paternal OT levels correlated with stimulatory behavior, including physical contact and tactile stimulation (Gordon et al., 2010). Similarly to the present study's findings, these results demonstrate that gender differences in OT may be evident in different categories of social behaviors.
Furthermore, the gender-specific outcome reported here points to some stereotypical characteristics of the effect that intranasal administration of OT has on individuals’ social perception. These results coincide with the biosocial origin theory presented by Wood and Eagly (2002), suggesting that biological factors, mainly hormonal, together with social and cultural factors, act to influence behavioral sex differences. These differences are expressed in women's pro-social behavioral tendencies, by which they tend to be more communal and familial in their behavior, whereas men are more inclined to be agentic, that is, masterful, competitive and striving to improve their social status. According to Wood and Eagly's theory, OT is suggested as a key hormone influencing pro-social behavior.
However, contrary to Wood and Eagly's proposition, who ascribe OT behavioral influences primarily to women, our results clearly show that this hormone impacts the social behavior of both sexes, though the type of behavior influenced in this context is different and in line with gender roles. As such, OT was shown to improve women's cognition of familial relationships, corresponding to their stereotypical tendency to be more communal and relational, whereas men were found to display an enhanced perception of competitive relationships, correlating with their more agentic attitude.
This phenomenon is further supported by the recent study of De Dreu et al. (2010), which demonstrated a vital role for OT in the way that men regulate competition and aggression among in-group and out-group members. In their study, men who received OT displayed aggressive behaviors toward competing out-groups. Although De Dreu et al. (2010) did not include women in their sample, their results may suggest that OT may have a critical role for the accurate perception and identification of competitive relationships in men.
Nonetheless, it is important to note that exogenous OT, as used in the current study, may have a different action as compared to the endogenous peptide. To the best of our knowledge, few studies to date have examined the relationship between intranasal administration of OT and the endogenous oxytocinergic system, and there is still no consensus regarding the existence of a correlation between OT levels in plasma and brain (Amico et al., 1990; Neumann et al., 1993; Wotjak et al., 1998; Engelmann et al., 2000; Winslow et al., 2003). Domes et al. (2010) have recently demonstrated that intranasal administration of OT elevates its plasma levels. Still, some studies suggest an association between OT administration intranasally and salivary cortisol levels, similar to the natural effect of endogenous OT release during suckling (Heinrichs et al., 2003), as well as an association with human amygdaloid functioning (Kirsch et al., 2005; Domes et al., 2007, 2010; Baumgartner et al., 2008; Labuschagne et al., 2010). In addition, Born et al. (2002) reported a slight but non-significant raise in plasma levels of vasopressin (AVP) following intranasal administration of this hormone, which is closely similar to OT, suggesting an association between exogenous administration of this peptide and its internal system. Yet, the consistent behavioral changes observed following the administration of OT indicates that exogenous administration of OT intranasally simulates an extreme adaptation of the naturally occurring neural condition. Utilization of a cross-over experimental design, as done in other studies investigating OT (Kirsch et al., 2005; Kosfeld et al., 2005; Domes et al., 2007, 2010; Guastella et al., 2008) is crucial in order to establish causal relationship between OT and behavior. Nevertheless, this unknown gap between these two conditions may suggest that findings from exogenous studies should be treated with caution.
In this study we focus on the role of OT in social perception both in men and women. While it is beyond the scope of the current work, future studies should also explore the effect of AVP in the context of complex human social behaviors, in light of its well-documented function in both male and female social behavior (Bielsky et al., 2004; Walum et al., 2008; Arakawa et al., 2010; Domes et al., 2010; Kessler et al., 2010; Tobin et al., 2010). In addition, although our results did not indicate that taking contraceptives interact with the effects of OT, it is recommended that future studies should examine the interactions between the administration of OT and menstrual cycle. It is important to note that previous reports in the literature did not find an association between the use of contraceptives and menstrual cycle phase and the effect of OT on women's performance (Ditzen et al., 2009; Theodoridou et al., 2009; Cardoso et al., 2011; Ellenbogen et al., 2011). Yet, it is possible that since there are indications of interactions between OT and levels of estrogen among women (Razzoli et al., 2003), levels of estrogen should be monitored in future studies using OT.
In summary, our results show that OT facilitates social perception of real-life interpersonal interactions. Moreover, our results show a distinctive differentiation between men's and women's behavioral response to the intranasal administration of OT, a phenomenon that has only recently been in relevant research.
FUNDING
Israeli Scientific Foundation (ISF) (grant number 489/08) and Israel Ministry of Health, Chief Scientists Office.
Conflict of Interest
None declared.
REFERENCES
- Alvares GA, Hickie IB, Guastella AJ. Acute effects of intranasal oxytocin on subjective and behavioral responses to social rejection. Experimental and Clinical Psychopharmacology. 2010;18(4):316–21. doi: 10.1037/a0019719. [DOI] [PubMed] [Google Scholar]
- Amico JA, Challinor SM, Cameron JL. Pattern of oxytocin concentrations in the plasma and cerebrospinal fluid of lactating rhesus monkeys (Macaca mulatta): evidence for functionally independent oxytocinergic pathways in primates. The Journal of Clinical Endocrinology and Metabolism. 1990;71(6):1531–35. doi: 10.1210/jcem-71-6-1531. [DOI] [PubMed] [Google Scholar]
- Arakawa H, Arakawa K, Deak T. Oxytocin and vasopressin in the medial amygdala differentially modulate approach and avoidance behavior toward illness-related social odor. Neuroscience. 2010;171(4):1141–51. doi: 10.1016/j.neuroscience.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Archer D, Akert R, Costanzo M. The accurate perception of nonverbal behavior: Questions of theory and research design. In: Blank PD, editor. Interpersonal expectations. New York: Cambridge university press; 1993. pp. 242–60. [Google Scholar]
- Barraza JA, Zak PJ. Empathy toward strangers triggers oxytocin release and subsequent generosity. Annals of the New York Academy of Sciences. 2009;1167:182–9. doi: 10.1111/j.1749-6632.2009.04504.x. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, et al. Oxytocin selectively improves empathic accuracy. Psychological Science. 2010;21(10):1426–8. doi: 10.1177/0956797610383439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58(4):639–50. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25(9):1565–74. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nature Neurosceince. 2002;5(6):514–16. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Buss DM, Schmitt DP. Sexual strategies theory: an evolutionary perspective on human mating. Psychological Review. 1993;100(2):204–32. doi: 10.1037/0033-295x.100.2.204. [DOI] [PubMed] [Google Scholar]
- Cardoso C, Ellenbogen MA, Linnen AM. Acute intranasal oxytocin improves positive self-perceptions of personality. Psychopharmacology. 2011 doi: 10.1007/s00213-011-2527-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Costanzo M, Archer D. Interpreting the expressive behavior of others: the Interpersonal Perception Task. Journal of Nonverbal Behavior. 1989;13(4):225–45. [Google Scholar]
- De Dreu CK, Greer LL, Handgraaf MJ, et al. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;328(5984):1408–11. doi: 10.1126/science.1189047. [DOI] [PubMed] [Google Scholar]
- De Dreu CK, Greer LL, Van Kleef GA, Shalvi S, Handgraaf MJ. Oxytocin promotes human ethnocentrism. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(4):1262–6. doi: 10.1073/pnas.1015316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B, Finkelmeyer A, Eickhoff S, et al. Multidimensional assessment of empathic abilities: neural correlates and gender differences. Psychoneuroendocrinology. 2010;35(1):67–82. doi: 10.1016/j.psyneuen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biological Psychiatry. 2009;65(9):728–31. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biological Psychiatry. 2007;61(6):731–3. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, et al. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35(1):83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Ellenbogen MA, Linnen AM, Grumet R, Cardoso C, Joober R. The acute effects of intranasal oxytocin on automatic and effortful attentional shifting to emotional faces. Psychophysiology. 2011;49(1):128–37. doi: 10.1111/j.1469-8986.2011.01278.x. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Ebner K, Landgraf R. Behavioural impact of intraseptally released vasopressin and oxytocin in rats. Experimental Physiology. 2000;85:125–30. doi: 10.1111/j.1469-445x.2000.tb00015.x. [DOI] [PubMed] [Google Scholar]
- Fischer-Shofty M, Shamay-Tsoory SG, Harari H, Levkovitz Y. The effect of intranasal administration oxytocin on fear recognition. Neuropsychologia. 2010;48(1):179–84. doi: 10.1016/j.neuropsychologia.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Geary DC. Functional organization of the human mind: implications for behavioral genetics research. Human Biology. 1998;70(2):185–98. [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin and the development of parenting in humans. Biological Psychiatry. 2010;68(4):377–82. doi: 10.1016/j.biopsych.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biological Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry. 2003;54(12):1389–98. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Hofer A, Siedentopf CM, Ischebeck A, et al. Gender differences in regional cerebral activity during the perception of emotion: a functional MRI study. Neuroimage. 2006;32(2):854–62. doi: 10.1016/j.neuroimage.2006.03.053. [DOI] [PubMed] [Google Scholar]
- Hofer A, Siedentopf CM, Ischebeck A, et al. Sex differences in brain activation patterns during processing of positively and negatively valenced emotional words. Psychological Medicine. 2007;37(1):109–19. doi: 10.1017/S0033291706008919. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, et al. Oxytocin enhances amygdale-dependent, socially reinforced learning and emotional empathy in humans. The Journal of Neuroscience. 2010;30(14):4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler MS, Bosch OJ, Bunck M, Landgraf R, Neumann ID. Maternal care differs in mice bred for high vs. low trait anxiety: impact of brain vasopressin and cross-fostering. Social Neuroscieince. 2010;6(2):156–68. doi: 10.1080/17470919.2010.495567. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25(49):11489–93. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–6. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, Stout JC, Nathan PJ. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychpharmacology. 2010;35(12):2403–13. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Hormones and Behavior. 2006;50(4):506–17. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Lubin B. Adjective checklists for measurement of depression. Archives of General Psychiatry. 1965;12:57–62. doi: 10.1001/archpsyc.1965.01720310059007. [DOI] [PubMed] [Google Scholar]
- Mah L, Arnold MC, Grafman J. Impairment of social perception associated with lesions of the prefrontal cortex. The American Journal of Psychiatry. 2004;161(7):1247–55. doi: 10.1176/appi.ajp.161.7.1247. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Yu HH, Pine DS, Blair RJ. Oxytocin improves specific recognition of positive facial expressions. Psychopharmacology. 2010;209(3):225–32. doi: 10.1007/s00213-010-1780-4. [DOI] [PubMed] [Google Scholar]
- Neumann I, Ludwig M, Engelmann M, Pittman QJ, Landgraf R. Simultaneous microdialysis in blood and brain: oxytocin and vasopressin release in response to central and peripheral osmotic stimulation and suckling in the rat. Neuroendocrinology. 1993;58(6):637–45. doi: 10.1159/000126604. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Penn DL, Perkins. DO, Lieberman J. Implications for the neural basis of social cognition for the study of schizophrenia. American Journal of Psychiatry. 2003;160:815–24. doi: 10.1176/appi.ajp.160.5.815. [DOI] [PubMed] [Google Scholar]
- Razzoli M, Cushing BS, Carter CS, Valsecchi P. Hormonal regulation of agonistic and affiliative behavior in female Mongolian gerbils (Meriones unguiculatus) Hormones and Behavior. 2003;43(5):549–53. doi: 10.1016/s0018-506x(03)00064-3. [DOI] [PubMed] [Google Scholar]
- Rimmele U, Hediger K, Heinrichs M, Klaver P. Oxytocin makes a face in memory familiar. The Journal of Neuroscience. 2009;29(1):38–42. doi: 10.1523/JNEUROSCI.4260-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockliff H, Karl A, McEwan K, Gilbert J, Matos M, Gilbert P. Effects of intranasal oxytocin on ‘compassion focused imagery’. Emotion, Epub ahead of print. 2011 doi: 10.1037/a0023861. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(50):21437–41. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Ruther M, Markowistch HJ, Shah NJ, Fink GR, Piefke M. Gender differences in brain networks supporting empathy. Neuroimage. 2008;42(1):393–403. doi: 10.1016/j.neuroimage.2008.04.180. [DOI] [PubMed] [Google Scholar]
- Schulze L, Lischke A, Greif J, Herpertz SC, Heinrichs M, Domes G. Oxytocin increases recognition of masked emotional faces. Psychoneuroendocrinology. 2011 doi: 10.1016/j.psyneuen.2011.03.011. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Silk JB. Social components of fitness in primate groups. Science. 2007;317(5843):1347–51. doi: 10.1126/science.1140734. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA. Biobehavioral responses to stress in females: tend–and-befriend, not fight-or-flight. Psychological Review. 2000;107(3):411–29. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Theodoridou A, Rowe AC, Penton-Voak IS, Rogers PJ. Oxytocin and social perception: oxytocin increases perceived facial trustworthiness and attractiveness. Hormones and Behaviors. 2009;56(1):128–32. doi: 10.1016/j.yhbeh.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Tobin VA, Hashimoto H, Wacker DW, Takayanagi Y, Langnaese K, Caquineau C, Noack J, Landgraf R, Onaka T, Leng G, Meddle SL, Engelmann M, Ludwig M. An intrinsic vasopressin system in the olfactory bulb is involved in social recognition. Nature. 2010;464(7287):413–17. doi: 10.1038/nature08826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vugt M. Sex differences in intergroup competition, aggression, and warfare: the male warrior hypothesis. Annals of the New York Academy of Sciences. 2009;1167:124–34. doi: 10.1111/j.1749-6632.2009.04539.x. [DOI] [PubMed] [Google Scholar]
- Van Vugt M, De Cremer D, Janssen DP. Gender differences in cooperation and competition: the male-warrior hypothesis. Psychological Science. 2007;18(1):19–23. doi: 10.1111/j.1467-9280.2007.01842.x. [DOI] [PubMed] [Google Scholar]
- Walum H, Westberg L, Henningsson S, Neiderhiser JM, Reiss D, Igl W, Ganiban JM, Spotts EL, Pedersen NL, Eriksson E, Lichtenstein P. Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(37):14153–56. doi: 10.1073/pnas.0803081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28(5):910–18. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- Wood W, Eagly AH. A cross-cultural analysis of the behavior of women and men: implications for the origins of sex differences. Psychological Bulletin. 2002;128(5):699–727. doi: 10.1037/0033-2909.128.5.699. [DOI] [PubMed] [Google Scholar]
- Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of peptidergic neurons. Neuroscience. 1998;85(4):1209–22. doi: 10.1016/s0306-4522(97)00683-0. [DOI] [PubMed] [Google Scholar]
- Zak PJ, Stanton AA, Ahmadi S. Oxytocin increases generosity in humans. PLoS One. 2007;2(11):e1128. doi: 10.1371/journal.pone.0001128. [DOI] [PMC free article] [PubMed] [Google Scholar]