Abstract
Following the two-stage model of disgust, ‘core disgust’ (e.g. elicited by rotten food) is extended to stimuli that remind us of our animal nature ‘AR disgust’ (e.g. mutilations, animalistic instincts). There is ample evidence that core and AR represent distinct domains of disgust elicitors. Moreover, people show large differences in their tendency to respond with disgust to potential disgust elicitors (propensity), as well as in their appraisal of experiencing disgust (sensitivity). Thus these traits may be important moderators of people's response patterns. Here, we aimed to find brain mechanisms associated with these distinct disgust domains and traits, as well as the interaction between them. The right ventrolateral occipitotemporal cortex, which preferentially responded to visual AR, was functionally coupled to the middle cingulate cortex (MCC), thalamus and prefrontal cortex (medial, dorsolateral), as a function of disgust domain. Coupling with the anterior part of MCC was modulated by disgust ‘propensity’, which was strongest during AR. Coupling with anterior insula and ventral premotor cortex was weaker, but relied fully on this domain–trait interaction. Disgust ‘sensitivity’ modulated left anterior insula activity irrespective of domain, and did not affect functional connectivity. Thus a frontal-posterior network that interacts with disgust ‘propensity’ dissects AR and core disgust.
Keywords: animal-reminder disgust, core disgust, propensity, sensitivity
INTRODUCTION
Disgust is conceptualized as an avoidant-defensive mechanism that evolved to prevent the body from contamination by spoiled foods. According to theories of disgust (Rozin and Fallon, 1987; Rozin et al., 1995; Curtis et al., 2004), this mechanism of ‘core disgust’ stretched to more complex domains such as socio-moral disgust and animal-reminder (AR) disgust. AR disgust may be triggered by reminders of mortality, mutilation and intrinsic animalistic instincts (Rozin et al., 2000); cues that remind us of our animal ancestry. This disgust-mediated rejection of our animal nature is argued to serve a defensive function for maintaining the hierarchical division between humans and animals (Haidt et al., 1994). Though often treated as a single category, core and AR disgust can be systematically distinguished as separate concepts in factor analytical studies (van Overveld et al., 2009), with distinct patterns of behavioural avoidance and associated psychopathologies like contamination-based Obsessive Compulsive Disorder (OCD) (Olatunji et al., 2007, 2008) versus blood–injury fears (de Jong and Merckelbach, 1998).
Brain areas consistently associated with disgust across neuroimaging studies include anterior insula, frontal operculum, amygdala, occipitotemporal cortex, orbitofrontal cortex, caudate-putamen and globus pallidus (Phan et al., 2002, Zald et al., 2002; Murphy et al., 2003), but few studies have taken disgust domains into account. Yet, recent evidence suggests that core and AR-like disgust experiences draw upon distinct peripheral physiological activity and separable responses in the anterior insula (Harrison et al., 2010), whereas core and moral disgust have been shown to elicit differential activation at the level of the amygdala (Moll et al., 2005; Schaich Borg et al., 2008). These studies provide further support that disgust carries subcategories that are fundamentally distinct. In direct comparisons between AR and core conditions, brain responses to AR-like stimuli (e.g. mutilation) tend to be stronger, most notably in occipitotemporal (Sarlo et al. 2005) and parietal (Wright et al., 2004; Schienle et al., 2006) cortices.
Of note, stimuli in these studies may have been suboptimal, in that they had not meticulously represented and distinguished AR and core disgust. For instance, a movie of a clean surgical procedure (Harrison et al., 2010) does not necessarily fall within the realm of AR. Additional complexity is introduced when we consider that individual differences in disgust traits have been found to critically modulate subjective (van Overveld et al., 2009) and physiological disgust responses (Rohrmann and Hopp, 2008; van Overveld et al., 2010), and that these traits seem to interact with disgust domain in the origin and maintenance of psychopathological symptoms (de Jong and Merckelbach, 1998; Sawchuk et al., 2000; Olatunji et al., 2005). Disgust traits comprise of individual differences regarding people's threshold for experiencing disgust (propensity), as well as regarding their appraisal of the experienced disgust responses (sensitivity). That is, people not only vary in their tendency to experience the emotion of disgust more readily but also in their tendency to find the emotion of disgust unpleasant. High disgust propensity may increase the probability that stimuli acquire disgust-evoking properties that could lead to avoidance behaviour (e.g. de Jong and Muris, 2002). In turn, this avoidance would be specifically pronounced in individuals who appraise the experience of disgust as highly negative (e.g. van Overveld et al., 2010). Attesting further to the relevance of differentiating between disgust propensity and sensitivity, both traits have been shown to be differentially involved in psychopathology (e.g. Olatunji et al., 2007; Engelhard et al., 2011). Disgust-related brain responses that correlate with ‘propensity’ disgust trait include the insula, ventral pallidum and occipitotemporal cortex (Schienle et al., 2005; Calder et al., 2007; Mataix-Cols et al., 2008; Schafer et al., 2009), whereas ‘sensitivity’ disgust trait was negatively associated with medial and dorsolateral prefrontal activity (Schafer et al., 2009). However, the potentially important interaction between specific disgust traits (propensity, sensitivity) and disgust domains (AR, core) has so far been overlooked.
All these accounts support the claim that disgust extends far beyond filth. Disgust responses seem (at least partly) domain-specific, and people show remarkable differences in their responsivity to potential disgust elicitors, as well as in their appraisals of the experience of disgust. Here, we aim to reveal the complexities/intricacies of disgust by searching brain mechanisms that express an interaction between disgust domain (AR, core) and putative disgust traits.
MATERIALS AND METHODS
Participants
Twenty-one healthy women [mean age (±s.d.), 22 (±2.1)] participated in this study against modest financial reimbursement. All participants had normal or corrected-to-normal vision, no structural brain abnormality and no past neurological or psychiatric history. One volunteer was excluded because of excessive head motion and poor compliance. This experiment, which is part of a larger project, was approved by the local ethics committee and all procedures were conducted in accordance with its standard, which includes a written informed consent from all participants. Participants were scanned in the first half of their menstrual cycle and never during menstruation. About 20% of the sample did not make use of contraceptives, whereas 80% used oral contraceptives. All participants reported moderate alcohol and nicotine consumption at most, and all denied drug use. Apart from two participants, all were exclusively right-handed according to the Edinburgh Handedness Inventory (Oldfield, 1971). Participants were recruited via the local media, by means of a website designed for this study and by advertisements that were attached at various sites of the University Campus. All women self-selected themselves for participating as ‘healthy controls’ in an on-going study about cognitive processes in women with sexual pain disorders.
Self-report disgust trait questionnaires
The Disgust Propensity and Sensitivity Scale-Revised (DPSS-R) is a 16-item questionnaire that consists of two validated subscales that measure trait disgust propensity and trait disgust sensitivity, respectively (van Overveld et al., 2006). Participants read 16 propositions on the frequency of experiencing bodily sensations (e.g. ‘Disgusting things make my stomach turn’ for propensity, and ‘I think feeling disgust is bad for me, It scares me when I feel like fainting’ for sensitivity), and indicated which applied best to them on a scale from 1 (never) to 5 (always). The DPSS-R has been validated and used in a number of studies (e.g. van Overveld et al., 2006) and it is the first index that measures disgust propensity and disgust sensitivity irrespective of disgust elicitors (Cavanagh and Davey, 2000). The scale has been shown to be internally consistent (Van Overveld et al., 2006) and has shown predictive validity for experienced disgust in disgust-eliciting experimental tasks across all relevant disgust domains (Van Overveld et al., 2010). In previous studies, the scale was shown to be reliable, with the DPSS-R and its subscales’ internal consistency all above Cronbach's α of 0.78 (Fergus and Valentiner, 2009; van Overveld et al., 2011). In our sample, the Cronbach's α for disgust sensitivity was (0.54) and for disgust propensity (0.56). In our sample, the correlation between sensitivity and propensity was negligible (r = 0.06), which attest to the relative independence of these two constructs.
Paradigm and procedure
The stimuli consisted of 36 coloured photographs representing six emotional categories: ‘Neutral objects’, ‘Fear’, ‘Core Disgust’, ‘Animal-Reminder (AR) Disgust’, ‘Erotic’ and ‘Neutral bodies’. Core disgust stimuli mainly portrayed humans interacting with food and body products, whereas AR stimuli depicted body envelope violations (i.e. surgery, mutilation) and death. Fear stimuli mostly included humans under imminent threat. Stimuli chosen from the International Affective Picture System (Lang et al., 1999) included IAPS 3150, 3400 (AR disgust); 9320, 9300 (core disgust); 7010, 5520 (neutral) and 6550, 1300, 6350 (fear). Selection of non-IAPS pictures, which comprised the bulk of the stimuli, was done in a pre-structured process. Initially, more than 200 photographs were collected by the researchers themselves. Based on characteristics agreed on a priori, the research team selected 50 photographs that were sent for further validation conducted with 40 women via an online survey (www.esurveyspro.com). A second validation was conducted with a set of new pictures for AR disgust to reduce noise in the category of AR—due to significantly high mean on both dimensions of fear and disgust in the first validation process. The research team matched the scenes for physical features such as complexity, brightness, contrasts, ethnicity and colour. In all photographs, models were kept faceless or with minimal focus on the face. Stimuli were presented in a block design, with each block consisting of 10 pictures representing the same category. Each photograph was presented for 1.4 s, with a 1 s interval between consecutive stimuli. Six blocks (split by 16-s inter-block intervals), corresponding to the six stimulus categories, were run in a pseudo-randomized sequence. Six of these functional runs were acquired for each participant, separated by 30-s intervals, adding up to a total duration of the fMRI experiment of 1458 s. For presentation of the experimental design, we used a psychtoolbox (http://psyctoolbox.org) application implemented in Matlab (Version R2009a). The stimuli were presented on a translucent screen at the end of the scanner by means of a mirror attached above the participants’ head. Preceding the experiment, a training task was done inside the scanner with the aim to familiarize the participants with the procedure. Participants were instructed to look at the pictures presented and ‘melt’ with their emotions and not to suppress their emotions. Given the passive nature of the design participants were asked to respond (i.e. press a button) to an ‘*’ that was over-imposed on a (fixed) randomly-selected number of photographs (two per block). These responses were recorded, but were not used in the analysis. Post-scanning, participants were accompanied to a computer room where a Visual Analogue Scale (VAS) implemented on a computer was explained to them and then they were left in their own privacy to rate the stimuli. The VAS ranging from 0 (not at all—in Dutch language ‘helemaal niet’) to 100 (very much—in Dutch language ‘heel erg’) was included to rate their subjective evaluation, on two dimensions: disgust and fear. All stimuli were rated subjectively on the dimension of ‘general arousal’ post hoc from an independent sample of 25 women who did not differ in other demographic data. This was done post hoc due to connotations to sexual and positive arousal for the Dutch word (‘opwinding’) that we used in the experiment. When the experiment was completed, participants had a debriefing session with refreshments.
Image acquisition
Images were acquired on a Philips Intera 3T MR-scanner. A sense 8-channel head coil was used for radio frequency reception. A series of echo planar imaging (EPI) volumes were acquired to measure the blood oxygen level dependent (BOLD) effect, which entailed a T2*-weighted gradient echo sequence with a repetition time (TR) of 2000 ms and an echo time of 30 ms. Flip angle was 70° using whole-brain acquisition (matrix size 64 × 64 voxels) and interleaved slice acquisition order, with an inter-slice gap of 0 mm and plane thickness of 3 mm. EPIs were acquired at 3 × 3 mm in-plane resolution. The (axial) images (volumes) were acquired parallel to the anterior–posterior commissure plane. In total, 740 volumes were obtained per participant. A T1-weighted anatomical MRI (TR = 9 ms, TE = 3.5 ms, matrix size 256 × 256) and two diffusion tensor imaging (DTI) volumes of 55 slices each of 620 ms duration (with scan resolution of 96 × 96, flip angle 70°) were acquired after the EPI runs. The DTI measurements were not used in this manuscript.
Behavioural and self-report analysis
For the DPSS-R, two main variables namely, disgust propensity and disgust sensitivity are generated from the sum of the corresponding items provided on the scale (van Overveld et al., 2006).
fMRI pre-processing
For image pre-processing and analysis, we used the Statistical Parametric Mapping software (SPM8; University College London, UK; http://www.fil.ion.ucl.ac.uk) implemented in Matlab 7.2 (The MathWorks Inc., http://www.mathworks.com). For each participant, all EPI volumes were realigned to the first volume acquired, and a mean EPI image was created. The realignment parameters were inspected and if movements exceeded 2 mm in any direction, the participant was excluded from further analysis. The anatomical (T1) scan was manually co-registered to the mean EPI image, and subsequently all EPI images and the T1 image were spatially normalized to MNI (Montreal Neurological Institute) standard stereotactic space (Friston et al., 1995). Data were re-sampled to 2 × 2 × 2 mm (8 mm3) isotropic voxels. All volumes were smoothed with an isotropic Gaussian kernel of 8 mm full-width at half-maximum.
Image analysis
After pre-processing, analyses were performed using general linear models (GLM) at the first (subject) and second (group) level (Friston et al., 1995). Two analytic tracks were followed. First, we identified brain regions predominantly associated with core and AR disgust. Furthermore, parametric modulation was used to find BOLD responses correlating with individual disgust trait. Second, we analysed the functional connectivity and its interaction with disgust trait, of areas showing a clear and consistent bias towards AR and core disgust processing.
For the first strategy, we computed a GLM for each participant, which included regressors for the six conditions (including conditions that are not used in any of the contrasts) and also one for the inter-run instructions, convolved with a canonical haemodynamic response function. Rotational and translational head movements were added as nuisance variables (six covariates). For each voxel a high-pass filter (cut-off 128 s) was applied to remove low-frequency noise. A binarized version of the standard grey matter mask provided by SPM8 was used as an explicit mask. The following contrasts (contrast images) were computed: core > neutral, AR > neutral, fear > neutral. To assess haemodynamic changes at the group level (random effects) the results of these weighted contrasts were entered into a second-level flexible factorial model. We specified two factors, ‘Subject’ (independence ‘yes’; variance ‘equal’) and ‘Condition’ (independence ‘no’; variance ‘equal’). The factor ‘Condition’ contained three levels representing the three contrast images. As covariates, we entered the individual scores for DPSS-R disgust propensity and DPSS-R disgust sensitivity. We specified one main effect (Condition) and two interaction effects (propensity × Condition, sensitivity × Condition). All contrasts [(core > fear); (AR > fear); (core > AR); (AR > core)], as well as the interactions with disgust trait [(core > neutral) × propensity; (AR > neutral) × propensity; (core > neutral) × sensitivity; (AR > neutral) × sensitivity], were initially tested at P < 0.001, uncorrected.
For the functional connectivity analysis, we used psychophysiological interaction (PPI) to assess how activity in areas of interest covaries with that in other areas in the brain, as a function of the psychological context that the participants were exposed to (Friston et al., 1997). Since we aimed at distinguishing between core and AR disgust, we focused on the contrasts between these two disgust stimuli. PPI seed regions were selected as follows: BOLD responses were considered to be biased to AR when they showed consistent and significant activation in AR > core and AR > fear comparisons. Likewise, core-bias was inferred from significant and consistent activation in core > AR and core > fear comparisons. Only one area met these stringent criteria: the right ventrolateral occipitotemporal cortex (vlOT, MNI coordinates 50 −60 −8) showed a clear AR-bias. We used the AR>neutral contrast to identify this vlOT seed area at the subject level (P < 0.05, uncorrected), which could be achieved in 19 out of 20 women scanned. For each selected participant, a summary time course (first eigenvariate) was extracted from the right vlOT seed region using a sphere centred on the coordinates above (sphere radius: 5 mm). PPI's were calculated as the element by element product, convolved with the haemodynamic response function, of these summary time courses and a vector coding for the psychological context (AR vs core). Subsequently, this interaction term together with the summary time course of the seed region and the vector coding for the psychological context were entered as regressors in a first level GLM, which also included the six-head motion parameters as nuisance variables. The contrast images for each of these three regressors entered a random effects second level flexible-factorial model. Two factors were specified, ‘Subject’ (independence ‘yes’; variance ‘equal’) and ‘PPI’ (independence ‘no’; variance ‘equal’). The factor ‘PPI’ had three levels representing (i) the seed region's time course, (ii) the psychological vector and (iii) the interaction term (PPI). As covariates we entered the individual scores for DPSS-R disgust propensity and DPSS-R disgust sensitivity. We specified one main effect (factor ‘PPI’) and two interaction effects (propensity × ‘PPI’, sensitivity × ‘PPI’). By setting the appropriate contrasts to the parameter weights, we were able to explore areas whose functional connectivity (with vlOT) was different for AR and core disgust. Critically, we sought to investigate whether this functional connectivity would correlate with individual trait disgust propensity and sensitivity. The initial threshold was set to P < 0.001, uncorrected, for all PPI analyses.
Clusters were considered significant if they reached P < 0.05, Family-wise error (FWE) corrected for multiple comparisons, either for the whole brain, or within a reduced search space representing the most consistently reported areas in disgust studies (‘disgust mask’). The significance threshold was more stringent for correlations with disgust trait to reduce the risk of type I error (two traits tested, critical α/2 = P < 0.025). For correlations with disgust trait we also mention marginal effects (0.025 < P < 0.05). The ‘disgust mask’ (7798 voxels) comprised globus pallidus, caudate-putamen, anterior insula, amygdala, ventral occipitotemporal cortex, frontal operculum and orbitofrontal cortex. It was built using information from different sources. A bilateral frontal operculum Region of Interest, ROI (BA44) was adopted from the SPM Anatomy toolbox (Eickhoff et al., 2005), whereas a ROI representing bilateral anterior (agranular and dysgranular) insula was hand-drawn on a brain template following the anatomical description in Nanetti et al. (2009). Bilateral occipitotemporal ROIs were also hand-drawn, which was guided by the results of an F-test over disgust vs neutral contrasts of the present study (that gave very strong occipitotemporal effects). This strategy did not lead to ‘double dipping’ (Kriegeskorte et al., 2009), because task-relevant occipitotemporal (OT) activity reached significance using a threshold with a FWE correction for the whole brain (Table 3). Caudate, putamen, amygdala and globus pallidus ROIs were taken from the Harvard–Oxford Subcortical Atlas (http://www.cma.mgh.harvard.edu/). Because of substantial susceptibility artefact in the area of the orbitofrontal cortices (OFC), the latter was not included in the mask.
Table 3.
Areas predominantly associated with AR and core disgust processing
| Side region (MNI) | k | x | y | z | Z-score | FWE P |
|---|---|---|---|---|---|---|
| Core disgust > AR disgust | ||||||
| L Middle temporal gyrus | 1159 | −58 | −54 | 10 | 4.91 | 0.000 |
| R Middle temporal gyrus | 826 | 58 | −40 | 10 | 4.71 | 0.000 |
| L Posterior cingulate cortex | 906 | −12 | −42 | 48 | 4.18 | 0.000 |
| Core disgust > fear | ||||||
| L Fusiform gyrus | 1273 | −28 | −46 | −10 | 5.89 | 0.000 |
| R Ventral pallidum/amygdala | 1187 | 18 | 0 | −12 | 4.11 | 0.000 |
| R Fusiform gyrus | 1078 | 28 | −48 | −10 | 5.36 | 0.000 |
| AR disgust > core disgust | ||||||
| L Lingual gyrus/cerebellum | 1933 | −10 | −92 | −4 | 5.76 | 0.000 |
| R Postcentral gyrus | 462 | 42 | −30 | 40 | 4.11 | 0.002 |
| R Inferior temporal gyrus (vlOT) | 262 | 50 | −60 | −8 | 4.66 | 0.029 |
| R Middle occipital gyrus | 236 | 36 | −78 | 8 | 4.48 | 0.049 |
| AR disgust > fear | ||||||
| L Lingual gyrus/inferior temporal gyrus | 5059 | −8 | −92 | −4 | 6.55 | 0.000 |
| R Inferior temporal gyrus (vlOT) | 1130 | 52 | −58 | −8 | 5.67 | 0.000 |
| R Middle occipital gyrus | 478 | 36 | −82 | 14 | 5.89 | 0.002 |
| R Superior parietal lobule | 1549 | 24 | −64 | 56 | 4.76 | 0.000 |
| R Inferior frontal gyrus (frontal operculum) | 254 | 52 | 10 | 28 | 4.44 | 0.032 |
Core and AR were contrasted against each other and against fear. Listed clusters reached P < 0.05, FWE corrected. Clusters are listed with coordinates of the peak voxel in standard (MNI) space, and size (k voxels). The peak Z-scores are given along with FWE corrected P-values. MNI, Montreal Neurological Institute; R, right hemisphere; vlOT, ventrolateral occipitotemporal cortex; Z, Z-value.
RESULTS
Self-report measures
Table 1 illustrates the subjective evaluation of each stimulus-type on the dimensions of disgust and fear. A mixed between-within subject ANOVA to assess the appraisal of categories of pictures (AR, core, fear, neutral) on two participants’ emotional ratings (fear and disgust) showed a significant interaction of Picture*Emotion Wilk's λ = 0.06, F(3,17) = 85.38, P < 0.001.
Table 1.
Subjective evaluation for each dimension as a function of stimulus type
| Emotion | Core, M (s.d.) | AR, M (s.d.) | Fear, M (s.d.) | Neutral, M (s.d.) |
|---|---|---|---|---|
| Disgust | 79.3 (13.2)a,y | 84.7 (14.5)b,x | 33.7 (23.5)c | 0.60 (0.7)d |
| Fear | 27.5 (19.4)a | 54.7 (29.6)b | 74.2 (19.4)c,y | 1.2 (3.1)d |
a,b,c,d Indicate significant difference between stimulus categories within a dimension. For instance, the ‘a’ on Core and the ‘b’ on AR elicitors on the first row indicates that they do differ significantly from each other on the dimension of disgust. The second letter (x, y) applies to relevant comparisons across columns. For instance the ‘x’ of the AR on the dimension of disgust with the ‘y’ on Fear elicitors on the dimension of fear indicates significant difference between the two. Means (M) and standard deviations (s.d.) of the subjective ratings for each stimulus-type (AR disgust, core disgust, fear and neutral) on two dimensions (disgust, fear).
The general pattern of subjective ratings attests to the validity of the stimulus materials (Table1). To examine in more detail whether the fearful stimuli were as effective in eliciting fear as the disgust stimuli were in eliciting disgust, we evaluated the relevant comparisons by means of t-tests, corrected for multiple testing: AR disgust stimuli were rated significantly higher on the dimension of disgust (M = 84.75, s.d. = 14.53) than of fear (M = 54.73, s.d. = 29.62), t(19) = 6.23, P < 0.001, r = 0.81 and similarly core disgust stimuli were rated significantly higher on the dimension of disgust (M = 79.30, s.d. = 13.16) than on fear (M = 27.45, s.d. = 19.43), t(19) = 13.39, P < 0.001, r = 0.82. The Fear stimuli were rated significantly higher on fear (M = 74.20, s.d. = 19.36) than on disgust (M = 33.71, s.d. = 23.52), t(19) = −8.43, P < 0.001, r = 0.62; all with a large effect size.
On the other hand, AR stimuli elicited stronger subjective disgust (M = 84.74, s.d. = 14.53) than fear stimuli elicited subjective fear (M = 74.20, s.d. = 19.36), t(19) = 2.52, P < 0.05, r = 0.06. Similarly, core stimuli elicited higher levels of disgust (M = 79.29, s.d. = 13.16) than fear stimuli elicited subjective fear (M = 74.20, s.d. = 19.36), t(19) = 1.31, P < 0.20 (α/6 = 0.03), r = 0.01. AR disgust stimuli (M = 84.74, s.d. = 14.53) differed significantly from core disgust stimuli (M = 79.29, s.d. = 13.16), t(19) = −2.28, P < 0.03 (α/6 = 0.01), r = 0.05, in that the category of AR stimuli elicited higher level of subjective disgust compared with core disgust elicitors.
On the dimension of fear, AR stimuli (M = 54.73, s.d. = 29.62) were rated as more fearful than core disgust stimuli (M = 27.45, s.d. = 19.43), t(19) = −7.05, P < 0.001, r = 0.50.1
To test the alleged relationships between both indices of trait and state disgust, we computed bivariate Pearson correlations between DPSS (propensity and sensitivity) on the one hand and the subjective disgust elicited by both types of disgusting stimuli on the other. Supporting the validity of the DPSS, and in line with the constructs of propensity there were positive correlations with the experienced intensity of disgust for both core (r = 0.26) and AR (r = 0.45) disgust elicitors. Although only for the latter the correlation reached the conventional level of significance. Also in line with the construct of sensitivity there were no correlations with sensitivity and core disgust elicitors (r = 0.01) and neither with sensitivity and AR disgust elicitors (r = 0.01).
Brain areas showing interactions between disgust domain and individual disgust trait
The only significant interaction was observed in the dorsal-most part of the left anterior insula (Figure 1 and Table 2). The specific nature of this interaction was that both when subjects watched AR and core stimuli, their activity in this part of the insula was modulated by disgust sensitivity, but not propensity. In the right counterpart, this effect was only marginal. No significant or meaningful negative correlations were identified.
Fig. 1.
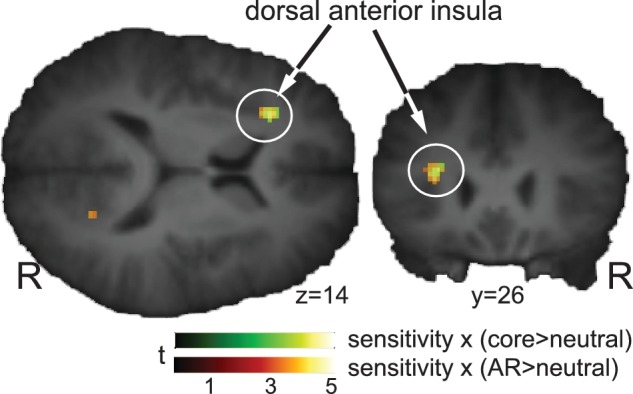
Correlation between BOLD activity induced by AR and core disgust elicitors and self-reported disgust sensitivity. Note that correlates of disgust sensitivity occur in the same area of the left anterior dorsal anterior insula irrespective of disgust domain.
Table 2.
Interaction between disgust domain and disgust trait
| Side region (MNI) | k | x | y | Z | Z-score | FWE P |
|---|---|---|---|---|---|---|
| Disgust Sensitivity × core disgust | ||||||
| L Anterior insula | 37 | −26 | −28 | 16 | 4.23 | 0.022* |
| Disgust Sensitivity × AR disgust | ||||||
| L Anterior insula | 37 | −28 | 26 | 14 | 4.17 | 0.028*‡ |
*P < 0.025 FWE, for a disgust-relevant reduced search volume. ‡Marginally significant (0.025 < P < 0.05). Activity in AR > neu and core > neu contrasts was correlated against self-reported disgust ‘propensity’ and ‘sensitivity’, following the DPSS-R questionnaire. Significance threshold was P < 0.025 FWE corrected because of two traits tested (critical α /2). Clusters are listed with coordinates of the peak voxel in standard (MNI) space and size (k voxels). The peak Z-scores are given along with FWE corrected P-values. No significant or expected correlations were found with disgust propensity. MNI, Montreal Neurological Institute.
Areas predominantly associated with core and AR disgust
For core > AR, significant clusters were found in the posterior part of the middle temporal gyrus (pMTG), bilaterally and in the most-dorsal part of the posterior cingulate cortex. For the reverse contrast, AR > core, significant clusters were found in the left lingual gyrus, the posterior part of the right inferior temporal gyrus (ventrolateral occipitotemporal cortex, vlOT), the right middle occipital gyrus, the right postcentral gyrus extending into supramarginal gyrus and bilaterally in the cerebellar hemisphere.
To further specify the disgust-related brain responses, we included Fear as an aversive and arousing, but not disgusting control category. For core > fear, significant clusters were found in bilateral fusiform gyrus and the right ventral pallidum (that spread to include the right amygdala and contra-lateral ventral pallidum). For AR > fear, results largely resembled the AR > core comparison. Significant clusters were present in the left lingual gyrus, vlOT, inferior parietal lobule and inferior frontal gyrus (frontal operculum) corresponding to the location of the premotor cortex in the right hemisphere. All effects are listed in Table 3.
Right vlOT functional connectivity
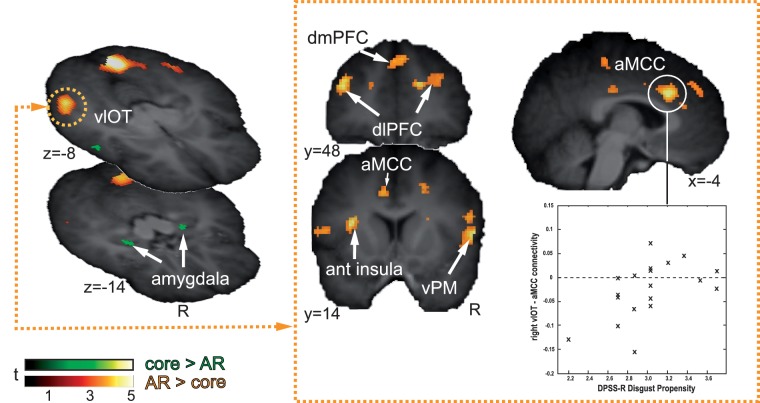
No area correlated stronger with the right vlOT during AR disgust relative to core (Table 4). However, we identified a network of areas whose functional connectivity with the right vlOT was weaker during AR than during core disgust. Clusters where the strength of this correlation was significant were the left thalamus (extending into the striatum), anterior (aMCC) and posterior (pMCC) parts of the middle cingulate cortex, the right superior frontal gyrus (dlPFC, dorsolateral prefrontal cortex). Marginal effects were observed in the right superior medial gyrus (dmPFC, dorsomedial prefrontal cortex) and right parietal operculum. At least for part of the cingulate and PFC, we could establish (via additional PPI's with the baseline) that the weaker coupling with the right vlOT during AR relative to core was due to decreased coupling during AR relative to baseline, which was unaltered for core disgust.
Table 4.
Right vlOT functional connectivity (PPI) of core relative to AR disgust
| Side region (MNI) | k | x | y | Z | Z-score | FWE P | |
|---|---|---|---|---|---|---|---|
| Core disgust > AR disgust | |||||||
| L | Thalamus/putamen/globus pallidus | 586 | −14 | −8 | 20 | 4.41 | 0.000 |
| R/L | Middle cingulate cortex, ant (aMCC) | 439 | 2 | 28 | 22 | 4.29 | 0.002 |
| R/L | Middle cingulate cortex, pos (pMCC) | 340 | 8 | −18 | 40 | 3.71 | 0.007 |
| R | Superior frontal gyrus (dlPFC) | 318 | 22 | 38 | 36 | 4.14 | 0.010 |
| R | Rolandic operculum | 230 | 60 | −20 | 18 | 3.94 | 0.035 |
| R | Superior medial gyrus (mPFC) | 228 | 6 | 62 | 8 | 3.53 | 0.036 |
The psychophysiological interaction (ppi) of the right vlOT with the rest of the brain was calculated, with the psychological context vector set to AR > core. No area correlated stronger with the right vlOT during AR relative to core disgust. Clusters are listed with appropriate anatomical label, size (k voxels) and peak voxel location in MNI coordinates (relative to anterior commissure, AC). Negative sign for x, y and z indicates left, posterior and ventral to AC, respectively. AR, animal-reminder disgust elicitors; core, core disgust elicitors; ant, anterior; aMCC, anterior mid cingulate cortex; dlPFC, dorsolateral prefrontal cortex; L, left hemisphere; MNI, Montreal Neurological Institute; mPFC; medial prefrontal cortex; R, right hemisphere; sup, superior; vlOT; ventrolateral occipitotemporal cortex: Z, Z-value.
Trait disgust propensity, but not sensitivity, modulated correlations with the right vlOT and this modulation was stronger during AR than during core disgust (Figure 2 and Table 5). This effect was significant for the aMCC, and marginal for the right inferior frontal gyrus (frontal operculum, ventral premotor cortex) and the left anterior insula.
Fig. 2.
Frontal-posterior connectivity is modulated by self-reported disgust propensity as a function of disgust domain. On the left side, areas are shown that preferentially responded to AR (orange colouring). The amygdala on both sides showed a statistically non-significant preferential response to core disgust stimuli (green). Because right vlOT activated significantly relative to fear and core disgust (Table 3), AR bias was inferred and this area was selected as seed region for PPI analysis. Functional connectivity of the right vlOT (right half of the figure) correlated with individual disgust propensity in a number of areas, and this modulation was stronger under conditions of AR. In the bottom right, individual vlOT–aMCC functional connectivity is plotted against individual disgust propensity, which illustrates that in all likelihood the AR-disgust propensity interaction effect was driven by women with low disgust propensity who had weak functional connectivity with the right vlOT. aMCC, middle cingulate cortex, anterior part; dlPFC, dorsolateral prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; vlOT, ventrolateral occipitotemporal cortex; vPM, ventral premotor cortex. SPM's threshold at P < 0.001, uncorrected, for display purposes.
Table 5.
Right vlOT functional connectivity modulated by disgust trait (PPI)
| Side region (MNI) | k | x | y | z | Z-score | FWE P |
|---|---|---|---|---|---|---|
| Disgust Propensity: AR > core | ||||||
| L aMCC | 570 | −4 | 24 | 30 | 4.35 | 0.000 |
| R Inferior frontal gyrus (frontal operculum) | 198 | 60 | 14 | 6 | 4.11 | 0.038*‡ |
| L Anterior insula | 71 | −32 | 12 | 14 | 4.06 | 0.046*‡ |
*P < 0.025 FWE, for a disgust-relevant reduced search volume, ‡marginally significant (0.025 < P < 0.05). The psychophysiological interaction (PPI) of the right vlOT with the rest of the brain was calculated, with the psychological vector set to AR > core. Modulation by disgust propensity was calculated by calling the interaction term (PPI × disgust propensity) in the flexible factorial model. Significance threshold was P < 0.025 FWE corrected because of two traits tested (critical α /2). Clusters are listed with coordinates of the peak voxel in standard (MNI) space, and size (k voxels). The peak Z-scores is given along with FWE corrected P-values. Disgust sensitivity did not modulate right vlOT connectivity. aMCC, anterior part of middle cingulate cortex; MNI, Montreal Neurological Institute; R, right hemisphere; vlOT, ventrolateral occipitotemporal cortex; Z, Z-value.
DISCUSSION
In the present study, we used brain imaging to shed more light on the complex nature of disgust, a complexity that has recently also gained interest as a relevant factor in the origin and maintenance of psychopathology (Olatunji and McKay, 2009). Specifically, we aimed to find brain mechanisms associated with distinct disgust domains (core, AR) and traits (propensity, sensitivity), as well as the interaction between them. A series of behavioural and psychometric studies already showed that core and AR disgust represent distinct classes of disgust elicitors (e.g. van Overveld et al., 2009). Here, we present evidence that core and AR can also be discerned at the brain network level. The right vlOT, which responded most robustly to visual AR, showed disgust domain-dependent functional coupling with a network comprising thalamus, aMCC and pMCC and dorsal prefrontal areas. Disgust propensity, but not sensitivity, modulated the strength of vlOT coupling and this modulation was strongest during AR. Specifically, this modulation was found to be strongest for the vlOT–aMCC coupling, which is interesting in the light of behavioural flexibility and other functions ascribed to the aMCC. The same domain–trait interaction revealed coupling with anterior insula and ventral premotor cortex (areas known to play a pivotal role in disgust processing), though this modulation was found to be only marginally significant (0.025 < P < 0.05). On the other hand, disgust sensitivity modulated left anterior insula activity irrespective of domain, and did not affect functional connectivity. These results not only support the idea that AR and core disgust are fundamentally distinct (Haidt et al., 1994), they also stress the significance of disgust traits as modulators of the disgust response.
The anterior insula generally responds to elicitors of both types of disgust (Heining et al., 2003; Schienle et al., 2006) and this response tracks with the severity of both individual traits (Schienle et al., 2005; Calder et al., 2007) and experienced disgust (Harrison et al., 2010). We concur with these findings, showing that left dorsal anterior insula activity correlated with individual disgust sensitivity, but not propensity (Figure 1). These findings are also in line with the suggestion that the dorsal anterior insula processes disgust experience (Harrison et al., 2010) rather than recognition (of facial disgust expressions), which preferentially recruits ventral anterior insula (Phillips et al., 1998; Jabbi et al., 2008; Von dem Hagen et al., 2009).
Recently, evidence was presented to argue that the psychophysiological origins of AR and core disgust responses are fundamentally distinct. Specifically, the left anterior insula showed conjugated activity between experienced AR disgust and AR-induced reduction of parasympathetic heart activity (‘dizziness’), whereas core-induced dysregulated gastric muscular activity (‘stomach turning’) and experienced core disgust shared activity in the right anterior insula (Harrison et al., 2010). We followed a different approach, exploiting individual differences in disgust trait (which is likely to be connected to higher-order cortical functions) to further set apart AR and core disgust at the level of brain networks.
As mentioned in the Introduction section, previous studies comparing core and AR-like disgust elicitors have found neocortical responses generally inclined to AR and restricted to posterior neocortical areas like the occipitotemporal cortex (Sarlo et al., 2005) and inferior (Schienle et al., 2006) and superior parietal lobule (Wright et al., 2004). We identified a cluster in the right vlOT cortex that activated more prominently during exposure to AR than to core or fear stimuli that made us conclude that this area exhibited ‘AR-specific activity’. The finding that subjective arousal was not significantly different between AR and core, argues against the suggestion that occipitotemporal activity reflects emotion-induced arousal and, therefore, does not carry a specific emotional signature (Mourao-Miranda et al., 2003; Wright et al., 2004). The location of the vlOT cluster corresponds with that of the extrastriate and fusiform body areas (EBA/FBA), which contain neurons that preferentially respond to body parts (Downing et al., 2001; Orlov et al., 2010) and bodily shapes, but also body scheme distortions (Wagner et al., 2003; Uher et al., 2005).
The only systematic bodily difference between stimuli was that distortions were absent in all stimuli except AR—where they were abundant (injury, mutilation). Enhanced vlOT responses during AR may therefore reflect the severe violation of the general body scheme that is characteristic of mutilated bodies or body envelope injury. If the vlOT indeed contributes to decoding of the danger message conveyed by body injury (Pessoa, 2005), it seems reasonable to propose that the vlOT constitutes a cortical gateway of major interest in the context of AR.
Functional coupling of the right vlOT with other parts of the brain was driven not only by disgust domain, but also by disgust trait. Specifically, individual disgust propensity, but not sensitivity, modulated the psychophysiological interaction—coupling with the right vlOT being stronger during AR than core disgust—especially in the aMCC. For left dorsal anterior insula and the interior frontal gyrus (ventral premotor cortex), functional connectivity with the right vlOT was ‘only’ revealed when disgust propensity was taken into account. Though less prominent than the modulation of vlOT–aMCC connectivity, this finding is capturing from the perspective that the ventral premotor cortex (vPM), insula and vlOT are part of a putative network implicated in social cognition, action imitation and empathic ability (Caspers et al., 2010; Keysers and Gazzola, 2010; Zaki et al., 2010).
Disgust propensity is likely to depend on cognitive resources more than disgust sensitivity. It is interesting that individual disgust propensity, but not sensitivity, modulated functional connectivity between right vlOT and aMCC, an area heavily associated with behavioural flexibility (Bush et al., 2002; Georgiadis et al., 2010; Zaki et al., 2010). During certain expectation of a negative stimulus, e.g. the aMCC gets activated and aMCC–occipitotemporal coupling gains strength (Herwig et al., 2007; Onoda et al., 2008). This could serve to prevent a motor response, like withdrawal or avoidance, which does not meet environmental demands.
Based on the network of areas communicating with the right vlOT, and recalling that those high in disgust propensity rated AR stimuli as more disgusting, what could be the mechanism that determines AR disgust? A recent study used a response conflict design with both contextual and nonverbal (bodily) information, and demonstrated that individuals who relied more on nonverbal cues to resolve a conflict not only activated the vlOT more strongly, but also had stronger positive functional coupling between aMCC and ventral premotor cortex (Zaki et al., 2010). Though not an exact copy of our result, it does present a remarkably similar network that correlates with cognitive decisions based on interpretation of bodily information, and such interpretations may be important to the effectiveness of AR stimuli. When we plotted the strongest functional connection, vlOT–aMCC, we made an important observation. It appeared that women with ‘low’ disgust propensity were driving the correlation, showing weak vlOT–aMCC functional connectivity during AR relative to core disgust (Figure 2). This may have been due to AR-induced ‘vlOT uncoupling’ in these women. First, the correlation between right vlOT and aMCC was positive across all conditions (result not shown), so we assume right vlOT to exhibit positive connectivity both during AR and core disgust. Second, across subjects, this coupling was reduced during AR, relative to baseline, whereas during core disgust it was similar to baseline (see Results section). This suggests that AR—and not core—was the condition driving the observed modulation of vlOT connectivity by disgust propensity.
Assuming other functional connections were modulated in a similar way, but in appreciation of the fact that this modulation was not equally prominent for all areas (Table 5), one might take the liberty to argue that individuals with low disgust propensity (who report infrequent disgust responses) are ‘safeguarded’ against AR-disgust. Weaker coupling between vlOT and ventral premotor cortex could indicate different interpretation of this particular kind of bodily information. In turn, this would less likely necessitate enhanced behavioural flexibility (e.g. to adhere to the experiment), represented by reduced coupling with aMCC. These individuals could be less liable to experience disgust, which is supported by reduced coupling with the dorsal anterior insula, an area strongly related to disgust experience (Harrison et al., 2010).
No area could be specifically linked to core disgust processing, at least not according to the criteria used here. An interesting observation is that the amygdala and dorsally-adjacent ventral pallidum exhibited a subsignificant (i.e. significant for core > fear, but only a trend for core > AR) preference for core disgust stimuli (Figure 2). This supports findings that the amygdala responds strongly to disgusting tastes and smells (Zald and Pardo, 1997; Zald et al., 2002), but not to higher-order moral disgust (Moll et al., 2005; Schaich Borg et al., 2008). Together with evidence that the posterior part of ventral pallidum processes food-related disgust (Calder et al., 2007), a more primal route in the brain for contamination-related input may be suggested. This may represent yet another neural distinction between AR and core disgust, even when the present results are not sufficiently consistent to fully resolve this issue.
Several comments are in order with respect to the current study: first, in this study we preferred the DPSS-R over content-dependent trait disgust measures, as it prevents artificial relationships between the measure of trait disgust and actual disgust responding during the experiment due to content overlap of stimuli (van Overveld et al., 2010). Moreover, it is the only instrument available that differentiates between disgust propensity and disgust sensitivity. Attesting to the relevance of differentiating between both disgust traits, the correlation between both subscales approached zero. Furthermore, sustaining both the validity and reliability of the DPSS-R, the subscales showed a meaningful pattern with state disgust showing that only disgust propensity was associated with the level of subjective disgust that was elicited in the present experiment. It should be acknowledged, however, that the internal consistency of the DPSS-R subscales for our sample was relatively low, which might have reduced the sensitivity of our study to detect relevant interactions between trait disgust and particular patterns of disgust-related brain activity. In addition, it points to the possibility that the trait–brain interactions that were evident in this study may only depend on particular aspects of the propensity and sensitivity constructs.
Second, core and AR differed in their potency to elicit fear; AR stimuli were rated as more fearful than core disgust stimuli. Thus, where we find differences between AR and core at the central level, this could, at least partially, be due to fear-related processing. Yet, fear may be an intrinsic component of the emotions that are elicited by stimuli generally considered representatives of core, and particularly AR disgust. Note that imprecise labelling of emotional feelings is not uncommon for fear and disgust (e.g. Woody and Teachman, 2000). If indeed fear is an intrinsic component of the emotional feelings that are elicited particularly by AR disgust stimuli (compared with core disgust) it is impossible to control for that. Perhaps it is also relevant to note that fear and disgust are not properties of the stimulus materials per se. Stimuli might elicit fear because they relate to impending harm, but also because they may give rise to contamination. Hence, in the present context it may not be very helpful to think in terms of pure emotional feelings—rather it seems more worthwhile to use proper stimuli well representative of the categories of interest.
The final considerations concern the brain imaging data. PPI is not a measure of effective connectivity (Friston et al., 1997), making inferences about causality impossible. Moreover, the current implementation of PPI in SPM does not allow one to check the sign of the correlation between regions ‘within’ a task. In that respect, claims about weaker connectivity between right vlOT and other areas are somewhat speculative. In this study we were specifically interested in two distinct disgust traits, but we had no a priori hypothesis about how their distinctiveness would affect brain effects and connectivity patterns. Some of the effects (indicated in Tables 2 and 5) should therefore be approached with more caution. In the light of current interpretations of the results, we further acknowledge that we did not gather subjective information on, e.g. social cognition, or reliance on bodily information. Finally, due to the homogeneity of our participants in terms of gender, age, education, menstrual phase and no sexual complaints, generalizability may be restricted.
Taken together, the present findings advance our understanding of disgust by showing that AR and core disgust can be distinguished at the brain network level. Specifically, functional connectivity in a frontal-posterior network was modulated by trait disgust propensity, and this seemed to be driven by the AR condition. It is possible that this finding reflects the different attitudes and emotional responses (disgust, fascination, enjoyment) people may show towards AR.
Conflict of Interest
None declared.
Acknowledgments
The authors would like to thank George Azzopardi for technical support; Renske Bosman and Josefien Breedvelt for assisting in the experiments and all the participants that made this study possible. This study is supported by the University of Groningen and University Medical Centre Groningen along with the Malta Government Scholarship Scheme (Grant Number MGSS_PHD_2008-12, partial funding to C.B.).
Footnotes
1From the independent sample of 25 women, core stimuli (m = 36.28, s.d. = 18.43) did not differ from AR stimuli (m = 40.25, s.d. = 20.56) t(24) = 1.25, P = 0.23, r = 0.01 on the dimension of arousal. Moreover, the pattern of findings on the dimension of fear and disgust on AR, core and fear stimuli was very similar to the ratings reported by the participants of the actual study.
REFERENCES
- Bush G, Vogt BA, Holmes J, et al. Dorsal anterior cingulate cortex. A role in reward-based decision making. Proceedings of the National Academy of Sciences. 2002;99:523–8. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder AJ, Beaver JD, Davis MH, van Ditzhuijen J, Keane J, Lawrence AD. Disgust sensitivity predicts the insula and pallidal response to pictures of disgusting foods. European Journal Neuroscience. 2007;25:3422–8. doi: 10.1111/j.1460-9568.2007.05604.x. [DOI] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. NeuroImage. 2010;50:1148–67. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh K, Davey GCL. Winchester, UK: Paper presented to the British Psychological Society; 2000. The development of a measure of individual differences in disgust. [Google Scholar]
- Cremers HR, Demenescu LR, Aleman A, et al. Neuroticism modulates amygdala-prefrontal connectivity in response to negative emotional facial expressions. NeuroImage. 2010;49:963–70. doi: 10.1016/j.neuroimage.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Curtis V, Aunger R, Rabie T. Evidence that disgust evolved to protect from risk of disease. Proceedings of the Royal Society of London Series B. Biological Sciences. 2004;271:S131–3. doi: 10.1098/rsbl.2003.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong PJ, Merckelbach H. Blood-injection-injury phobia and fear of spiders. Domain specific individual differences in disgust sensitivity. Personality and Individual Differences. 1998;24:153–8. [Google Scholar]
- de Jong PJ, Muris P. Spider phobia: Interaction of disgust and perceived likelihood of involuntary physical contact. Journal of Anxiety Disorders. 2002;16:51–65. doi: 10.1016/s0887-6185(01)00089-5. [DOI] [PubMed] [Google Scholar]
- Downing PE, Jiang Y, Shuman M, Kanwisher N. A cortical area selective for visual processing of the human body. Science. 2001;293:2470–3. doi: 10.1126/science.1063414. [DOI] [PubMed] [Google Scholar]
- Eickhoff S, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–35. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Engelhard MI, Olatunji BO, de Jong PJ. Disgust and the development of posttraumatic stress among soldiers deployed to Afghanistan. Journal of Anxiety Disorders. 2011;25:58–63. doi: 10.1016/j.janxdis.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Fergus TA, Valentiner DP. Reexamining the domain of hypochondriasis. Comparing the illness attitudes scale to other approaches. Journal of Anxiety Disorders. 2009;23:760–6. doi: 10.1016/j.janxdis.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes KJ, Worsley J, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging. A general linear approach. Human Brain Mapping. 1995;2:89–210. [Google Scholar]
- Georgiadis JR, Farrell MJ, Boessen R, et al. Dynamic subcortical blood flow during male sexual activity with ecological validity, a perfusion fMRI study. NeuroImage. 2010;50:208–16. doi: 10.1016/j.neuroimage.2009.12.034. [DOI] [PubMed] [Google Scholar]
- Haidt J, McCauley C, Rozin P. Individual differences in sensitivity to disgust. A scale sampling seven domains of disgust elicitors. Personality and Individual Differences. 1994;16:701–13. [Google Scholar]
- Harrison NA, Marcus A, Gray MA, Gianaros PJ, Critchley HD. The embodiment of emotional feelings in the brain. Journal of Neuroscience. 2010;30:12878–84. doi: 10.1523/JNEUROSCI.1725-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heining MAIK, Young AW, Ioannou GLAV, et al. Disgusting smells activate human anterior insula and ventral striatum. Annals of the New York Academy of Sciences. 2003;1000:380–4. doi: 10.1196/annals.1280.035. [DOI] [PubMed] [Google Scholar]
- Herwig U, Abler B, Walter H, Erk S. Expecting unpleasant stimuli - An fMRI study. Psychiatry Research Neuroimaging. 2007;154:1–12. doi: 10.1016/j.pscychresns.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Bastiaansen J, Keysers C. A common anterior insula representation of disgust observation. Experience and imagination shows divergent functional connectivity pathways. PLoS One. 2008;3:e2939. doi: 10.1371/journal.pone.0002939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Social neuroscience. Mirror neurons recorded in humans. Current Biology. 2010;20:R353–4. doi: 10.1016/j.cub.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nature Neuroscience. 2009;12:535–40. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-4. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 1999. International Affective Picture System (IAPS) Instruction Manual and Affective Ratings. [Google Scholar]
- Mataix-Cols D, An SK, Lawrence NS, et al. Individual differences in disgust sensitivity modulate neural responses to aversive/disgusting stimuli. European Journal of Neuroscience. 2008;27:3050–8. doi: 10.1111/j.1460-9568.2008.06311.x. [DOI] [PubMed] [Google Scholar]
- Matchett G, Davey GCL. A test of a disease avoidance model of animal phobias. Behaviour Research and Therapy. 1991;29:91–4. doi: 10.1016/s0005-7967(09)80011-9. [DOI] [PubMed] [Google Scholar]
- Merckelbach H, de Jong PJ, Arntz A, Schouten E. The role of evaluative learning and disgust sensitivity in the etiology and treatment of spider phobia. Advances in Behaviour Research and Therapy. 1993;15:243–55. [Google Scholar]
- Moll J, de Oliveira-Souza R, Moll FT, et al. The moral affiliations of disgust: a functional MRI study. Cognitive and Behavioural Neurology. 2005;18:68–78. doi: 10.1097/01.wnn.0000152236.46475.a7. [DOI] [PubMed] [Google Scholar]
- Mourao-Miranda J, Volchan E, Moll J, et al. Contributions of stimulus valence and arousal to visual activation during emotional perception. NeuroImage. 2003;20:1955–63. doi: 10.1016/j.neuroimage.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith IAN, Lawrence AD. Functional neuroanatomy of emotions. A meta-analysis. Cognitive. Affective & Behavioral Neuroscience. 2003;3:207–33. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Nanetti L, Cerliani L, Gazzola V, Renken RJ, Keysers C. Group analyses of connectivity-based cortical parcellation using repeated k-means clustering. Neuroimage. 2009;47:1666–77. doi: 10.1016/j.neuroimage.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Lohr JM, Sawchuk CN, Tolin DF. Multimodal assessment of disgust in contamination-related obsessive-compulsive disorder. Behaviour Research and Therapy. 2007;45:263–276. doi: 10.1016/j.brat.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Olatunji BO, McKay D. Disgust and its Disorders: Theory, Assessment and Treatment Implications. Washington DC: American Psychological Association; 2009. pp. 167–285. [Google Scholar]
- Olatunji BO, Williams NL, Lohr JM, Sawchuk CN. The structure of disgust., domain specificity in relation to contamination ideation and excessive washing. Behaviour Research and Therapy. 2005;43:1069–86. doi: 10.1016/j.brat.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Olatunji B, Cisler J, Meunier S, Connolly K, Lohr J. Expectancy bias for fear and disgust and behavioral avoidance in spider fearful individuals. Cognitive Therapy and Research. 2008;32:460–9. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Toki S, et al. Anterior cingulate cortex modulates preparatory activation during certain anticipation of negative picture. Neuropsychologia. 2008;46:102–10. doi: 10.1016/j.neuropsychologia.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Orlov T, Makin TR, Zohary E. Topographic representation of the human body in the occipitotemporal cortex. Neuron. 2010;68:586–600. doi: 10.1016/j.neuron.2010.09.032. [DOI] [PubMed] [Google Scholar]
- Pessoa L. To what extent are emotional visual stimuli processed without attention and awareness? Current Opinion in Neurobiology. 2005;15:188–96. doi: 10.1016/j.conb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion., A meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phillips M, Young AW, Scott SK, et al. Neural responses to facial and vocal expressions of fear and disgust. Proceedings of the Royal Society of London. Series B. Biological Sciences. 1998;7:1809–17. doi: 10.1098/rspb.1998.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrmann S, Hopp H. Cardiovascular indicators of disgust. International Journal of Psychophysiology. 2008;68:201–8. doi: 10.1016/j.ijpsycho.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Rozin P, Fallon AE. A perspective on disgust. Psychological Review. 1987;94:23–41. [PubMed] [Google Scholar]
- Rozin P, Haidt J, McCauley C. Disgust. In: Lewis M, Haviland M, editors. Handbook of Emotions. UK: Guilford; 2000. pp. 637–53. [Google Scholar]
- Rozin P, Nemeroff C, Horowitz M, Gordon B, Voet W. The borders of the self. Contamination sensitivity and potency of the body apertures and other body parts. Journal of Research in Personality. 1995;29:318–40. [Google Scholar]
- Sarlo M, Buodo G, Poli S, Palomba D. Changes in EEG alpha power to different disgust elicitors the specificity of mutilations. Neuroscience Letters. 2005;382:291–6. doi: 10.1016/j.neulet.2005.03.037. [DOI] [PubMed] [Google Scholar]
- Sawchuk CN, Lohr JM, Tolin DF, Lee TC, Kleinknecht RA. Disgust sensitivity and contamination fears in spider and blood-injection-injury phobias. Behaviour Research and Therapy. 2000;38:753–62. doi: 10.1016/s0005-7967(99)00093-5. [DOI] [PubMed] [Google Scholar]
- Schafer A, Leutgeb V, Reishofer G, Ebner F, Schienle A. Propensity and sensitivity measures of fear and disgust are differentially related to emotion-specific brain activation. Neuroscience Letters. 2009;465:262–6. doi: 10.1016/j.neulet.2009.09.030. [DOI] [PubMed] [Google Scholar]
- Schaich Borg J, Lieberman D, Kiehl KA. Infection, incest, and iniquity: investigating the neural correlates of disgust and morality. Journal of Cognitive Neuroscience. 2008;20:1529–46. doi: 10.1162/jocn.2008.20109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienle A, Schafer A, Hermann A, Walter B, Stark R, Vaitl D. fMRI responses to pictures of mutilation and contamination. Neuroscience Letters. 2006;393:174–8. doi: 10.1016/j.neulet.2005.09.072. [DOI] [PubMed] [Google Scholar]
- Schienle A, Schafer A, Stark R, Walter B, Vaitl D. Relationship between disgust sensitivity, trait anxiety and brain activity during disgust induction. Neuropsychobiology. 2005;51:86–92. doi: 10.1159/000084165. [DOI] [PubMed] [Google Scholar]
- Uher R, Murphy T, Friederich HC, et al. Functional neuroanatomy of body shape perception in healthy and eating-disordered women. Biological Psychiatry. 2005;58:990–7. doi: 10.1016/j.biopsych.2005.06.001. [DOI] [PubMed] [Google Scholar]
- van Overveld WJM, de Jong PJ, Peters ML. Digestive and cardiovascular responses to core and animal-reminder disgust. Biological Psychology. 2009;80:149–57. doi: 10.1016/j.biopsycho.2008.08.002. [DOI] [PubMed] [Google Scholar]
- van Overveld WJM, de Jong PJ, Peters ML. The Disgust Propensity and Sensitivity Scale - revised. Its predictive value for avoidance behavior. Personality and Individual Differences. 2010;49:706–11. [Google Scholar]
- van Overveld M, de Jong JP, Peters ML. The multi-dimensional blood/injury phobia inventory: its psychometric properties and relationship with disgust propensity and disgust sensitivity. Journal of Anxiety Disorders. 2011;25:319–25. doi: 10.1016/j.janxdis.2010.10.004. [DOI] [PubMed] [Google Scholar]
- van Overveld WJM, de Jong PJ, Peters ML, Cavanagh K, Davey GCL. Disgust propensity and disgust sensitivity, separate constructs that are differentially related to specific fears. Personality and Individual Differences. 2006;41:1241–52. [Google Scholar]
- von dem Hagen EA, Beaver JD, Ewbank MP, et al. Leaving a bad taste in your mouth but not in my insula. Social Cognitive and Affective Neuroscience. 2009;4:379–86. doi: 10.1093/scan/nsp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A, Ruf M, Braus DF, Schmidt MH. Neuronal activity changes and body image distortion in anorexia nervosa. NeuroReport. 2003;14:2193–7. doi: 10.1097/00001756-200312020-00012. [DOI] [PubMed] [Google Scholar]
- Woody SR, Teachman BA. Intersection of disgust and fear: normative and pathological views. Clinical Psychology, Science and Practice. 2000;7:291–311. [Google Scholar]
- Wright P, He G, Shapira NA, Goodman WK, Liu Y. Disgust and the insula., fMRI responses to pictures of mutilation and contamination. Neuroreport. 2004;15:2347–51. doi: 10.1097/00001756-200410250-00009. [DOI] [PubMed] [Google Scholar]
- Zaki J, Hennigan K, Weber J, Ochsner KN. Social cognitive conflict resolution. Contributions of domain-general and domain-specific neural systems. The Journal of Neuroscience. 2010;30:8481–8. doi: 10.1523/JNEUROSCI.0382-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Hagen MC, Pardo JV. Neural correlates of tasting concentrated quinine and sugar solutions. Journal of Neurophysiology. 2002;87:1068–75. doi: 10.1152/jn.00358.2001. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4119–24. doi: 10.1073/pnas.94.8.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]