Abstract
Studies in psychiatry and cognitive neuroscience have reported an important relationship between individual interoceptive accuracy and anxiety level. This indicates that greater attention to one’s bodily state may contribute to the development of intense negative emotions and anxiety disorders. We hypothesized that reactivity in the anterior insular cortex underlies the intensity of interoceptive awareness and anxiety. To elucidate this triadic mechanism, we conducted functional magnetic resonance imaging (fMRI) and mediation analyses to examine the relationship between emotional disposition and activation in the anterior insular cortex while participants evaluated their own emotional and bodily states. Our results indicated that right anterior insular activation was positively correlated with individual levels of social anxiety and neuroticism and negatively correlated with agreeableness and extraversion. The results of the mediation analyses revealed that activity in the right anterior insula mediated the activity of neural correlates of interoceptive sensibility and social fear. Our findings suggest that attention to interoceptive sensation affects personality traits through how we feel emotion subjectively in various situations.
Keywords: interoception, anxiety, neuroticism, body, emotion
INTRODUCTION
Anxiety disorders are one of the most common types of psychiatric disorder, including specific phobias, social anxiety disorder, panic disorders and general anxiety disorders. Psychological and somatic physiological symptoms are associated with anxiety disorders, and pathology provides important evidence for understanding the relationship between subjective emotion and somatic sensation.
The relationship between subjective emotion and associated somatic responses has been the subject of research for a long time. James (1884) proposed that the experience of emotion results from the perception of specific and unique patterns in the somatovisceral response. This hypothesis has been tested by numerous studies over the past few decades (Schachter and Singer, 1962; Plutchik and Ax, 1967; Ekman et al., 1983; Levenson et al., 1990; Damasio, 1994; Critchley et al., 2005; Rainville et al., 2006).
For understanding the mechanism of emotion, psychological and neural correlates of the subjective experience of emotion have been investigated, leading to influential models such as the somatic marker hypothesis (Damasio, 1994). Although it remains unclear which aspects of the neural and peripheral response fundamentally determine our emotional experiences, the results of recent psychological and brain imaging studies indicate that the perception of bodily signals contribute to and at least partially mediate emotional experience (Bechara et al., 1996; Pollatos et al., 2005; Lane 2008; Dunn et al., 2010).
The perception of afferent information arising from anywhere and everywhere within the body has been termed ‘interoception’ (Sherrington, 1906; Cameron, 2001). Some methods have been developed as measurements of an individual’s ability to perceive interoception, such as the heartbeat detection task (Schandry, 1981), water load test (Herbert et al., 2012) and questionnaires like Body Perception Questionnaire (Porges, 1993) and Modified Somatic Perceptions Questionnaire (Main et al., 1992). The accuracy of interoception and subjective emotion is intimately associated (Pollatos et al., 2005). Some studies have suggested that sensitivity to interoception represents the disposition of a person’s emotional experience, such as its intensity (Wiens et al., 2000; Critchley et al., 2004; Werner et al., 2009) or the tendency to focus on arousal (Barrett et al., 2004). These findings indicate that the way in which interoceptive information is processed can affect emotional experience in daily life.
Importantly, a significant relationship between individual accuracy of interoception and higher levels of anxiety has been reported in psychiatry and cognitive neuroscience (Domschke et al., 2010). When bodily sensation is measured using self-report questionnaires, patients with increased anxiety sensitivity (AS) as well as panic disorder and other anxiety disorders, typically report hypervigilance for bodily sensations (Ludewig et al., 2005; Olatunji et al., 2007; Anderson and Hope, 2009). Studies using the heartbeat detection task have provided evidence of increased cardiac interoceptive sensitivity in individuals with higher levels of anxiety (Pollatos et al., 2007; Dunn et al., 2010; Stevens et al., 2011) and patients with anxiety related disorders, such as panic disorder and social anxiety disorder (Domschke et al., 2010). These findings indicate that greater attention to one’s own bodily state may contribute to the development of intense negative emotions and anxiety disorders. However, the psychological and neurological mechanisms of this relationship are not well understood, and studies that disentangle the close connection between interoceptive awareness and subjective emotion will provide better understanding of the pathology of anxiety disorders.
Based on the data from previous studies, Paulus and Stein (2010) proposed that high interoceptive sensitivity and self-referential belief-based thought might lead to the development of anxiety disorders. Specifically, they proposed that patients with anxiety disorders are particularly sensitive to changes in bodily state when anticipating subsequent events, and the belief-based thought can strongly negatively influence the emotional valence of these amplified bodily signals. Recent studies have identified the insular cortex, especially the anterior part, as a key neural correlate of this mechanism, as well as an essential neural region for engendering subjective emotion from bodily signals (Phillips et al., 2003; Critchley et al., 2004; Craig, 2009). The saliency of subjective emotion and the occurrence of interoceptive awareness are associated with activation in the anterior insular cortex (Critchley et al., 2004; Phan et al., 2004; Iaria et al., 2008). Interestingly, the anticipatory experience of subsequent emotion-laden events causes insular activation along with the uncertainty or emotional valence of the events (Singer et al., 2009). Neuroimaging studies have reported increased activity in anterior insular cortex associated with increasing task instability, complexity and ambiguity with decision-making context, indicating that this area integrates exteroceptive and interoceptive signals concerning uncertainty, and improves learning and guidance of behavioral choice (Huettel et al., 2005; Feinstein et al., 2006; Singer et al., 2009).
Reactivity in the right anterior insula during anticipation is closely associated with an individual’s emotional traits, such as levels of anxiety (Simmons et al., 2011) and scores on the neuroticism scale of the Big Five Inventory (Feinstein et al., 2006). In addition, insular cortex activity during emotional task performance has been proposed as a potentially significant biomarker for detection of anxiety concern disorders (Paulus and Stein, 2006; Simmons et al., 2011). Conversely, our recent study revealed that right anterior insular cortical activation increases when people evaluate their own emotional and bodily states, even if prominent changes in these states do not occur (Terasawa et al., 2011). Thus, accessing the internal state itself would be a fundamental function of this region, underpinning subjective experience of anxiety and emotion. According to Paulus and Stein’s (2006) theory, hypersensitivity to changes in bodily state when anticipating subsequent events yields anticipatory anxiety, because sensitivity increases to detect subtle changes of bodily signals that are associated with the predicted events. As a specific phobia represents an anticipatory anxiety, the association between anxiety and right anterior insular activation would be expected for a particular phobia rather than a general anxiety disorder.
Based on the previous findings, we hypothesized that high-anxiety individuals may exhibit accurate interoceptive sensation underpinned by reactivity in the anterior insular cortex. To elucidate this triadic mechanism, we conducted functional magnetic resonance imaging (fMRI) and mediation analyses to examine the relationship between activation in the anterior insular cortex while participants evaluated their own emotional and bodily states and emotional dispositions. To test our hypotheses, we chose particular social anxiety traits and the Big Five Inventory as indices of personality traits.
METHODS
Participants
A total of 19 undergraduate and graduate students (eight male and 11 female) participated in our study (mean 22.9 years ± 2.11 s.d.). No subjects had a current diagnosis of psychiatric disorder or were taking any medication. All subjects were right-handed and had normal or corrected normal vision. The experiment was performed with the approval of the Keio University Research Ethics Committee (No. 09006). Before participation, all participants read and signed a written informed consent form explaining (i) the purpose and procedure of the study and (ii) that they were able to cease their participation in the study at any time. All participants completed the experiment.
Questionnaires
Before fMRI scanning, participants’ anxiety traits and personality traits were assessed using several questionnaires: Japanese version of NEO Five-Factor Inventory (NEO-FFI) (Shimonaka et al., 1999) and the Social Anxiety Disorder Scale (SADS) (Kaiya, 2009). SADS is a Japanese questionnaire to assess individuals’ social anxiety traits with four subscales: social fear, avoidance, somatic symptoms and daily life interference. Participants completed all questionnaires by themselves.
Procedures and materials
The procedures and materials used were similar to those reported in our previous study (Terasawa et al., 2011). During fMRI scanning, participants were required to answer questions on three topics: emotional awareness, bodily awareness and personal possessions. The emotional awareness and bodily awareness conditions were designed to identify the neural regions related to the evaluation of emotion or the body, respectively. The possession condition was designed as a control condition to identify the regions of activation associated with general task components related to responding to questions about the self. In addition to the sentence type condition, we prepared two temporal constraint conditions: ‘now’ and ‘usual’. The now condition required participants to perform online monitoring, whereas the usual condition required them to perform offline monitoring.
After presenting a fixation point for 4–6 s on a monitor placed in the fMRI scanner, we presented a cue (‘now’ or ‘usual’), followed by a statement, such as ‘I’m happy (for emotional awareness)’, ‘I have a fast pulse (for bodily awareness)’ and ‘I have money (for possessions)’. The cue was presented for 3 s and the sentence was presented for 8 s. In the now condition, participants evaluated the appropriateness of the statement as a description of their current state. In the usual condition, they evaluated the appropriateness of the statement as a description of their usual disposition. Participants’ evaluation regarding the appropriateness of the statement was chosen from four options: ‘not at all’, ‘somewhat’, ‘very’ and ‘definitely’. Stimuli were generated by a control computer using Cogent 2000 (http://www.vislab.ucl.ac.uk/cogent_2000.php) implemented in MATLAB (MathWorks Inc.). Participants responded using a four-button MRI-compatible keypad connected to the control computer, which recorded the responses and reaction times (RTs).
The sentences presented in each trial were identical to our previous study. Sentences for the emotional awareness conditions were selected from the Positive and Negative Affect Scale (Watson et al., 1988) and translated into Japanese based on Sato and Yasuda’s (2001) report. For the bodily awareness condition, sentences were selected from the Body Perception Questionnaire (Porges, 1993) and the Modified Somatic Perceptions Questionnaire (Main et al., 1992). Items used in the possession condition were selected by interviewing undergraduate students about what items they were usually carrying. We prepared 16 sentences for each condition. Thus, there were 96 trials in total (two cues, three conditions and 16 sentences). The full list of stimuli used in each condition is available as the appendix of Terasawa et al. (2011). The 96 trials were divided into three blocks, meaning that each block comprised 32 trials. The order of the blocks and trials was counterbalanced across participants.
fMRI data acquisition and analyses
fMRI scanning employed a 3 T Siemens Tim Trio scanner with an eight-channel head coil for data acquisition. Scanning consisted of three experimental functional runs and a high-resolution T1-weighted structural scan (1 mm isotropic resolution three-dimensional magnetization-prepared rapid acquisition gradient echo). Each functional run consisted of 274 whole-brain T2*-weighted single-shot gradient echo-planar imaging (EPI) images, collected in an oblique axial orientation [time to repetition 2.35 s, time to echo 30 ms, flip angle 90°, voxel size 3.5 × 3.5 × 2 mm, 44 slices (descending) and slice gap 1 mm]. The structural scan was co-registered to the subject’s mean EPI image.
Individual data were pre-processed and analyzed using SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Functional time images from each participant were spatially corrected for head movement, and temporally corrected for slice timing, spatially normalized to the Montreal Neurological Institute (MNI) template and smoothed with a three-dimensional Gaussian filter (8 mm full width half maximum). In addition, a high-pass temporal filter with a cutoff of 128 s was applied to remove low-frequency drift in signals, and global changes were removed by proportional scaling.
First-level analyses were performed to determine each subject’s voxel-wise activation, while participants were choosing the appropriate options. The analyses were performed using an event-related model with six trial types: ‘body-now’, ‘body-usual’, ‘emotion-now’, ‘emotion-usual’, ‘possession-now’ and ‘possession-usual’. A canonical set of three functions, the hemodynamic response function (HRF), its temporal derivatives and its dispersion derivatives (Friston et al., 1998), characterized the neural response. The general linear model was used to create statistical parametric maps. Subsequent second-level group random effects analyses were performed on the SPM contrast images of the first-level canonical HRF responses. In accord with previous fMRI studies, a statistical threshold of P < 0.001, uncorrected, with an extent threshold of 10 voxel for multiple spatial comparisons across the whole brain was used, except for conjunction analyses in which the threshold was set at P < 0.005 with family wise error (FWE) correction.
Time constraint conditions (now/usual) were used to distinguish between online experience-based evaluation and offline knowledge-based evaluation. As we reported similarities and the difference between these two conditions in our previous study (Terasawa et al., 2011), in this study, we focused attention on the neural activity underlying online experience-based evaluation (now condition). In addition, the possession condition was prepared similarly to our previous study. The neural activity in this condition was outside the focus of this study, therefore it is not mentioned further below.
RESULTS
Behavioral results
RTs were recorded from the time of presentation of the sentences until the time the response button was pressed. The mean RT and SEM for each condition were as follows: emotion-now 2.42 (±0.11) s, emotion-usual 2.60 (±0.15) s, body-now 2.38 (±0.10) s, body-usual 2.31 (±0.11) s, possession-now 2.47 (±0.11) s and possession-usual 2.54 (±0.09) s. Two-way analysis of variance (ANOVA) of RTs was conducted for question types (emotion, body and possession) and cue conditions (now and usual). Neither the main effect of question type nor the cue conditions were significant.
To assess rating responses, we calculated mean rating values from the participants’ average responses by replacing the rating options with numerical quanta. That is, ‘not at all’ was replaced with 0, ‘somewhat’ with 1, ‘very’ with 2 and ‘definitely’ with 3. The mean rating value and SEM for each condition were as follows: emotion-now 1.43 (±0.04), emotion-usual 1.52 (±0.06), body-now 1.47 (±0.05), body-usual 1.45 (±0.08), possession-now 1.94 (±0.07) and possession-usual 2.06 (±0.06). In addition, we performed a two-way ANOVA to investigate differences in rating value among the three question types (emotional awareness, bodily awareness and possession) and two cue conditions (now/usual). The main effects of question type and cue condition were not significant. We intentionally eliminated factors that evoked prominent emotional or bodily responses in all conditions. The participants’ questionnaire responses indicated that they did not perceive prominent changes in their emotional or bodily states.
Questionnaires
To assess individual differences in anxiety traits and personality, we used SADS (Kaiya, 2009) and the Japanese version of NEO-FFI (Shimonaka et al., 1999). The mean score and standard deviation of each questionnaire are shown in Supplementary Table S1.
fMRI data
Shared neural substrates of emotional and bodily awareness
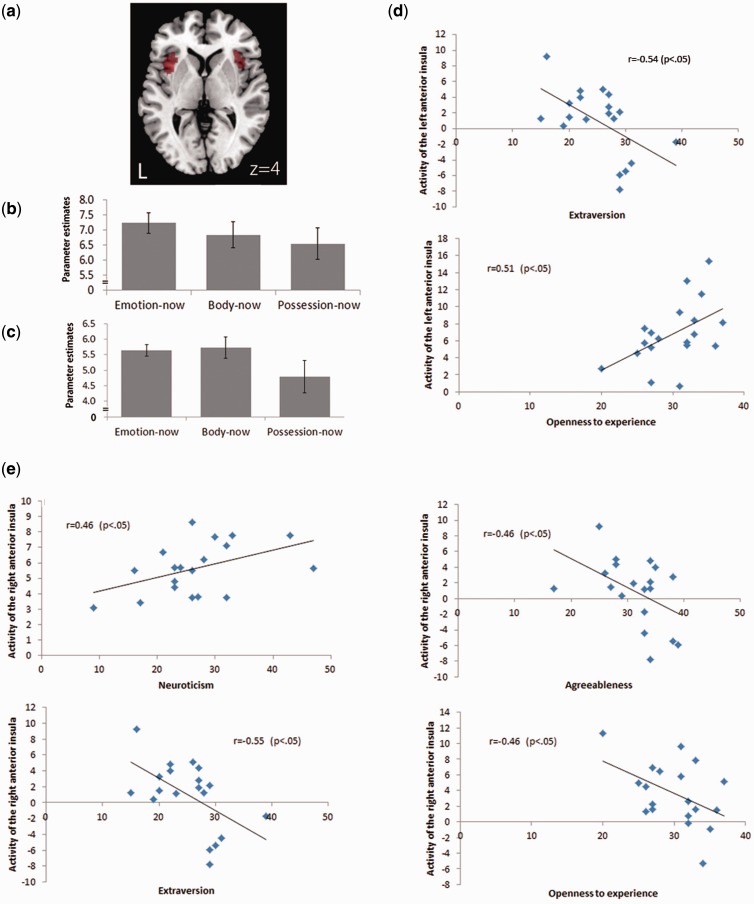
We analyzed the fMRI data using a random-effects group analyses (n = 19) to identify the shared neural substrates of emotional and bodily awareness. For this purpose, the intersection of main effect images of the body-now and the emotion-now conditions identified the commonly activated regions. As shown in Table 1, the bilateral anterior insula cortex, the bilateral temporo-parietal junction, the lingual gyrus, the medial frontal cortex and some parts of the brain stem were commonly activated for both contrasts and thus identified as the neural substrates of emotional and bodily awareness [P < 0.005 (FWE), k > 10]. Both this study and our previous report (Terasawa et al., 2011) revealed that the anterior insular cortex is an important area for accessing bodily and emotional awareness. Parameter estimates of the bilateral anterior insula for each condition were calculated as shown in Figure 1B and C.
Table 1.
Shared neural substrates of emotional and bodily awareness (P < 0.005, FWE, k > 10)
| Regions of activation | L/R | BA | Number of voxels in cluster | z | MNI |
||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Insula | L | 13 | 3106 | 7.71 | −34 | 22 | 4 |
| R | 13 | 472 | 6.4 | 34 | 26 | 4 | |
| Medial frontal gyrus | L/R | 8 | 1973 | 7.63 | 6 | 20 | 48 |
| L | 23 | 93 | 5.85 | −2 | −16 | 30 | |
| Inferior parietal gyrus | L | 7 | 147 | 5.81 | −30 | −60 | 50 |
| Lingual gyrus | L | 19 | 7287 | 7.72 | −18 | −62 | −8 |
| Cerebellum | L | – | 17 | 5.65 | −6 | −76 | −26 |
| Brain stem | R | 70 | 5.65 | 22 | −26 | −6 | |
| L | 14 | 5.92 | −20 | −28 | −4 | ||
| L | 12 | 5.5 | −6 | −26 | −10 | ||
Fig. 1.
Activity of the insular cortex and personality scores. Bilateral anterior insular cortex was strongly activated in both body-now and emotion-now conditions (P < 0.005, FWE) and we set these regions as ROIs to understand the relationship between emotional and interoceptive awareness. (A) ROIs on the bilateral insular cortex. The peak voxel of right anterior insula is x = 34, y = 26, z = 4 and left anterior insula is x = −34, y = 22, z = 4. (B) Parameter estimates of the left anterior insula for each condition. (C) Parameter estimates of the right anterior insula for each condition. (D) The activation extracted from ROIs of the anterior insular cortex was correlated with some personality factors. Left anterior insular cortex activity during emotion-now condition was negatively correlated with the levels of extraversion (r = −0.54, P < 0.05) but positively correlated with openness to experience (r = 0.51, P < 0.05). (E) Right anterior insular cortex activity during emotion-now condition was positively correlated with levels of neuroticism (r = 0.46, P < 0.05). In addition, the activity in body-now condition was negatively correlated with levels of extraversion (r = −0.55, P < 0.05), agreeableness (r = −0.46, P < 0.05) and openness to experience (r = −0.46, P < 0.05).
In accord with previous studies, we set the bilateral anterior insula as a region of interests (ROIs) to examine the relationship between emotional and interoceptive awareness (emotion-now and body-now). Parts of anterior insular cortex exhibiting common activation for the body- and emotion-now conditions were set as ROIs using MarsBar (http://marsbar.sourceforge.net/). The peak voxels of the bilateral insula were as follows: right anterior insula, x = 34, y = 26, z = 4 and left anterior insula, x = −34, y = 22, z = 4 (Figure 1A).
We investigated the association between anterior insular cortex activation and personality traits. The activation measured in the ROIs of the anterior insular cortex was correlated with some personality factors (Figure 1). Left anterior insular cortex activity in the emotion-now condition was negatively correlated with levels of extraversion (r = −0.54, P < 0.05) but positively correlated with openness to experience (r = 0.51, P < 0.05) (Figure 1D). In addition, right anterior insular cortex activity in the emotion-now condition was positively correlated with levels of neuroticism (r = 0.46, P < 0.05) and right anterior insular activation in the body-now condition was negatively correlated with levels of extraversion (r = −0.55, P < 0.05), agreeableness (r = −0.46, P < 0.05) and openness to experience (r = −0.46, P < 0.05) (Figure 1E).
Neural correlates of anxiety
Regression analyses were performed on images of the emotion-now condition to find the neural activity correlated with levels of social anxiety. The SADS comprises four subcategories: social fear, social avoidance, somatic sensation and daily life interference. Scores of each subcategory were used as regressors. The analyses revealed that right anterior insular activation was correlated with social fear score. Other regions in the middle and superior frontal gyrus, cuneus, parahippocampal and inferior parietal gyrus were also correlated with social fear (Table 2). Avoidance scores were associated with activation in the precuneus, superior frontal gyrus and mediodorsal nucleus of the left thalamus (Table 3). Only two areas, the left insular and the medial frontal gyrus, were identified as somatic processing-related areas.
Table 2.
Regions associated with social fear scores (P < 0.001, uncorrected)
| Regions of activation | L/R | BA | Number of voxels in cluster | z | MNI |
||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Middle frontal gyrus | R | 8 | 64 | 4.42 | 38 | 30 | 44 |
| Superior frontal gyrus | R | 6 | 39 | 3.78 | 30 | 8 | 66 |
| Cuneus/calacarine | L | 23 | 90 | 3.75 | −10 | −76 | 6 |
| Cuneus | L | 17 | 3.26 | −18 | −82 | 10 | |
| Insular cortex | R | 13 | 24 | 3.6 | 38 | 16 | −2 |
| R | 18 | 3.45 | 34 | 22 | −14 | ||
| Insular/superior temporal cortex | L | 13 | 27 | 3.58 | −56 | −34 | 20 |
| L | − | 3.23 | −52 | −36 | 12 | ||
| Parahippocampal gyrus | L | 16 | 3.54 | −32 | −18 | −24 | |
| Superior parietal gyrus | L | 7 | 10 | 3.5 | −22 | −64 | 64 |
| Inferior parietal gyrus | R | 40 | 25 | 3.47 | 46 | −52 | 48 |
| Inferior parietal gyrus | L | 13 | 3.28 | −38 | −38 | 44 | |
Table 3.
Regions associated with social avoidance scores (P < 0.001, uncorrected)
| Regions of activation | L/R | BA | Number of voxels in cluster | z | MNI |
||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Precuneus | L | 7 | 136 | 4.82 | −20 | −64 | 46 |
| Superior frontal gyrus | L | 6 | 15 | 3.6 | −24 | 14 | 66 |
| L | 14 | 3.46 | −38 | 6 | 58 | ||
| Thalamus | L | 10 | 3.56 | 0 | −24 | 2 | |
Neural correlates associated with interoceptive sensibility
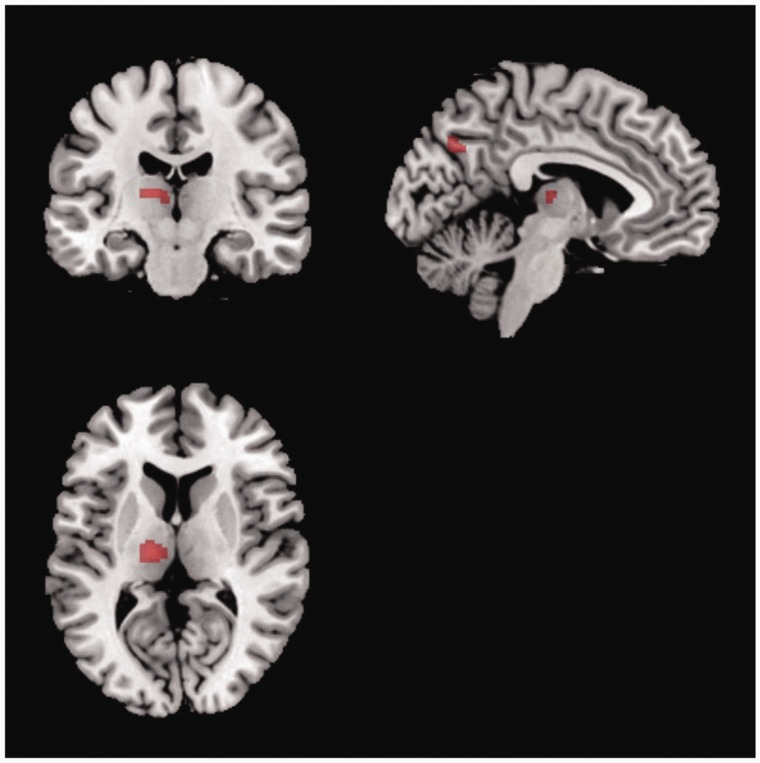
To identify the neural correlates associated with interoceptive sensibility, the association between the level of sensibility to somatic sensation and neuronal activity during the emotion-now condition was investigated using regression analysis. Each participant’s somatic sensibility level was calculated by summing the scores in each trial in the body-now condition. The results revealed that the left ventral posterior and mediodorsal thalamus (x = −23, y = −18, z = 10, k = 106) exhibited a strong association with interoceptive sensibility. The precuneus (x = −8, y = −66, z = 34, k = 55) and supramarginal gyrus (x = −42, y = −46, z = 34, k = 32) exhibited a similar pattern (Figure 2).
Fig. 2.
Neural correlates associated with interoceptive sensibility. Somatic sensibility levels of each participant were correlated with activity in left ventral posterior and mediodorsal thalamus, precuneus and supramarginal gyrus (P < 0.001, uncorrected).
Interoceptive sensibility and social anxiety
In this study, we sought to clarify the way in which interoceptive sensibility modulates anxiety level. To disentangle the relationship, we focused on the insular modulation of the association between the level of social anxiety and the activity of neural correlates of interoceptive sensibility while accessing emotional state. As such, we employed mediation analyses to examine the activity of regions that were associated with individuals’ interoceptive sensibility, and identified the regions representing the extent of interoceptive processing. In the first step of the mediation analyses used with regression analyses, we examined the neural regions in which activity predicted the level of social anxiety. The activation of the left thalamus, but not the precuneus and supramarginal gyrus, was significantly associated with individual levels of social fear [t(17) = 2.62, P < 0.05]. As we hypothesized that the insular cortex plays an essential role in the association between interoception and the levels of social anxiety, we focused on the relationship between the activation of the extracted ROI of the anterior insular cortex and the left thalamus. The results of the regression analyses revealed a statistically significant association between these two regions [t(17) = 2.97, P < 0.01].
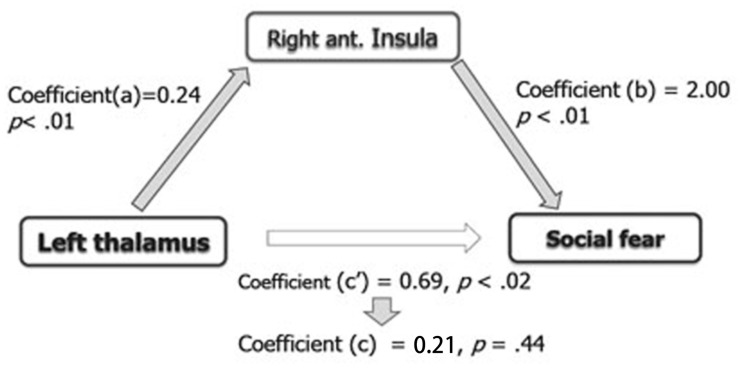
Second, we assessed whether the activation of the right anterior insular cortex mediates the correlation between the activation of the left thalamus and the level of social fear. In brief, mediation analyses provided an estimation of the effect of an independent variable on a mediator (a), the effect of a mediator on a dependent variable (b), the direct effect of an independent variable on a dependent variable (c ′) and the indirect effect of an independent variable on a dependent variable via a mediator (ab). As shown in Figure 3, the analyses revealed a significant indirect effect of the left thalamus on the level of social fear via the right anterior insula (ab = 0.48, s.e. = 0.22, z = 2.16, P < 0.05) (Sobel test, http://quantpsy.org/sobel/sobel.htm). Higher activation in the left thalamus was associated with higher activation in the right anterior insula [a = 0.24, s.e. = 0.08, t(17) = 2.97, P < 0.01] and higher activation in the right anterior insula was associated with higher levels of social fear [b = 2.00, s.e. = 0.64, t(17) = 3.11, P < 0.01]. Importantly, the direct pathway between the left thalamus and social fear became non-significant [c = 0.21, s.e. = 0.27, t(17) = 0.79, P = 0.44 (n.s.)] when the right anterior insula mediated these two factors indicating that this association was fully mediated by the right anterior insula.
Fig. 3.
Outline of the mediation analysis focused on the insular modulation of the association between the levels of social anxiety and the activity of the left thalamus, in which activity was correlated with somatic sensibility level. The analysis revealed that activity in the right anterior insular cortex mediated the activity of the left thalamus and the level of social fear (P < 0.05).
DISCUSSION
This study sought to clarify the relationship between the properties of subjective experience of emotion and interoceptive processing. As such, we investigated activation in the anterior insular cortex while participants evaluated their own emotional and bodily states, and found that the activation was positively correlated with individual levels of anxiety and neuroticism and negatively correlated with agreeableness and extraversion. The results of the mediation analyses revealed that activity in the right anterior insula mediated the activity of the neural correlates of interoceptive sensibility and social fear. In accord with our hypotheses, these results indicate that interoceptive processing mediates bodily sensation and anxiety level. The anterior insular cortex is considered as a critical area for subjective experience of emotion, combining interoceptive information with information about the participants’ environment (Critchley et al., 2004; Craig, 2009). The strong association between insular activation and emotional traits, such as anxiety and neuroticism, would be important in understanding the role of the insula in the subjective experience of emotion.
It has been proposed that an increase in anxious affect, worrisome thought and other avoidance behaviors are triggered by predictive signals of aversive bodily state associated with negative consequences (Paulus and Stein, 2006). According to this hypothesis, high-anxiety individuals tend to be sensitive to their own interoceptive information, so they experience a body state with exaggerated interoceptive predictive signals. These findings suggest that the anterior insula may play an important role in this process. A recent study by Killgore et al. (2011) reported that individual levels of AS were correlated with insular activity while the participants viewed negative facial expression stimuli. High-AS individuals were found to exhibit a strong defensive response and enhanced autonomic response to subsequent threats (Melzig et al., 2008). These studies present the difficulty of generating a clear understanding of the function of insular activity in negative emotional situations and whether it represents interoceptive sensitivity or an exaggerated emotional autonomic response.
In this study, we did not employ any emotion elicitation process for focusing on the neural response of afferent processing in accord with emotional experience. This novel procedure revealed that the anterior insular cortex was closely related with anxiety trait scores, and that its activation mediated social anxiety and activation of the left thalamus, which is related to somatic perception. The brain regions most closely related to somatic perception in the left thalamus were the ventral posterior lateral and mediodorsal areas. Relay nuclei are gathered in the ventral posterior lateral region, and these nuclei relay information from the cranial nerve and medulla spinal area to the primary sensory and somatosensory areas (Blumenfeld, 2002). In addition, these areas are involved in the pathogenic mechanisms of intractable pain, and stimulation to this brain region induces visceral pain. The onset of ischemia without subjective symptoms enhances the activation of this area (Rosen et al., 1996). These reports support the notion that these regions play an important role in the neural network underlying interoception by monitoring the internal bodily state (Cameron, 2001). Damasio et al. (2012) recently reported that a patient with bilateral insula damage was still able to feel emotion and self-awareness because of the residual function of subcortical regions. These findings suggest that the brain stem and hypothalamus contribute to the perception of internal states at a primary level, and that the thalamus relays signals from these regions to the telencephalon.
Using mediation analyses, we found that activity in the right anterior insula mediated the activity of the neural correlates of interoceptive sensibility and social fear. These results indicated that the level of attention to interoceptive information can impact on subjective feelings of anxiety, rather than the perception of bodily state per se. Lovero et al. (2009) revealed that the anterior insula was activated during the anticipation of touch but not during the actual tactile sensation, and that the intensity of anticipated touch was predicted by its activity. Although the results indicated the involvement of the left thalamus and right anterior insula in social anxiety, right thalamus activity was found to be associated with interoceptive sensibility when the threshold was lowered. These findings shed light on the connection between the insula and the thalamus in terms of the expression of social fear, rather than hemispheric lateralization. The thalamic nuclei in the ventral posterior region are anatomically part of the pathway carrying visceral afferent information from the brainstem to the insular cortex (Cameron, 2002). In addition, enhanced functional connectivity of thalamo-cortical circuits, including the insula and the medial prefrontal cortex, has been observed in social anxiety disorders (Etkin and Wager, 2007; Gimenez et al., 2012). These findings support our hypothesis that the anterior insular cortex represents the process of attending to interoceptive information, not only bodily perception, and emphasizes the significance of interoceptive processing for the awareness of subjective experience of emotion.
Interestingly, our results indicated that activity in the right anterior insular cortex was correlated with anxiety and some personality traits. Specifically, we found associations between anterior insular activation and neuroticism, extraversion, agreeableness and openness to experience. Several previous studies elicited negative emotion by presenting emotion-laden stimuli or situations, and observed enhanced activity of the anterior insular cortex in people with high anxiety or neuroticism trait scores (Drabant et al., 2011; Killgore et al., 2011). The high reactivity of the anterior insula is thought to guide a person to safer choices in risky decision-making situations (Feinstein et al., 2006; Paulus and Stein, 2006). Personality traits and levels of anxiety are thought to represent individual differences in emotional experience (McCrae and Costa, 1991). It is known that individuals with high levels of neuroticism are at an increased risk for developing depressive and anxiety disorders (Kendler and Gardner, 1998; Bienvenu et al., 2001). For example, Cox et al. (1999) reported a positive correlation between AS and self-consciousness and a negative correlation between AS and extraversion. Our findings suggest that the level of attention to interoceptive sensation affects personality traits through how we feel emotion subjectively in various situations.
Our findings revealed that anterior insular activity predicted individual levels of social fear and somatic concern, subscales of the SADS. Social avoidance is another subscale, reflecting the inclination to select social avoidance behavior when people feel social fear. As most of the participants in this study had not experienced extreme disturbance due to social anxiety in their life, social avoidance levels were low overall. The extent to which anterior insula activity is involved appears to depend on which subscales of social anxiety are used. Practical behavioral selection is a product of complex decision making, and emotional sensibility is not the only factor affecting behavioral selection. The attenuation of functional connectivity between the dorsolateral prefrontal cortex and insular cortex in high-anxiety individuals implies that their cognitive regulation ability may be weakened in emotional situations (Simmons et al., 2011). Further studies examining this issue will be valuable for understanding the pathogenic mechanisms of social anxiety.
Our results indicate that attention reorientation may help to reduce anxiety in individuals suffering from social anxiety disorders, and that reorientation from interoceptive to exteroceptive environmental information may reduce anxiety levels. In accord with this prediction, a previous study reported the effectiveness of task concentration training or attention training for reducing anxiety in individuals suffering from anxiety disorders (Bogels and Mansell, 2004). This training is based on the reorientation of attention away from bodily autonomic response such as accelerated heartbeat and shallow breathing, toward executing tasks or social situations per se.
In addition, our results are in accord with clinical observations indicating the involvement of the anterior insular cortex in some other psychiatric symptoms related to disrupted subjective feelings, such as anosognosia (Orfei et al., 2007), alexithymia (Kano et al., 2007; Moriguchi et al., 2007), depersonalized disorder (Phillips et al., 2001) and schizophrenia (Phillips et al., 2003; Makris et al., 2006). Taken together, these findings suggest that the way in which we perceive and interpret physiological changes in the body can strongly impact on our health through emotional experience. To clarify the relationship between interoception, emotional experience and neural activity in patients with psychiatric disorders could improve our understanding of the mechanisms underlying these clinical symptoms, and may aid the development of effective treatment. Future studies with psychiatric patients should thus be conducted to provide further insight into emotional experience and its psychological and neural mechanisms.
This study has several limitations. First, our results focused on the relationship between social anxiety and interoceptive processing and its neural correlates. However, it should be noted that the associations between these factors vary across different types of anxiety. For example, findings regarding the association between levels of trait anxiety and interoceptive sensibility have been inconsistent (Domschke et al., 2010). Although enhanced activity of insular cortex in people suffering from anxiety has been consistently observed (Etkin and Wager, 2007), patients with generalized anxiety disorder have been found to exhibit significantly less insular activation in response to fearful relative to neutral stimuli, because of enhanced responses to ‘neutral’ stimuli (Blair et al., 2008). These various associations may represent the variety of pathological mechanisms of each anxiety disorder. The applicability of our findings to other anxiety-related disorders should be examined in future studies. Second, we observed hemispheric lateralization in the relationship between insular activation and scores of openness to experience. Although the functional laterality of the insula remains contentious, Craig (2005) hypothesized that the left insula is associated with affiliative emotions, whereas the right insula is associated with aroused emotions. It is unclear whether these findings support this hypothesis, but the laterality of the insula should be carefully considered. Finally, we defined participants’ somatic sensibility based on their subjective evaluations rather than psychophysiological assessments such as the heartbeat detection task (Schandry, 1981). Thus, our findings are primarily concerned with the component of the relationship between somatic sensibility and anxiety that is accessible to verbal report. Other aspects of the relationship should be considered in future research.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
This work was supported by the JSPS KAKENHI grant numbers, 23700318, 24330210, the Global COE Program ‘Centre for Advanced Research on Logic and Sensibility’ by the MEXT of Japan and Program for the Advancement of Next Generation Research Projects, Keio University.
REFERENCES
- Anderson ER, Hope DA. The relationship among social phobia, objective and perceived physiological reactivity, and anxiety sensitivity in an adolescent population. Journal of Anxiety Disorders. 2009;23(1):18–26. doi: 10.1016/j.janxdis.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Quigley KS, Bliss-Moreau E, Aronson KR. Interoceptive sensitivity and self-reports of emotional experience. Journal of Personality and Social Psychology. 2004;87(5):684–97. doi: 10.1037/0022-3514.87.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cerebral Cortex. 1996;6(2):215–25. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- Bienvenu OJ, Nestadt G, Samuels JF, Costa PT, Howard WT, Eaton WW. Phobic, panic, and major depressive disorders and the five-factor model of personality. Journal of Nervous and Mental Disease. 2001;189(3):154–61. doi: 10.1097/00005053-200103000-00003. [DOI] [PubMed] [Google Scholar]
- Blair K, Shaywitz J, Smith BW, et al. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. The American Journal of Psychiatry. 2008;165(9):1193–202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H. Neuroanatomy through Clinical Cases. Sunderland, MA: Sinauer Associates, Inc; 2002. [Google Scholar]
- Bogels SM, Mansell W. Attention processes in the maintenance and treatment of social phobia: hypervigilance, avoidance and self-focused attention. Clinical Psychology Review. 2004;24(7):827–56. doi: 10.1016/j.cpr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Cameron OG. Interoception: the inside story—a model for psychosomatic processes. Psychosomatic Medicine. 2001;63(5):697–710. doi: 10.1097/00006842-200109000-00001. [DOI] [PubMed] [Google Scholar]
- Cameron OG. Visceral Sensory Neuroscience, Interoception. New York: Oxford University Press; 2002. [Google Scholar]
- Cox BJ, Borger SC, Taylor S, Fuentes K, Ross LM. Anxiety sensitivity and the five-factor model of personality. Behaviour Research and Therapy. 1999;37(7):633–41. doi: 10.1016/s0005-7967(98)00174-0. [DOI] [PubMed] [Google Scholar]
- Craig AD. Forebrain emotional asymmetry: a neuroanatomical basis? Trends in Cognitive Sciences. 2005;9(12):566–71. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Rotshtein P, Nagai Y, O'Doherty J, Mathias CJ, Dolan RJ. Activity in the human brain predicting differential heart rate responses to emotional facial expressions. NeuroImage. 2005;24(3):751–62. doi: 10.1016/j.neuroimage.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7(2):189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio A, Damasio H, Tranel D. Persistence of feelings and sentience after bilateral damage of the insula. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs077. doi: 10.1093/cercor/bhs077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR. Descarte's Error. New York: Penguin Putnam; 1994. [Google Scholar]
- Domschke K, Stevens S, Pfleiderer B, Gerlach AL. Interoceptive sensitivity in anxiety and anxiety disorders: an overview and integration of neurobiological findings. Clinical Psychology Review. 2010;30(1):1–11. doi: 10.1016/j.cpr.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Drabant EM, Kuo JR, Ramel W, et al. Experiential, autonomic, and neural responses during threat anticipation vary as a function of threat intensity and neuroticism. NeuroImage. 2011;55(1):401–10. doi: 10.1016/j.neuroimage.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn BD, Galton HC, Morgan R, et al. Listening to your heart: how interoception shapes emotion experience and intuitive decision making. Psychological Science. 2010;21(12):1835–44. doi: 10.1177/0956797610389191. [DOI] [PubMed] [Google Scholar]
- Ekman P, Levenson RW, Friesen WV. Autonomic nervous system activity distinguishes among emotions. Science. 1983;221(4616):1208–10. doi: 10.1126/science.6612338. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American Journal of Psychiatry. 2007;164(10):1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Stein MB, Paulus MP. Anterior insula reactivity during certain decisions is associated with neuroticism. Social Cognitive and Affective Neuroscience. 2006;1(2):136–42. doi: 10.1093/scan/nsl016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Josephs O, Rees G, Turner R. Nonlinear event-related responses in fMRI. Magnetic Resonance in Medicine. 1998;39(1):41–52. doi: 10.1002/mrm.1910390109. [DOI] [PubMed] [Google Scholar]
- Gimenez M, Pujol J, Ortiz H, et al. Altered brain functional connectivity in relation to perception of scrutiny in social anxiety disorder. Psychiatry Research. 2012;202(3):214–23. doi: 10.1016/j.pscychresns.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Herbert BM, Muth ER, Pollatos O, Herbert C. Interoception across modalities: on the relationship between cardiac awareness and the sensitivity for gastric functions. PLoS ONE. 2012;7(5):e36646. doi: 10.1371/journal.pone.0036646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA, Song AW, McCarthy G. Decisions under uncertainty: probabilistic context influences activation of prefrontal and parietal cortices. The Journal of Neuroscience. 2005;25(13):3304–11. doi: 10.1523/JNEUROSCI.5070-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaria G, Committeri G, Pastorelli C, Pizzamiglio L, Watkins KE, Carota A. Neural activity of the anterior insula in emotional processing depends on the individuals' emotional susceptibility. Human Brain Mapping. 2008;29(3):363–73. doi: 10.1002/hbm.20393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. What is an emotion? Mind. 1884;19:188–205. [Google Scholar]
- Kaiya Y. Social Anxiety Disorder Scale. Tokyo: Kaneko Syobo; 2009. [Google Scholar]
- Kano M, Hamaguchi T, Itoh M, Yanai K, Fukudo S. Correlation between alexithymia and hypersensitivity to visceral stimulation in human. Pain. 2007;132(3):252–63. doi: 10.1016/j.pain.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO. Boundaries of major depression: an evaluation of DSM-IV criteria. American Journal of Psychiatry. 1998;155(2):172–7. doi: 10.1176/ajp.155.2.172. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Britton JC, Price LM, Gold AL, Deckersbach T, Rauch SL. Neural correlates of anxiety sensitivity during masked presentation of affective faces. Depression and Anxiety. 2011;28(3):243–9. doi: 10.1002/da.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD. Neural substrates of implicit and explicit emotional processes: a unifying framework for psychosomatic medicine. Psychosomatic Medicine. 2008;70(2):214–31. doi: 10.1097/PSY.0b013e3181647e44. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Ekman P, Friesen WV. Voluntary facial action generates emotion-specific autonomic nervous system activity. Psychophysiology. 1990;27(4):363–84. doi: 10.1111/j.1469-8986.1990.tb02330.x. [DOI] [PubMed] [Google Scholar]
- Lovero KL, Simmons AN, Aron JL, Paulus MP. Anterior insular cortex anticipates impending stimulus significance. NeuroImage. 2009;45(3):976–83. doi: 10.1016/j.neuroimage.2008.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig S, Geyer MA, Ramseier M, Vollenweider FX, Rechsteiner E, Cattapan-Ludewig K. Information-processing deficits and cognitive dysfunction in panic disorder. Journal of Psychiatry and Neuroscience. 2005;30(1):37–43. [PMC free article] [PubMed] [Google Scholar]
- Main CJ, Wood PL, Hollis S, Spanswick CC, Waddell G. The distress and risk assessment method. A simple patient classification to identify distress and evaluate the risk of poor outcome. Spine. 1992;17(1):42–52. doi: 10.1097/00007632-199201000-00007. [DOI] [PubMed] [Google Scholar]
- Makris N, Goldstein JM, Kennedy D, et al. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophrenia Research. 2006;83(2–3):155–71. doi: 10.1016/j.schres.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Mccrae RR, Costa PT. The NEO Personality-Inventory—Using the 5-Factor Model in Counseling. Journal of Counseling and Development. 1991;69(4):367–72. [Google Scholar]
- Melzig CA, Michalowski JM, Holtz K, Hamm AO. Anticipation of interoceptive threat in highly anxiety sensitive persons. Behaviour Research and Therapy. 2008;46(10):1126–34. doi: 10.1016/j.brat.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Decety J, Ohnishi T, et al. Empathy and judging other's pain: an fMRI study of alexithymia. Cerebral Cortex. 2007;17(9):2223–34. doi: 10.1093/cercor/bhl130. [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Cisler JM, Tolin DF. Quality of life in the anxiety disorders: a meta-analytic review. Clinical Psychology Review. 2007;27(5):572–81. doi: 10.1016/j.cpr.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Orfei MD, Robinson RG, Prigatano GP, et al. Anosognosia for hemiplegia after stroke is a multifaceted phenomenon: a systematic review of the literature. Brain. 2007;130(Pt 12):3075–90. doi: 10.1093/brain/awm106. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biological Psychiatry. 2006;60(4):383–7. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Structure and Function. 2010;214(5–6):451–63. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. NeuroImage. 2004;21(2):768–80. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biological Psychiatry. 2003;54(5):515–28. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Medford N, Senior C, et al. Depersonalization disorder: thinking without feeling. Psychiatry Research. 2001;108(3):145–60. doi: 10.1016/s0925-4927(01)00119-6. [DOI] [PubMed] [Google Scholar]
- Plutchik R, Ax AF. A critique of Determinants of Emotional state by Schachter and Singer (1962) Psychophysiology. 1967;4(1):79–82. doi: 10.1111/j.1469-8986.1967.tb02740.x. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Kirsch W, Schandry R. On the relationship between interoceptive awareness, emotional experience, and brain processes. Brain Research Cognitive Brain Research. 2005;25(3):948–62. doi: 10.1016/j.cogbrainres.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Traut-Mattausch E, Schroeder H, Schandry R. Interoceptive awareness mediates the relationship between anxiety and the intensity of unpleasant feelings. Journal of Anxiety Disorders. 2007;21(7):931–43. doi: 10.1016/j.janxdis.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Porges S. Body Perception Questionnaire. University of Maryland: Laboratory of Developmental Assessment; 1993. [Google Scholar]
- Rainville P, Bechara A, Naqvi N, Damasio AR. Basic emotions are associated with distinct patterns of cardiorespiratory activity. International Journal of Psychophysiology. 2006;61(1):5–18. doi: 10.1016/j.ijpsycho.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Rosen SD, Paulesu E, Nihoyannopoulos P, et al. Silent ischemia as a central problem: regional brain activation compared in silent and painful myocardial ischemia. Annals of Internal Medicine. 1996;124(11):939–49. doi: 10.7326/0003-4819-124-11-199606010-00001. [DOI] [PubMed] [Google Scholar]
- Sato A, Yasuda A. Development of the Japanese version of Positive and Negative Affect Schedule (PANAS) scales. The Japanese Journal of Personality. 2001;9:138–9. [Google Scholar]
- Schachter S, Singer JE. Cognitive, social, and physiological determinants of emotional state. Psychological Review. 1962;69:379–99. doi: 10.1037/h0046234. [DOI] [PubMed] [Google Scholar]
- Schandry R. Heart beat perception and emotional experience. Psychophysiology. 1981;18(4):483–8. doi: 10.1111/j.1469-8986.1981.tb02486.x. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. The Integrative Action of the Nervous System. New Haven: Yale University Press; 1906. [Google Scholar]
- Shimonaka Y, Nakazato K, Gondo Y, Takayama M. NEO-PI-R, NEO-FFI Manual for the Japanese Version. Tokyo: Tokyo Shinri; 1999. [Google Scholar]
- Simmons AN, Stein MB, Strigo IA, Arce E, Hitchcock C, Paulus MP. Anxiety positive subjects show altered processing in the anterior insula during anticipation of negative stimuli. Human Brain Mapping. 2011;32(11):1836–46. doi: 10.1002/hbm.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Sciences. 2009;13(8):334–40. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Stevens S, Gerlach AL, Cludius B, Silkens A, Craske MG, Hermann C. Heartbeat perception in social anxiety before and during speech anticipation. Behaviour Research and Therapy. 2011;49(2):138–43. doi: 10.1016/j.brat.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Terasawa Y, Fukushima H, Umeda S. How does interoceptive awareness interact with the subjective experience of emotion? An fMRI study. Human Brain Mapping. 2011 doi: 10.1002/hbm.21458. doi: 10.1002/hbm.21458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Werner NS, Duschek S, Marttern M, Schandry R. Interoceptive sensitivity modulates anxiety during public speaking. Journal of Psychophysiology. 2009;23(2):85–94. [Google Scholar]
- Wiens S, Mezzacappa ES, Katkin ES. Heartbeat detection and the experience of emotions. Cognition and Emotion. 2000;14(3):417–27. [Google Scholar]