Abstract
In this issue, Terasawa and colleagues used functional neuroimaging to test for common neural substrates supporting conscious appraisal of subjective bodily and emotional states and explored how the relationship might account for personality and experience of anxiety symptoms. Their study highlights a role for the same region of anterior insula cortex in appraisal of emotions and bodily physiology. The reactivity of this region also mediated the relationship between ‘bodily sensibility’ and social fear, translating a cognitive representation of subjective physical state into an individual personality trait that influences social interaction. The task used by Terasawa and colleagues taps into conscious aspects to the expression of this dynamic. These findings add to increasing evidence for the role of anterior insula as the interface between physiologically driven internal motivational states, emotional awareness and interpersonal behaviour.
With advances in affective neuroscience, there is increasing empirical knowledge about the relationship between bodily sensations and emotional experience. Somatic sensitivity and interoceptive awareness are relevant, and at times controversial, concepts that emerge from both clinical experience and theoretical models of emotion. Interoception refers to sensitivity to stimuli originating from within the body. Interoception is distinguished in Sherrington’s original definition from the sensing of stimulation from outside the body (exteroception) and of the body’s position in space (proprioception) (Sherrington, 1948). While interoception encompasses both skeletomuscular and circulating (humoral) signals, more emphasis is generally given to afferent information from visceral organs and vascular system. The viscerosensory nerves that carry this information typically also form the afferent limb of autonomic nervous reflexes and higher levels of autonomic regulation. Correspondingly, interoception is linked to low-level homeostatic control processes that to a large extent are managed pre-consciously by peripheral, brainstem and subcortical (e.g. hypothalamic) structures and adequately describable in animal studies. However, interception also encompasses conscious visceromotor sensations, e.g. the fullness of bladder or bowel that motivates controlled voiding, or the abdominal sensations that accompany nausea or hunger.
The notion that emotional feelings arise from internal bodily sensations is influential, and continues to drive an interest in interoception. Peripheral theories of emotion, e.g. that formulated in the 19th century by William James and Carl Lange (Lange and James, 1967) have qualified empirical support (Cannon, 1927; Schachter and Singer, 1962; Damasio et al., 1991; Lazarus, 1991). It is broadly accepted that visceral bodily states (of arousal) at the very least can contribute to (and intensify) many emotional feelings. It follows that emotions may be more subjectively powerful at times when an individual is more sensitive to changes their bodily arousal state. Moreover, individual differences in ‘interoceptive sensitivity’ may predict differences in the expression of emotional traits or responses. Interoceptive sensitivity is generally viewed as an invariant constitutional trait (somewhat like personality), that is hard wired hence stable over long time periods. Individuals with heightened interoceptive awareness (as quantified objectively from performance in a heartbeat detection task) report more intense emotional experiences (Wiens et al., 2000). This and related observations endorse the notion that internal physiological and subjective emotional states are interdependent. Motivated initially by the work of Damasio and others, the ascendancy of neuroimaging techniques in human neuroscience, has led to renewed interest into brain mechanisms underlying the generation of emotions. A key question is the nature and degree of shared neurocircuitry underlying perception of bodily response and emotion judgements. The paper by Terasawa and colleagues in this issue extends a growing literature on this subject, providing insight into the neural mediation of social and emotional behaviour, implicating in particular anterior insula cortex.
INTEROCEPTIVE REPRESENTATION AND THE NEURAL SUBSTRATES OF EMOTIONS
Insular cortex, particularly anterior sectors, is commonly activated across a range of cognitive and emotional neuroimaging tasks. Phillips et al. (1998) highlighted the role insula plays in the processing of the visceral emotion of disgust. Subsequent studies have shown more generalized reactivity of insula (along with ‘visceromotor’ anterior cingulate) to salience (e.g. Seeley et al., 2007). Nevertheless, salient stimuli are by definition motivationally and emotionally important; hence, typically evoke changes in internal state (e.g. orienting response). One neuroimaging study, developing from work on central autonomic control, identified anterior insula cortex as a likely neural substrate for the conscious representational of internal bodily signals (e.g. Critchley et al., 2004). Participants were required to focus on the timing of their own heartbeats at rest (a widely used test of interoceptive awareness/sensitivity). Activity in a number of brain regions was enhanced during performance of this heartbeat detection task (relative to an exteroceptive task). However, in this study, activity within right anterior insular corresponded best to performance accuracy, a proxy for interoceptive awareness. The link to emotion was established indirectly, showing that the activation (and size) of right anterior insula correlated with both interoceptive sensitivity (task and questionnaire measures) and score on a questionnaire that probed day-to-day experiences of anxiety. Similar studies also implicate dorsal/anterior cingulate cortex in both bodily arousal and interoceptive sensitivity (Pollatos et al., 2007) or emotional awareness (Lane et al., 1998) but the link to affective feelings remained circumstantial. The role of insula, in conjunction with anterior cingulate cortex, in the representation and control of internal state is backed by influential interpretations of central neuroanatomical pathways (Craig 2002).
A recent study directly investigated whether interoceptive performance and emotional assessments are indeed supported by the same functional neural architecture (Zaki et al., 2012). Here, participants watched videos of people recounting emotional stories and then rated their own emotional experience. The same participants also completed an interoceptive task in which they monitored their own heartbeat. Strikingly, across a number of conjunction analyses, they found that brain activation subserving both emotion and interoceptive judgements was limited to anterior insula and adjacent inferior frontal operculum (Zaki et al., 2012). These observations lend credence to the notion that the assessment of one’s own emotional feeling states is supported neural processes that underlying detection of internal bodily changes. In this issue, the paper of Terasawa and colleagues supports and extends this finding: Using a paradigm that orientates attention either to the participants’ own internal bodily state or to their own emotion state during fMRI scanning, the researchers identified bilateral anterior insular cortex as a neural substrate active in both the cognitive evaluation of bodily state and appraisal of self-emotion.
RELATIONSHIP BETWEEN INTEROCEPTION, ANXIETY AND PERSONALITY
Terasawa and colleagues took these observations further by testing if the shared neural architecture underlying judgements of body and emotion also account for individual differences in affective style. They analysed how relationships between interoceptive sensibility/emotion/social anxiety and personality factors might be mediated functionally by the underlying neurocircuitry. This line of investigation was motivated by recognition that interoceptive sensitivity has long been implicated in the expression and pathophysiology of anxiety disorders: Cognitive models of anxiety, such as those conceptualized by Clark and colleagues, identify the perception and misattribution of bodily sensations as a key component in the development of panic and related anxiety symptoms (Clark et al., 1997). Implicit in these models is the assumption that those vulnerable to anxiety have a heightened propensity to detect internal bodily changes which then forms the basis of a misinterpreted/misascribed signal. Across clinical populations there appears to be a tendency for better interoceptive awareness among patients with clinical anxiety disorders (Ehlers and Breuer, 1992; Zoellner and Craske, 1999; Pollatos et al., 2009; Dunn et al., 2010b; Stevens et al., 2011). Even anxiety patients whose symptoms are in remission can still display heightened interoceptive sensitivity (Ehlers et al., 1995), suggesting that interoception is a constitutional trait that underlies vulnerability to anxiety. With brain anatomy notably ‘interoceptive’ insular cortex in mind, Paulus and Stein (2006, 2010), posited that sub-threshold afferent interoceptive signals are detected by high anxiety individuals, and that these signals are then amplified, and associated with potential aversive or negative outcomes (Paulus and Stein, 2010). By investigating underlying mediating neurocircuitry, the study of Terasawa and colleagues reinforces our mechanistic understanding of the way in which insular cortex integrates interoceptive information toward anxiety responses (Paulus and Stein, 2006). Moreover, Terasawa and colleagues also identify the thalamus as an area related to social fear (which in fact replicates other findings, e.g. Stark et al., 2003). Importantly, they go on to demonstrate that this relationship between thalamic activation and social fear was fully mediated by ‘interoceptive’ right anterior insula cortex activity. Future work can build further upon this approach, to define using functional connectivity analyses how the circuitry involved in interoception supports, though relationships with other neural areas, a range of emotions and affective symptoms, yet also mediates the expression and influence of personality factors.
In pursing the neural correlates of individual differences, Terasawa and colleagues explore how different emotion dimensions correspond to differences in the magnitude of activation within the insula. To do this they examined separately the insula activity evoked during emotion and bodily assessments, and regressed this against quantified scores on different dimensions of personality. Interestingly, the engagement of left anterior insula when appraising current emotion was negatively correlated with extraversion and positively correlated with openness to experience. Activity within right anterior insula cortex positively correlated with neuroticism. Moreover, when focusing on bodily state, engagement of right anterior cortex was negatively correlated with extraversion, agreeableness and openness to experience. These observations suggest a representational and neural architecture (including hemispheric laterality) linked to the expression of different personality dimensions, and fundamentally grounded on emotion and interoception. This presented work remains exploratory and further research is needed to help elucidate the interplay between individual differences in personality, emotion, bodily assessment and underlying differences in neural activity in key interoceptive areas.
THE RELATIONSHIP BETWEEN INTEROCEPTION AND COGNITION
Beyond emotion, there is increasing evidence to suggest that detection of bodily sensations also guides our cognitive processes. Following on from the work of Damasio and colleagues and their formulation of the influential Somatic Marker Hypothesis (Damasio et al., 1991), efforts have been made to map the central contribution of autonomic response to brain activity underlying decision making (e.g. Critchley et al., 2001; Coricelli et al., 2005). The Somatic Marker Hypothesis proposes that fluctuations in bodily arousal contribute to cognitive processes themselves by feeding back to bias thoughts, judgements and behaviours (Damasio et al., 1991). This process may be particularly relevant for correcting suboptimal behaviours and guiding complex decision making in the face of uncertainty (Bechara et al., 1997). The hypothesis also suggests that individuals with high interoceptive sensitivity may be particularly gifted at utilizing this bodily information to guide cognition as associated behavioural choices. Emerging evidence suggests that individuals with better interoceptive sensitivity have an enhanced implicit memory (Werner et al., 2010) and display improved decision making on the Iowa Gambling Task (Dunn et al., 2010a). Neuroimaging studies report increasing activity within anterior insula cortex with increasing task instability, complexity and ambiguity in decision-making contexts. This has been interpreted as showing that anterior insula integrates exteroceptive and interoceptive signals concerning uncertainty to improve learning and guide behavioural choice (Huettel et al., 2005; Feinstein et al., 2006; Singer et al., 2009). Interpretation of insula in cognitive risk and decision making is relevant to Terasawa and colleagues’ neuroimaging inferences concerning social anxiety. The naturalistic real-world need for managing behavioural choices a complex uncertain and potentially risky environments lies within the social domain of interpersonal interactions with friends, acquaintances and strangers. There is a need to validate these inferences to understand how this integration of interoceptive cues with emotional and cognitive representations works at the level of the neuronal ensembles and distributed network circuitry.
MULTIPLE ELEMENTS OF INTEROCEPTION
Terasawa and colleagues examined the neural substrates of ‘interoceptive sensibility’ through self-report answers to bodily-awareness questions such as ‘I have a fast pulse’. Many other experimental studies assessing interoception have used self-report methodologies, typically questionnaires [(e.g. the Body Perception Questionnaire (Porges, 1993)]. Self-report measures of interoception are necessary to gauge individual differences in perceived subjective sensitivity to (and preoccupation with) internal bodily fluctuations. However, biases and alterations in subjective thresholds of perceived sensitivity tend to be included without an objective demonstration that actual detection levels are indeed different. Heartbeat detection tasks have emerged as the dominant method to objectively assess interoceptive sensitivity (e.g. Mandler and Kahn, 1960; Whitehead et al., 1977; Schandry, 1981; Katkin et al., 1983; Brener and Kluvitse, 1988; Critchley, et al., 2004). These tests are generally assumed to map onto other measures of interoceptive sensitivity, evidenced by correlations between heart beat and gastric awareness demonstrated in one or two studies (Whitehead and Drescher, 1980; Herbert et al., 2012). Despite some psychometric issues, heartbeat detection approaches demonstrate good validity with respect to predictions and inferences about subjective emotional behaviour. The heartbeat counting approach in particular is relatively easy to implement.
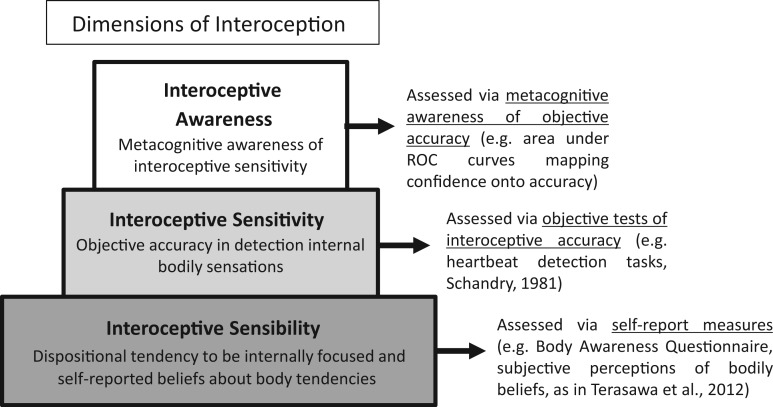
Many mental processes can be dissected in terms of measures of objective behaviour/performance and subjective awareness. Emotion (Lane, 2008) and knowledge (Dienes and Perner, 1999) are no exception, where behaviour and awareness may closely correlate or markedly diverge depending on context. A similar distinction can be made in relation to interoception: Interoceptive sensitivity (as measured by objective tests of interoceptive proficiency, e.g. performance on heartbeat detection tests) can be distinguished from interoceptive awareness—a metacognitive measure that quantifies individuals’ explicit knowledge of (and confidence in) their interoceptive accuracy. Few studies have made this distinction and investigated directly these measures simultaneously. Studies that have explored the relationship between participants’ self-reported heartbeat awareness (using questionnaires) with their actual (experimentally measured) heartbeat awareness tend not to find a strong or significant correlations (e.g. Mcfarland, 1975; Whitehead, et al., 1977), indicating that a preoccupation with internal bodily sensations and a belief in one’s own interoceptive sensitivity does not necessary predict actual interoceptive ability. Thus, a focus on internal bodily sensations, accurate detection of bodily sensations, and being aware/confident that one is perceiving these bodily sensations accurately, represent distinct processes and should not be conflated (see Figure 1). There is, however, a need to further characterize these distinct facets of interoception to fully understand the way in which they are (or are not) interdependent, their shared and distinct neural substrates, their relationship to different disorders, such as anxiety and depression and their relative predictive value for measures of emotion and cognition. A systematic assessment of these facets of interoception will help build a fully comprehensive understanding of the wider contributions of bodily representation to emotion and cognition.
Fig. 1.
Dimensions of interoception: Schematic figure depicting layered representation of internal bodily state and sensation. These facets may have distinct and dissociable contributions to affective behavior. Approaches for assessment these facets are suggested to the right hand side.
CONCLUSION
In this issue, the paper of Terasawa and colleagues draws attention to many of these concerns. The authors probe the substrates of (verbalized) interoceptive sensibility without specifically gauging objective ability, but nevertheless their findings are consistent with what is known about interoceptive representation and its translation into emotional feeling states. Importantly, they link the neural substrates of ongoing bodily condition and emotional state to pervasive features of personality that set the range of affective responsivity and interpersonal interaction. These findings enrich our understanding of the mechanisms supporting affective cognition and have implications for the understanding of the genesis of anxiety disorders.
Acknowledgments
SNG and HDC are supported by Brighton and Sussex Medical School, University of Sussex. Their work is supported by the Dr. Mortimer and Theresa Sackler Foundation. HDC is a co-recipient of a grant from the Medical Research Council UK
REFERENCES
- Bechara A., Damasio H., Tranel D., Damasio A.R. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275(5304):1293–5. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Brener J., Kluvitse C. Heartbeat detection—judgments of the simultaneity of external stimuli and heartbeats. Psychophysiology. 1988;25(5):554–61. doi: 10.1111/j.1469-8986.1988.tb01891.x. [DOI] [PubMed] [Google Scholar]
- Cannon W.B. Bodily Changes in Pain, Hunger, Fear and Rage: An Account of Recent Researches into the Function of Emotional Excitement. New York: D. Appleton; 1927. [Google Scholar]
- Clark D.M., Salkovskis P.M., Ost L.G., Breitholtz E., Koehler K., Westling B.E., Jeavons A., Gelder M. Misinterpretation of body sensations in panic disorder. Journal of Consulting and Clinical Psychology. 1997;65(2):203–213. doi: 10.1037//0022-006x.65.2.203. [DOI] [PubMed] [Google Scholar]
- Coricelli G., Critchley H.D., Joffily M., O'Doherty J.P., Sirigu A., Dolan R.J. Regret and its avoidance: a neuroimaging study of choice behavior. Nature Neuroscience. 2005;8(9):1255–62. doi: 10.1038/nn1514. [DOI] [PubMed] [Google Scholar]
- Craig A.D. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Mathias C.J., Dolan R.J. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;29(2):537–45. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Wiens S., Rotshtein P., Ohman A., Dolan R.J. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7(2):189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio A.R., Tranel D., Damasio H.C. Somatic markers and the guidance of behavior: theory and preliminary testing. In: Levin H.S., Eisenberg H.M., Benton A.L., editors. Frontal Lobe Function and Dysfunction. New York: Oxford University Press; 1991. pp. 217–229. [Google Scholar]
- Dienes Z., Perner J. A theory of implicit and explicit knowledge. Behavioral and Brain Sciences. 1999;22(5):735–55. doi: 10.1017/s0140525x99002186. [DOI] [PubMed] [Google Scholar]
- Dunn B.D., Galton H.C., Morgan R., et al. Listening to your heart: how interoception shapes emotion experience and intuitive decision making. Psychological Science. 2010a;21(12):1835–44. doi: 10.1177/0956797610389191. [DOI] [PubMed] [Google Scholar]
- Dunn B.D., Stefanovitch I., Evans D., Oliver C., Hawkins A., Dalgleish T. Can you feel the beat? Interoceptive awareness is an interactive function of anxiety- and depression-specific symptom dimensions. Behaviour Research and Therapy. 2010b;48(11):1133–8. doi: 10.1016/j.brat.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers A., Breuer P. Increased cardiac awareness in panic disorder. Journal of Abnormal Psychology. 1992;101(3):371–82. doi: 10.1037//0021-843x.101.3.371. [DOI] [PubMed] [Google Scholar]
- Ehlers A., Breuer P., Dohn D., Fiegenbaum W. Heartbeat perception and panic disorder—possible explanations for discrepant findings. Behaviour Research and Therapy. 1995;33(1):69–76. doi: 10.1016/0005-7967(94)e0002-z. [DOI] [PubMed] [Google Scholar]
- Feinstein J.S., Stein M.B., Paulus M.P. Anterior insula reactivity during certain decisions is associated with neuroticism. Social Cognitive and Affective Neuroscience. 2006;1(2):136–42. doi: 10.1093/scan/nsl016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert B.M., Muth E.R., Pollatos O., Herbert C. Interoception across modalities: on the relationship between cardiac awareness and the sensitivity for gastric functions. Plos One. 2012;7(5):e36646. doi: 10.1371/journal.pone.0036646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel S.A., Song A.W., McCarthy G. Decisions under uncertainty: probabilistic context influences activation of prefrontal and parietal cortices. Journal of Neuroscience. 2005;25(13):3304–11. doi: 10.1523/JNEUROSCI.5070-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katkin E.S., Reed S.D., Deroo C. A methodological analysis of 3 techniques for the assessment of individual-differences in heartbeat detection. Psychophysiology. 1983;20(4):452. [Google Scholar]
- Lane R.D. Neural substrates of implicit and explicit emotional processes: a unifying framework for psychosomatic medicine. Psychosomatic Medicine. 2008;70(2):214–31. doi: 10.1097/PSY.0b013e3181647e44. [DOI] [PubMed] [Google Scholar]
- Lane R.D., Reiman E.M., Axelrod B., Yun L.S., Holmes A., Schwartz G.E. Neural correlates of levels of emotional awareness: evidence of an interaction between emotion and attention in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 1998;10(4):525–35. doi: 10.1162/089892998562924. [DOI] [PubMed] [Google Scholar]
- Lange C.G., James W. In: The Emotions (Reprinted) Dunlap K., editor. New York: Hafner Publishing Co.; 1967. [Google Scholar]
- Lazarus R.S. Progress on a cognitive motivational relational theory of emotion. American Psychologist. 1991;46(8):819–34. doi: 10.1037//0003-066x.46.8.819. [DOI] [PubMed] [Google Scholar]
- Mandler G., Kahn M. Discrimination of changes in heart-rate—2 unsuccessful attempts. Journal of the Experimental Analysis of Behavior. 1960;3(1):21–5. doi: 10.1901/jeab.1960.3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcfarland R.A. Heart-rate perception and heart-rate control. Psychophysiology. 1975;12(4):402–5. doi: 10.1111/j.1469-8986.1975.tb00011.x. [DOI] [PubMed] [Google Scholar]
- Paulus M.P., Stein M.B. An insular view of anxiety. Biological Psychiatry. 2006;60(4):383–7. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Paulus M.P., Stein M.B. Interoception in anxiety and depression. Brain Structure & Function. 2010;214(5–6):451–63. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Young A.W., Scott S.K., et al. Neural responses to facial and vocal expressions of fear and disgust. Proceedings Biological Sciences. 1998;265:1809–17. doi: 10.1098/rspb.1998.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O., Schandry R., Auer D.P., Kaufmann C. Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Research. 2007;1141:178–87. doi: 10.1016/j.brainres.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Pollatos O., Traut-Mattausch E., Schandry R. Differential effects of anxiety and depression on interoceptive accuracy. Depression and Anxiety. 2009;26(2):167–73. doi: 10.1002/da.20504. [DOI] [PubMed] [Google Scholar]
- Porges S. Body Perception Questionnaire: Laboratory of Development Assessment. Baltimore, MD: University of Maryland; 1993. [Google Scholar]
- Schachter S., Singer J.E. Cognitive, social, and physiological determinants of emotional state. Psychological Review. 1962;69:379–99. doi: 10.1037/h0046234. [DOI] [PubMed] [Google Scholar]
- Schandry R. Heart beat perception and emotional experience. Psychophysiology. 1981;18(4):483–8. doi: 10.1111/j.1469-8986.1981.tb02486.x. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., et al. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27(9):2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington C.S. The Integrative Action of the Nervous System. Cambridge, UK: Cambridge University Press; 1948. [Google Scholar]
- Singer T., Critchley H.D., Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Sciences. 2009;13(8):334–40. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Stark R., Schienle A., Walter B., et al. Hemodynamic responses to fear and disgust-inducing pictures: an fMRI study. International Journal of Psychophysiology. 2003;50(3):225–34. doi: 10.1016/s0167-8760(03)00169-7. [DOI] [PubMed] [Google Scholar]
- Stevens S., Gerlach A.L., Cludius B., Silkens A., Craske M.G., Hermann C. Heartbeat perception in social anxiety before and during speech anticipation. Behaviour Research and Therapy. 2011;49(2):138–43. doi: 10.1016/j.brat.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Werner N.S., Peres I., Duschek S., Schandry R. Implicit memory for emotional words is modulated by cardiac perception. Biological Psychology. 2010;85(3):370–6. doi: 10.1016/j.biopsycho.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Whitehead W.E., Drescher V.M. Perception of gastric contractions and self-control of gastric-motility. Psychophysiology. 1980;17(6):552–8. doi: 10.1111/j.1469-8986.1980.tb02296.x. [DOI] [PubMed] [Google Scholar]
- Whitehead W.E., Drescher V.M., Heiman P., Blackwell B. Relation of heart-rate control to heartbeat perception. Biofeedback and Self-Regulation. 1977;2(4):371–92. [PubMed] [Google Scholar]
- Wiens S., Mezzacappa E.S., Katkin E.S. Heartbeat detection and the experience of emotions. Cognition and Emotion. 2000;14(3):417–27. [Google Scholar]
- Zaki J., Davis J.I., Ochsner K.N. Overlapping activity in anterior insula during interoception and emotional experience. Neuroimage. 2012;62(1):493–9. doi: 10.1016/j.neuroimage.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoellner L.A., Craske M.G. Interoceptive accuracy and panic. Behaviour Research and Therapy. 1999;37(12):1141–58. doi: 10.1016/s0005-7967(98)00202-2. [DOI] [PubMed] [Google Scholar]