Abstract
Background & Aims
Celiac disease (CD) is associated with an increased risk of lymphoma. However, relatively few studies have assessed the outcome of patients diagnosed with both CD and lymphoma. We evaluated the temporal association between lymphoma and CD, along with clinical presentation, response to therapy, and prognosis.
Methods
Patients diagnosed with both CD and lymphoma were identified retrospectively in a tertiary referral center. Clinical characteristics and survival were analyzed.
Results
Sixty-three patients (36 men) were identified who had been diagnosed with lymphoma and CD. Thirty-six (57%) were diagnosed with CD before they were diagnosed with lymphoma. The most common histologic entity was diffuse, large, B-cell lymphoma, which affected 18 (29%) patients. Complete information for staging was available in 59 patients; 24 (38%) had stage IV disease. Only chemotherapy or only radiation therapy was used for 43 (68%) and 11 (17%) patients, respectively. The 5- and 10-year cumulative survival rates for the entire cohort were 58% and 39%, respectively. Survival of patients with T-cell lymphoma was shorter than for all other lymphomas (119.4 vs 22.8 mo; P = .02).
Conclusions
CD is associated with B- and T-cell lymphomas. Patients with B-cell lymphomas had a better prognosis than those with T-cell lymphoma. Therapy is unsatisfactory for enteropathy-type T-cell lymphoma.
Keywords: Cancer, Enteropathy, GI Disease, Gluten
Celiac disease (CD) is a common autoimmune systemic disorder resulting from ingestion of gluten-containing foods, a major storage protein of wheat, barley, and rye.1 CD may affect as many as 1% of the general population, and the prevalence appears to be increasing over time.2–4 Most intestinal and extraintestinal manifestations of CD are reversible after treatment with a gluten-free diet.1 Severe complications and increased mortality may develop in the absence of treatment of symptomatic CD.5 Indeed, untreated CD has been associated with various hematologic complications, including lymphoma.6
The association of CD and lymphoma has been known for decades, and earlier studies suggested the risk of contracting lymphoma was high.6–13 The risk of lymphoma in the setting of CD likely is lower than previously thought because of the inclusion of high-risk patients in the historical estimates (eg, patients with refractory sprue) and a higher background prevalence of subclinical CD.11 Recent studies using large databases have suggested the risk of lymphoma in patients with clinically diagnosed CD may be as high as 5 times what is seen in the general population, and this risk seems to have diminished over the past decades.8,9 The association of CD (especially refractory CD type 2) with enteropathy-type T-cell lymphoma (ETL) appears particularly strong with standardized incidence ratios being as high as 24 and odds ratios of 28.9,10,14 ETL is a very rare disease,15 thus, despite the strong association of CD with ETL, the majority of lymphomas associated with CD are of the non-ETL type.7,9,10 Other types of lymphomas observed in patients with CD include B-cell lymphomas (eg, diffuse large B-cell lymphoma, DLBCL) and non-ETL T-cell lymphomas (eg, peripheral T-cell lymphoma).10,11 About 35% of ETL patients are associated with a previous clinical history of adult-onset CD.16 In more than 90% of ETL there is histologic evidence of enteropathy-like alterations in the gastrointestinal mucosa adjacent to the invasive tumor.12,16 Strict adherence to a gluten-free diet may decrease the risk of lymphomagenesis and development of other cancers associated with untreated CD.17,18 Finally, lymphomagenesis can occur in a minority of patients with CD and persistent villous atrophy despite treatment with a gluten-free diet, even in the absence of symptoms related to CD.19,20
The mechanisms of lymphomagenesis in CD are poorly understood but likely multifactorial. Change to a monoclonal phenotype, clonal expansion, and increased survival of intraepithelial lymphocytes induced at least in part by the uncontrolled overexpression of interleukin-15 by enterocytes in patients with refractory CD type 2, may promote the emergence of T-cell clonal proliferations and lymphoma.21 Thus, refractory CD associated with clonal intraepithelial lymphocytes is associated with a high risk for T-cell lymphoma over time.22–24 Indeed, lymphomagenesis (eg, ETL) may occur in 60% to 80% of patients with refractory CD type 2 (characterized by clonal intraepithelial lymphocytes) and occasionally in patients with refractory CD type 1.14,25–27 There is no established therapy to prevent progression to lymphoma in refractory CD type 2.28,29 Allelic imbalances with complex chromosomal gains at 9q or losses at 16q are present in most cases of ETL.30 Partial trisomy of the 1q region (1q22-q44) is strongly associated with refractory CD and can be present in 16% of patients with ETL.31,32 Homozygosity for HLA-DQ2 and myosin IXB gene (MYO9B) polymorphism (rs7259292) are associated with increased risk of ETL in European patients with CD.33,34 The mechanisms underlying extraintestinal lymphomagenesis in CD are unknown.
Although the literature on the epidemiology of CD-associated lymphomas is ample, relatively few studies have assessed the outcome of individual patients.11,16,35 The clinical course of ETL is highly aggressive, with most patients dying of the disease within months of diagnosis.14,16 The results of chemotherapy alone or chemotherapy and autologous stem cell transplantation for ETL are very poor with few long-term survivors.16,36
The aim of our study was to retrospectively review all cases of lymphoma in patients who also carried the diagnosis of CD and determine the temporal association of the lymphoma with CD, the clinical presentation, response to therapy, and prognosis.
Materials and Methods
Patients
Patients carrying the diagnosis of both CD and lymphoma were identified through the Mayo Clinic patient registry and the Mayo Clinic Lymphoma Database. Patients diagnosed with lymphoma in the period from 1970 to 2005 who also had a diagnosis of CD were included. Details of presenting history, physical examination, staging investigations, treatment, and clinical outcome were extracted from patient records. We collected information necessary to perform accurate lymphoma staging and to apply the International Prognostic Index (IPI) to all patients.37 Lymphoma staging was performed according to the Ann Arbor staging system.38 Information on therapy and to assess response was also collected where available. Complete remission was defined as a return to normal by all documented tumor sites. Partial remission was defined as a more than 50% reduction in disease bulk for at least 1 month. All other patients were regarded as nonresponders.
Statistical Analysis
Data were summarized using descriptive statistics. Overall survival was used as the primary outcome. Overall survival was defined as the number of months between the date of diagnosis of lymphoma and date of death (if the patient had died) or last follow-up evaluation. The date of death was obtained from the Mayo Clinic records. Survival was estimated using the Kaplan–Meier method, and log-rank statistics were computed to detect differences between survival curves for various prognostic factors. The χ2 test was used for comparing categoric variables where appropriate. The nonparametric Wilcoxon rank-sum test was used for comparing the median of continuous variables between groups. All statistical analyses were performed using JMP 8.0.2 (SAS Institute, Cary, NC).
Results
Patients and Clinical Presentation
Sixty-three patients carrying both a diagnosis of lymphoma and CD were identified. Thirty-six (57%) were men, and 27 (43%) were women. The median age at the time of diagnosis of lymphoma was 62 years (range, 11–91 y). The lymphoma diagnosis was confirmed from 1975 to 1985 in 16 (25%), from 1986 to 1996 in 22 (35%), and from 1997 to 2005 in 25 (40%) patients. Thirty-six (57%) patients had CD diagnosed before being diagnosed with lymphoma. Four (6%) patients were diagnosed with CD and lymphoma simultaneously. The remaining 23 patients (37%) were diagnosed with CD after being diagnosed with lymphoma. The median time from diagnosis of CD to the diagnosis of lymphoma in those patients in whom CD preceded the lymphoma was 8.6 years (range, 0.1–45 y). All 8 patients with ETL either had a pre-existing diagnosis of CD or were diagnosed with CD at the time of the lymphoma diagnosis (median time from CD diagnosis to ETL diagnosis, 3 y; range, 0–22 y). In the 23 patients in whom the lymphoma preceded the CD, the median time from the diagnosis of lymphoma to the diagnosis of CD was 2.7 years (range, 0.1–32.9 y). The diagnosis of CD was confirmed to be biopsy-proven in 53 (84%) patients. Subtotal or total villous atrophy were present in 49 (92%) patients, but partial villous atrophy was present in another 4 patients. An increased number of intraepithelial lymphocytes were described in 23 of 24 patients for whom this information was available in the pathology report. Positive tissue transglutaminase antibody or endomysial antibodies were documented in 9 patients at the time of CD diagnosis. HLA genotyping was available in 8 patients (DQ2 heterozygous, n =6; DQ2/DQ8, n = 1; and DQ8, n = 1). Seven patients had biopsy-proven dermatitis herpetiformis, including 3 with small-bowel biopsy compatible with CD and 4 without small-bowel biopsy. Lymphoma was diagnosed either after the diagnosis of dermatitis herpetiformis in 5 patients or before the diagnosis of dermatitis herpetiformis in 2 patients. Three were B-cell lymphomas (2 nodal, axilla and retroperitoneal nodes; and 1 small intestine), 2 were T-cell lymphomas (located in the colon and the liver), and 2 were unclassified lymphomas (located in the stomach and soft tissue).
Twenty-three (38%) patients presented with gastrointestinal lymphoma, 21 (33%) had nodal disease, and 18 (29%) had nongastrointestinal extranodal disease. The gastrointestinal lymphomas were located in the small bowel in 15 (65%) patients. Other gastrointestinal locations were the large intestine (n = 4), the liver (n = 3), and the stomach (n = 1). The pathology of small-bowel lymphomas included ETL (n = 8), B-cell lymphomas (n = 4), and unclassified lymphomas (n = 3). Ten (16%) patients presented with gastrointestinal emergencies, including 2 of the 8 patients with ETL. One of these patients presented with severe abdominal pain and possibly bowel perforation, and the other had severe gastrointestinal hemorrhage. Sixteen (25%) patients had B symptoms at the time of diagnosis (Table 1).
Table 1.
Clinical Characteristics in 63 Patients With Both Lymphoma and CD
| Feature | Number of patients (%) |
|---|---|
| Sex | |
| Male | 36 (57) |
| CD diagnosed before lymphoma | 36 (57) |
| Primary site of tumor | |
| Gastrointestinal tract | 23 (37) |
| Lymph nodes | 21 (33) |
| Cervical | 11 |
| Axillary | 2 |
| Mediastinal | 1 |
| Mesenteric | 4 |
| Retroperitoneal | 1 |
| Inguinal | 2 |
| Extranodal | 18 (29) |
| Not specified | 1 (1) |
| Gastrointestinal presentation | 10 (16) |
| Perforation | 3 |
| Hemorrhage | 3 |
| Bowel obstruction and/or abdominal pain | 4 |
| B symptoms at diagnosis | 16 (25) |
| Method of CD diagnosis | |
| Small-bowel biopsy (with or without CD serology) and response to GFD |
53 (84) |
| Malabsorption and response to GFD | 2 |
| Dermatitis herpetiformis without small bowel biopsy | 4 |
| Diagnostic code of CD but data at diagnosis not available |
4 |
GFD, gluten-free diet.
Pathology
The lymphoma histology varied among patients. The most common histologic entity was diffuse, large, B-cell lymphoma affecting 18 (29%) patients. Twelve (19%) patients had T-cell lymphoma other than ETL, 8 (13%) patients had ETL, and other histologies were less common (Table 2).
Table 2.
Type of Lymphoma and Relation Among the Time of CD Diagnosis and Lymphoma Diagnosis in 63 Patients With Both CD and Lymphoma
| Type of lymphoma | Number of patients (%) | Time of CD diagnosis in relation to lymphoma diagnosis | ||
|---|---|---|---|---|
|
| ||||
| Before | Simultaneously | After | ||
| Diffuse large B-cell lymphoma | 18 (29) | 12 | 0 | 6 |
| T-cell lymphoma | 12 (19) | 9 | 0 | 3 |
| ETL | 8 (13) | 5 | 3 | 0 |
| Follicular lymphoma | 4 (6) | 2 | 0 | 2 |
| Hodgkin lymphoma | 4 (6) | 0 | 0 | 4 |
| MALT lymphoma | 1 (2) | 0 | 0 | 1 |
| Other and unclassified lymphomas | 16 (25) | 8 | 1 | 7 |
| Total | 63 (100) | 36 | 4 | 23 |
MALT, mucosal-associated lymphoid tissue.
Staging and Prognostic Indicators
Sufficient information for staging was available for 59 (94%) patients. Fifteen (24%) patients presented with stage I disease, 18 (31%) presented with stage II, 2 (3%) presented with stage III, and 24 (41%) presented with stage IV according to the Ann Arbor criteria. B symptoms (fever, weight loss, and night sweats) were seen in 16 (25%) patients. Histologic type did not seem to affect the stage at diagnosis (data not shown). Thirty-two (54%) patients presented with IPI 0 to 1, 16 (27%) presented with IPI of 2, and 11 (19%) presented with IPI of 3 or higher. Patients with T-cell lymphoma had higher IPI and higher Eastern Cooperative Oncology Group performance status39 at presentation but the difference was not statistically significant (P = .07 and P = .06, respectively) (Table 3).
Table 3.
Clinical Stage and IPI in 59 Patients With Both CD and Lymphoma
| Number (%) | |
|---|---|
| Ann Arbor stage | |
| I | 15 (24.1) |
| II | 18 (31.0) |
| III | 2 (3.4) |
| IV | 24 (41.4) |
| IPI | |
| 0 | 13 (20.7) |
| 1 | 19 (32.8) |
| 2 | 16 (27.6) |
| 3 | 8 (13.8) |
| 4 | 3 (5.2) |
| Age-adjusted IPI | |
| 0 | 25 (41.4) |
| 1 | 23 (39.7) |
| 2 | 10 (17.2) |
| 3 | 1 (1.7) |
B symptoms were seen more commonly in T-cell lymphomas than B-cell lymphomas (47% vs 20%; P = .04).
Treatment and Clinical Response
Thirty-nine (63%) patients received chemotherapy at the time of diagnosis and an additional 4 patients received chemotherapy at the time of progression. Eleven patients received radiation therapy alone at the time of diagnosis, and an additional 3 patients received chemotherapy in addition to the radiation therapy. In 6 patients no therapy was given or data on therapy were not available.
Of the patients who received chemotherapy or radiation therapy, sufficient information to accurately assess clinical response was available for 41 patients: a complete response was observed in 33 patients (80%), a partial response in 4 patients, and progression despite treatment was seen in 4 patients.
Diffuse Large B-Cell Lymphoma
Of the 18 patients with DLBCL, 9 received cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) and 2 received rituximab plus CHOP. Two patients were treated with radiation therapy alone at diagnosis and 2 patients received comfort care only. One patient received bleomycin, doxorubicin, cyclophosphamide, vincristine, dexamethasone, methotrexate, and leucovorin, and another patient received doxorubicin, cyclophosphamide, etoposide, vincristine, bleomycin, methotrexate, cytarabine, and prednisone. One patient was treated with chemotherapy of unknown type. Twelve of the DLBCL patients achieved a complete response with first-line therapy with a median relapse-free survival of 26 months. Six patients with DLBCL eventually relapsed.
Nonenteropathy-Type T-Cell Lymphoma
Eight of the 12 patients with non-ETL T-cell lymphoma received therapy. Three patients died before receiving therapy, and 1 of those patients was diagnosed at the time of the autopsy. Information on therapy was missing for 1 patient. Three patients received CHOP, and an additional 2 patients received doxorubicin, cyclophosphamide, etoposide, vincristine, bleomycin, methotrexate, cytarabine, and prednisone. One patient with cutaneous T-cell lymphoma was observed initially but received radiation therapy with good results on 2 occasions and was still alive at the last follow-up evaluation 28.6 years after diagnosis. One patient treated with CHOP relapsed after 5 months and died without receiving further therapy. Another patient was refractory to CHOP, and salvage therapy with ifosfamide, carboplatin, and etoposide was unsuccessful. The third T-cell non-Hodgkin lymphoma patient receiving CHOP achieved a complete response and remained in complete remission 28 months after completing therapy. Of the 2 patients receiving doxorubicin, cyclophosphamide, etoposide, vincristine, bleomycin, methotrexate, cytarabine, and prednisone, 1 relapsed 25 months later and was refractory to further therapy. The other patient had a complete response and remained disease free 15 years later.
Enteropathy-Type T-Cell Lymphoma
Seven of the 8 patients with ETL received chemotherapy at the time of diagnosis. One patient was lost to follow-up evaluation. Four patients received first-line therapy with CHOP, and 3 had a complete response. These 3 patients all relapsed at a median of 6 months from finishing the treatment (range, 4.5–7 mo). One ETL patient receiving chemotherapy with 2-chlorodeoxyadenosine (cladribine) achieved a partial response that was not durable and was refractory to further therapy. Another ETL patient received cyclophosphamide chemotherapy with no response but achieved a partial response to CHOP in the second-line setting. This was followed by high-dose chemotherapy with carmustine, etoposide, cytarabine, melphalan, and autologous stem cell transplantation, and the patient was in complete remission almost 5 years after transplantation. A third ETL patient received mitoxantrone and fludarabine followed by CHOP and later dexamethasone at the time of recurrence.
Follicular Lymphoma
Four patients were diagnosed with follicular lymphoma. Two patients received no therapy at the time of diagnosis and 1 patient received radiotherapy at the time of diagnosis. Information regarding therapy was missing for 1 patient.
Hodgkin Lymphoma
Three of the 4 patients with Hodgkin lymphoma received radiotherapy at the time of diagnosis and 1 received chemotherapy. All patients had a durable response to chemotherapy with a median survival of 30 years.
Mucosal-Associated Lymphoid Tissue Lymphoma
One patient had a mucosal-associated lymphoid tissue lymphoma of the thyroid that was treated successfully with radiotherapy.
Other and Unclassified Lymphomas
Sixteen patients either had a lymphoma that did not fit the earlier-described categories or was of unusual or mixed histology. Eleven (69%) of these patients received the diagnosis of lymphoma before 1985, 3 (19%) from 1986 to 1996, and only 2 (12%) after 1997. The primary site of these lymphomas was nodal (n = 6), small bowel (n = 3), liver (n = 3), stomach (n = 1), and nongastrointestinal extranodal (n = 3). This group included 1 lymphoplasmacytic lymphoma (Waldenström macroglobulinemia) and 1 marginal zone lymphoma. These patients received a variety of treatments including radiation monotherapy and chemotherapy. Ten patients achieved a complete response with therapy but, of those, 7 recurred later. Three patients were long-term survivors after therapy.
Survival
The 5-year and 10-year cumulative survivals for the entire cohort were 58% (95% confidence interval, 45%–71%) and 39% (95% confidence interval, 25%–53%), respectively. The shortest median survival was seen in patients with T-cell lymphomas other than ETL (10.9 mo). The median survival for ETL patients was 27.4 months (Figure 1). When patients with T-cell lymphoma were compared with patients with either B-cell or unclassifiable lymphomas, the survival for T-cell lymphoma was inferior (119.4 vs 22.8 mo; P = .02).
Figure 1.
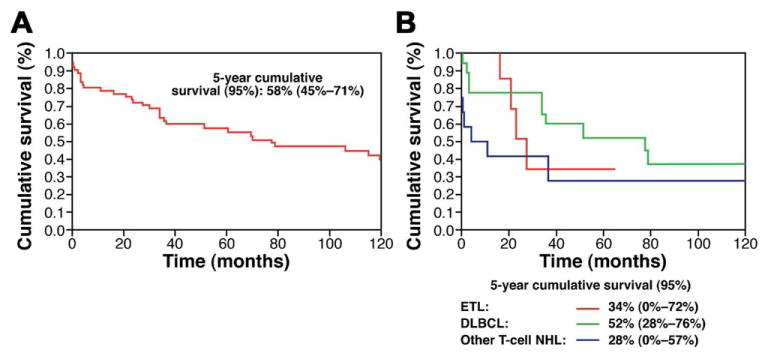
Cumulative survival by Kaplan–Meier analysis for the (A) entire cohort and according to (B) lymphoma subtype.
Discussion
We describe a single-center experience of 63 patients with lymphoma and CD. We included all patients who were seen at the Mayo Clinic carrying a diagnosis of both lymphoma and CD regardless of the temporal relationship between these 2 disorders. A slight majority of patients had a pre-existing diagnosis of CD at the time of the lymphoma diagnosis, though all of the cases of ETL either had an established diagnosis of CD before the diagnosis of lymphoma or coincident with the diagnosis of lymphoma, suggesting a close pathophysiologic association between these disorders. Thirty-seven percent of our patients had primary gastrointestinal lymphoma whereas the remainder had either predominantly nodal disease or extranodal disease outside of the gastrointestinal tract. Only a minority of the patients had ETL, the lymphoma most strongly associated with CD.11 The most frequently diagnosed lymphomas were diffuse large B-cell lymphoma and non-ETL T-cell lymphomas, a finding also reported by others.10 Although ETL has the strongest association with CD, it remains a rare type of lymphoma and other types of lymphoma are found more commonly in patients with CD.7,9,15,40 However, T-cell lymphomas are highly over-represented in this cohort of patients with lymphoma and CD as compared with the prevalence of T-cell lymphomas found in a large registry of lymphoid neoplasms diagnosed during 1992 to 2001 in 12 regions of the United States.41 Gastrointestinal emergencies were common among patients with digestive tract lymphoma although only 2 of 8 ETL patients had such presentation. Our analysis shows that celiac patients with T-cell lymphomas have an inferior prognosis when compared with celiac patients with B-cell lymphoma. Non-ETL T-cell lymphomas had a particularly poor prognosis, but the prognosis of all T-cell lymphomas combined was significantly inferior to B-cell lymphomas. The number of patients with ETL was small, precluding comparison of survival between ETL and other types of lymphomas. The observed survival of patients with ETL is slightly better than reported by others,16,42 but the low number of ETL patients in our study and the potential for selection bias makes all comparisons among studies unreliable. Long-term survivors with ETL were uncommon, and only 2 of the 8 ETL patients had a durable response to therapy. Therapy for ETL is expected to result in a 1-year survival rate ranging from 31% to 39%.16,42 More effective treatment options clearly are needed for this rare malignancy but, given the rarity of this lymphoma, clinical trials will be challenging to conduct. In recent years, high-dose chemotherapy followed by autologous stem cell transplantation has been used with variable results (often disappointing) for selected cases of ETL, but the number of patients treated is still very small.36,43 Recently, a group from England reported a 5-year progression-free survival rate of 52% in 26 patients with ETL after treatment with ifosfamide, etoposide, epirubicin, and methotrexate followed by autologous stem cell transplantation.44
We describe 7 patients with biopsy-proven dermatitis herpetiformis and either B-cell or T-cell lymphomas, a finding consistent with previous reports that have shown a significant increased risk of lymphoma in patients with dermatitis herpetiformis.13,45,46
The strengths of the study were the relatively complete follow-up data, large sample size, and the high number of patients with histologically confirmed CD. Our study had several potential limitations. It was retrospective in nature and may have suffered from significant biases, such as referral and selection bias. The study spanned a period from 1970 to 2005, and both the diagnostic accuracy of lymphomas and the treatment options have improved during this period. The diagnostic approach for CD at Mayo Clinic does not routinely include HLA genotyping. Serologic testing was not widely performed in Olmsted County until 1995.47 Thus, HLA-DQ status and follow-up serology are missing in most patients included in this series.
In conclusion, our study included patients with lymphomas of various types associated with CD. Although the prognosis of patients with T-cell lymphoma was poor, there were a few long-term survivors. Patients with B-cell lymphomas had a better prognosis than their T-cell counterparts. Therapy for ETL remains unsatisfactory.
Acknowledgments
Funding: This article was supported by the National Institutes of Health under the Ruth L. Kirschstein National Research Service Award/Training Grant in Gastrointestinal Allergy and Immunology (T32 AI-07047 to A.R.-T.) and National Institutes of Health grant DK-57892 (to J.A.M.).
Abbreviations used in this paper
- CD
celiac disease
- CHOP
cyclophosphamide, doxorubicin, vincristine, prednisone
- DLBCL
diffuse large B-cell lymphoma
- ETL
enteropathy-type T-cell lymphoma
- IPI
International Prognostic Index
Footnotes
Current addresses of T.R.H.: Department of Internal Medicine, Division of Hematology, Oncology and Blood and Marrow Transplantation, University of Iowa Hospitals and Clinics and Iowa City VA Medical Center, Iowa City, Iowa.
Conflicts of interest: The authors disclose no conflicts.
References
- 1.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–1743. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 2.Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286–292. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 3.Maki M, Mustalahti K, Kokkonen J, et al. Prevalence of celiac disease among children in Finland. N Engl J Med. 2003;348:2517–2524. doi: 10.1056/NEJMoa021687. [DOI] [PubMed] [Google Scholar]
- 4.Rubio-Tapia A, Kyle RA, Kaplan EL, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009;137:88–93. doi: 10.1053/j.gastro.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rostom A, Murray JA, Kagnoff MF. American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastroenterology. 2006;131:1981–2002. doi: 10.1053/j.gastro.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Halfdanarson TR, Litzow MR, Murray JA. Hematologic manifestations of celiac disease. Blood. 2007;109:412–421. doi: 10.1182/blood-2006-07-031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catassi C, Fabiani E, Corrao G, et al. Risk of non-Hodgkin lymphoma in celiac disease. JAMA. 2002;287:1413–1419. doi: 10.1001/jama.287.11.1413. [DOI] [PubMed] [Google Scholar]
- 8.Gao Y, Kristinsson SY, Goldin LR, et al. Increased risk for non-Hodgkin lymphoma in individuals with celiac disease and a potential familial association. Gastroenterology. 2009;136:91–98. doi: 10.1053/j.gastro.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mearin ML, Catassi C, Brousse N, et al. European multi-centre study on coeliac disease and non-Hodgkin lymphoma. Eur J Gastroenterol Hepatol. 2006;18:187–194. doi: 10.1097/00042737-200602000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Smedby KE, Akerman M, Hildebrand H, et al. Malignant lymphomas in coeliac disease: evidence of increased risks for lymphoma types other than enteropathy-type T cell lymphoma. Gut. 2005;54:54–59. doi: 10.1136/gut.2003.032094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catassi C, Bearzi I, Holmes GK. Association of celiac disease and intestinal lymphomas and other cancers. Gastroenterology. 2005;128:S79–S86. doi: 10.1053/j.gastro.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 12.Isaacson P, Wright DH. Intestinal lymphoma associated with malabsorption. Lancet. 1978;1:67–70. doi: 10.1016/s0140-6736(78)90004-1. [DOI] [PubMed] [Google Scholar]
- 13.Leonard JN, Tucker WF, Fry JS, et al. Increased incidence of malignancy in dermatitis herpetiformis. Br Med J (Clin Res Ed) 1983;286:16–18. doi: 10.1136/bmj.286.6358.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Toma A, Verbeek WH, Hadithi M, et al. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of single-centre experience. Gut. 2007;56:1373–1378. doi: 10.1136/gut.2006.114512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verbeek WH, Van De Water JM, Al-Toma A, et al. Incidence of enteropathy-associated T-cell lymphoma: a nation-wide study of a population-based registry in The Netherlands. Scand J Gastroenterol. 2008;43:1–7. doi: 10.1080/00365520802240222. [DOI] [PubMed] [Google Scholar]
- 16.Gale J, Simmonds PD, Mead GM, et al. Enteropathy-type intestinal T-cell lymphoma: clinical features and treatment of 31 patients in a single center. J Clin Oncol. 2000;18:795–803. doi: 10.1200/JCO.2000.18.4.795. [DOI] [PubMed] [Google Scholar]
- 17.Holmes GK, Prior P, Lane MR, et al. Malignancy in Coeliac disease--effect of a gluten free diet. Gut. 1989;30:333–338. doi: 10.1136/gut.30.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collin P, Reunala T, Pukkala E, et al. Coeliac disease-associated disorders and survival. Gut. 1994;35:1215–1218. doi: 10.1136/gut.35.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubio-Tapia A, Rahim MW, See JA, et al. Mucosal recovery and mortality in adults with celiac disease after treatment with a gluten-free diet. Am J Gastroenterol. 2010;105:1412–1420. doi: 10.1038/ajg.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaukinen K, Peraaho M, Lindfors K, et al. Persistent small bowel mucosal villous atrophy without symptoms in coeliac disease. Aliment Pharmacol Ther. 2007;25:1237–1245. doi: 10.1111/j.1365-2036.2007.03311.x. [DOI] [PubMed] [Google Scholar]
- 21.Mention JJ, Ben Ahmed M, Begue B, et al. Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology. 2003;125:730–745. doi: 10.1016/s0016-5085(03)01047-3. [DOI] [PubMed] [Google Scholar]
- 22.Cellier C, Patey N, Mauvieux L, et al. Abnormal intestinal intraepithelial lymphocytes in refractory sprue. Gastroenterology. 1998;114:471–481. doi: 10.1016/s0016-5085(98)70530-x. [DOI] [PubMed] [Google Scholar]
- 23.Cellier C, Delabesse E, Helmer C, et al. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma Coeliac Disease Study Group [French] Lancet. 2000;356:203–208. doi: 10.1016/s0140-6736(00)02481-8. [DOI] [PubMed] [Google Scholar]
- 24.Daum S, Hummel M, Weiss D, et al. Refractory sprue syndrome with clonal intraepithelial lymphocytes evolving into overt enteropathy-type intestinal T-cell lymphoma. Digestion. 2000;62:60–65. doi: 10.1159/000007779. [DOI] [PubMed] [Google Scholar]
- 25.Rubio-Tapia A, Kelly DG, Lahr BD, et al. Clinical staging and survival in refractory celiac disease: a single center experience. Gastroenterology. 2009;136:99–107. doi: 10.1053/j.gastro.2008.10.013. quiz 352–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malamut G, Afchain P, Verkarre V, et al. Presentation and longterm follow-up of refractory celiac disease: comparison of type I with type II. Gastroenterology. 2009;136:81–90. doi: 10.1053/j.gastro.2008.09.069. [DOI] [PubMed] [Google Scholar]
- 27.Daum S, Ipczynski R, Schumann M, et al. High rates of complications and substantial mortality in both types of refractory sprue. Eur J Gastroenterol Hepatol. 2009;21:66–70. doi: 10.1097/MEG.0b013e328307c20c. [DOI] [PubMed] [Google Scholar]
- 28.Rubio-Tapia A, Murray JA. Classification and management of refractory coeliac disease. Gut. 2010;59:547–557. doi: 10.1136/gut.2009.195131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cellier C, Cerf-Bensussan N. Treatment of clonal refractory celiac disease or cryptic intraepithelial lymphoma: a long road from bench to bedside. Clin Gastroenterol Hepatol. 2006;4:1320–1321. doi: 10.1016/j.cgh.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Deleeuw RJ, Zettl A, Klinker E, et al. Whole-genome analysis and HLA genotyping of enteropathy-type T-cell lymphoma reveals 2 distinct lymphoma subtypes. Gastroenterology. 2007;132:1902–1911. doi: 10.1053/j.gastro.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 31.Verkarre V, Romana SP, Cellier C, et al. Recurrent partial trisomy 1q22-q44 in clonal intraepithelial lymphocytes in refractory celiac sprue. Gastroenterology. 2003;125:40–46. doi: 10.1016/s0016-5085(03)00692-9. [DOI] [PubMed] [Google Scholar]
- 32.Zettl A, Ott G, Makulik A, et al. Chromosomal gains at 9q characterize enteropathy-type T-cell lymphoma. Am J Pathol. 2002;161:1635–1645. doi: 10.1016/S0002-9440(10)64441-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Toma A, Goerres MS, Meijer JW, et al. Human leukocyte antigen-DQ2 homozygosity and the development of refractory celiac disease and enteropathy-associated T-cell lymphoma. Clin Gastroenterol Hepatol. 2006;4:315–319. doi: 10.1016/j.cgh.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Wolters VM, Verbeek WH, Zhernakova A, et al. The MYO9B gene is a strong risk factor for developing refractory celiac disease. Clin Gastroenterol Hepatol. 2007;5:1399–1405. e1–2. doi: 10.1016/j.cgh.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 35.Lennard A. Combination chemotherapy followed by autologous stem cell transplant for enteropathy-associated T-cell lymphoma. Br J Haematol. 2007;137:170. doi: 10.1111/j.1365-2141.2007.06532.x. author reply 171. [DOI] [PubMed] [Google Scholar]
- 36.Al-Toma A, Verbeek WH, Visser OJ, et al. Disappointing outcome of autologous stem cell transplantation for enteropathy-associated T-cell lymphoma. Dig Liver Dis. 2007;39:634–641. doi: 10.1016/j.dld.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 37.A predictive model for aggressive non-Hodgkin's lymphoma.The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 38.Armitage JO. Staging non-Hodgkin lymphoma. CA Cancer J Clin. 2005;55:368–376. doi: 10.3322/canjclin.55.6.368. [DOI] [PubMed] [Google Scholar]
- 39.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 40.Smedby KE, Hjalgrim H, Askling J, et al. Autoimmune and chronic inflammatory disorders and risk of non-Hodgkin lymphoma by subtype. J Natl Cancer Inst. 2006;98:51–60. doi: 10.1093/jnci/djj004. [DOI] [PubMed] [Google Scholar]
- 41.Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Egan LJ, Walsh SV, Stevens FM, et al. Celiac-associated lymphoma. A single institution experience of 30 cases in the combination chemotherapy era. J Clin Gastroenterol. 1995;21:123–129. [PubMed] [Google Scholar]
- 43.Bishton MJ, Haynes AP. Combination chemotherapy followed by autologous stem cell transplant for enteropathy-associated T cell lymphoma. Br J Haematol. 2007;136:111–113. doi: 10.1111/j.1365-2141.2006.06371.x. [DOI] [PubMed] [Google Scholar]
- 44.Sieniawski M, Angamuthu N, Boyd K, et al. Evaluation of enteropathy associated T-cell lymphoma comparing standard therapies with a novel regimen including autologous stem cell transplant. Blood. 2010;115:3664–3670. doi: 10.1182/blood-2009-07-231324. [DOI] [PubMed] [Google Scholar]
- 45.Collin P, Pukkala E, Reunala T. Malignancy and survival in dermatitis herpetiformis: a comparison with coeliac disease. Gut. 1996;38:528–530. doi: 10.1136/gut.38.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sigurgeirsson B, Agnarsson BA, Lindelof B. Risk of lymphoma in patients with dermatitis herpetiformis. BMJ. 1994;308:13–15. doi: 10.1136/bmj.308.6920.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murray JA, Van Dyke C, Plevak MF, et al. Trends in the identification and clinical features of celiac disease in a North American community, 1950–2001. Clin Gastroenterol Hepatol. 2003;1:19–27. doi: 10.1053/jcgh.2003.50004. [DOI] [PubMed] [Google Scholar]


