Abstract
Our objective in this study was to develop and implement an effective intervention strategy to manipulate the amount and composition of dietary fat and carbohydrate (CHO) in free-living individuals in the RISCK study. The study was a randomized, controlled dietary intervention study that was conducted in 720 participants identified as higher risk for or with metabolic syndrome. All followed a 4-wk run-in reference diet [high saturated fatty acids (SF)/high glycemic index (GI)]. Volunteers were randomized to continue this diet for a further 24 wk or to 1 of 4 isoenergetic prescriptions [high monounsaturated fatty acids (MUFA)/high GI; high MUFA/low GI; low fat (LF)/high GI; and LF/low GI]. We developed a food exchange model to implement each diet. Dietary records and plasma phospholipid fatty acids were used to assess the effectiveness of the intervention strategy. Reported fat intake from the LF diets was significantly reduced to 28% of energy (%E) compared with 38%E from the HM and LF diets. SF intake was successfully decreased in the HM and LF diets to ≤10%E compared with 17%E in the reference diet (P = 0.001). Dietary MUFA in the HM diets was ∼17%E, significantly higher than in the reference (12%E) and LF diets (10%E) (P = 0.001). Changes in plasma phospholipid fatty acids provided further evidence for the successful manipulation of fat intake. The GI of the HGI and LGI arms differed by ∼9 points (P = 0.001). The food exchange model provided an effective dietary strategy for the design and implementation across multiple sites of 5 experimental diets with specific targets for the proportion of fat and CHO.
Introduction
Metabolic syndrome affects as many as 25% of the U.K. population (1) and confers substantial risk of cardiovascular disease. Evidence exists that the amount and quality of dietary fat can modify features of metabolic syndrome, including blood lipids (2), insulin resistance (3), hypertension, and endothelial function (4–7). A key scientific and public health question is whether reducing intakes of saturated fats (SF)10 via low-fat, high-carbohydrate (CHO) diets, or by moderate-fat diets in which SF are substituted with monounsaturated fatty acids (MUFA), have differential effects on risk factors for metabolic syndrome.
Controlled intervention trials in this area are difficult to perform, not least because of the challenge in designing a dietary manipulation strategy to simultaneously address the quality and type of fat and CHO. Few long-term studies have achieved good compliance with such extensive dietary changes in free-living individuals. This article describes the food exchange model, which was developed and used successfully to implement the dietary manipulation required for the RISCK study. It details the steps taken including an assessment of typical U.K. dietary intakes, consideration of the availability and ease of substitution of study foods with a desirable nutrient composition into an individual's habitual diets, and the development of dietary guidelines that were sustainable over the duration of the study, while allowing some flexibility for an individual's food preferences and lifestyle. The effectiveness of the dietary strategy is also assessed through analysis of dietary records and measurement of plasma phospholipid fatty acid status.
Methods
Study participants and design
The RISCK study was a randomized, controlled, parallel trial performed in free-living participants at 5 U.K. centers (University of Reading, Imperial College London, Kings College London, University of Surrey, and the Medical Research Council Human Nutrition Research [MRC HNR]). The study was approved by the South East Multi-Center Research Ethics Committee (ref: MREC04/MRE01/2). Written informed consent was obtained from all participants. The study design has been described elsewhere (8). In brief, a total of 720 participants selected on the basis of their increased risk or presence of metabolic syndrome were recruited. All participants followed a 4-wk run-in period during which they were prescribed a high-SF, high-glycemic index (GI) (HS/HGI) reference diet. A minimization procedure (controlling for age, gender, waist, and HDL cholesterol) was then used to assign participants to the reference diet for a further 24 wk or to 1 of 4 isoenergetic dietary prescriptions [high MUFA/high GI (HM/HGI), high MUFA/low GI (HM/LGI), low fat (LF)/high GI (LF/HGI), and LF/LGI]. The target intake for total fat was 38% of energy (%E) in the high-SF and both high-MUFA diets and 28%E in the 2 LF diets, which also had a higher target CHO intake (55 vs. 45%E). The 4 intervention diets were designed to reduce SF intake to 10%E, where protein and alcohol were unaltered and the remaining energy was derived from CHO. Participants attended the study centers for 2 half-days of measures after the run-in period and after the 24-wk intervention. The main outcome was a measure of insulin sensitivity with secondary outcomes, including a range of cardiovascular risk markers, the results of which will be reported elsewhere.
Overall dietary intervention strategy
A fundamental tenet of the study was to prescribe appropriate isoenergetic substitutions for the individual dietary regimens while allowing participants to eat ad libitum. To facilitate the required dietary changes and maximize compliance, specific study foods that were components of household meals or recipes were collected by each participant fortnightly from respective study centers in sufficient quantities for the whole household.
Strategy for achieving target fat intakes
A RISCK food exchange model was designed based on data from the National Diet and Nutrition Survey (NDNS) (9) and had a similar format to a strategy used previously (10). The survey showed dietary fat accounted for ∼34%E in the mean U.K. diet including alcohol consumption. Through the removal of the major exchangeable dietary sources of fat, it was calculated that ∼42% of total dietary fat was accessible (Table 1). The model requires exchangeable fat to be replaced by study foods with a specific fatty acid profile (Tables 2–4). Specially formulated fat spreads, cooking and baking fats, and mayonnaises were provided by Unilever Food and Health Research Institute (Unilever R&D) (Table 5). The products were provided to participants in plain packaging identifiable by a code and were accompanied by specifically chosen commercially available snack foods to replace those normally consumed. The remaining fat replacement was achieved through an appropriate exchange of full-fat or low-fat dairy products.
TABLE 1.
The RISCK food exchange model: removal of the major exchangeable sources of dietary fat, based on NDNS data
| Energy |
Fat |
SF |
MUFA |
PUFA |
CHO |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | |
| NDNS, %E from | 33.7 | 33.9 | 12.6 | 12.8 | 11.3 | 11.0 | 5.9 | 6.1 | 44.6 | 46.6 | ||
| NDNS intake | kJ/d | g/d | ||||||||||
| Added fats- oil | 376 | 263 | 10.0 | 7.0 | 0.9 | 0.7 | 3.9 | 2.7 | 4.7 | 3.3 | 0 | 0 |
| Added fats-spreads | 387 | 261 | 10.4 | 6.8 | 3.9 | 2.6 | 3.5 | 2.2 | 2.1 | 1.2 | 0 | 0 |
| All milk | 485 | 410 | 4.3 | 3.7 | 2.6 | 2.1 | 1.2 | 0.8 | 0.3 | 0.3 | 11 | 8.1 |
| Cheese | 290 | 205 | 5.2 | 3.7 | 3.3 | 2.3 | 1.2 | 0.8 | 0.1 | 0.03 | 0 | 0 |
| Biscuits, cakes, buns, pastries | 582 | 477 | 6.1 | 4.3 | 2.6 | 1.9 | 2.0 | 1.4 | 0.7 | 0.5 | 19.3 | 14.2 |
| Total of foods | 2120 | 1616 | 36.0 | 25.5 | 13.3 | 9.6 | 11.8 | 7.9 | 7.9 | 5.3 | 30.3 | 22.3 |
| Intake less fats, milk, and cheese | 7558 | 5212 | 50.5 | 35.9 | 19.2 | 13.7 | 17.3 | 12.3 | 7.3 | 5.8 | 244.7 | 180.7 |
TABLE 2.
Replacement of exchangeable sources of dietary fat with study foods: diet HS/HGI
| Amount |
Energy |
Fat |
SF |
MUFA |
PUFA |
CHO |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet HS/HGI | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female |
| g/d | kJ/d | g/d | ||||||||||||
| Study spread, high SF | 20 | 15 | 590 | 444 | 16.0 | 12.0 | 7.7 | 5.8 | 4.5 | 3.2 | 4.0 | 3.0 | 0 | 0 |
| Study cooking fat | 11 | 6 | 405 | 221 | 11.0 | 6.0 | 5.2 | 2.9 | 3.7 | 2.0 | 2.0 | 1.1 | 0 | 0 |
| Cheese (full fat) | 17 | 12 | 293 | 207 | 5.8 | 4.1 | 3.7 | 2.6 | 1.6 | 1.1 | 0.2 | 0.2 | 0.02 | 0.01 |
| Milk (whole) | 230 | 190 | 635 | 525 | 9.0 | 7.4 | 5.5 | 4.6 | 2.5 | 2.1 | 0.2 | 0.2 | 11.0 | 9.1 |
| CHO portion | −45 | −30 | −442 | −295 | −0.9 | −0.6 | −0.2 | −0.1 | −0.2 | −0.1 | −0.2 | −0.2 | −22.2 | −14.8 |
| Study snack (mean of 2) | 20 | 20 | 433 | 433 | 5.7 | 5.7 | 3.6 | 3.6 | 1.6 | 1.6 | 0.2 | 0.2 | 12 | 12 |
| Intake less fats, milk, and cheese | 7558 | 5212 | 50.5 | 35.9 | 19.2 | 13.7 | 17.3 | 12.3 | 7.3 | 5.8 | 244.7 | 180.7 | ||
| Total | 9472 | 6747 | 97.1 | 70.5 | 44.7 | 33.1 | 31.0 | 22.2 | 13.7 | 10.3 | 245.5 | 187.0 | ||
| %E | 38.6 | 39.3 | 17.8 | 18.5 | 12.3 | 12.4 | 5.4 | 5.7 | 40.7 | 43.5 | ||||
TABLE 3.
Replacement of exchangeable sources of dietary fat with study foods: diets HM/HGI and HM/LGI
| Amount |
Energy |
Fat |
SF |
MUFA |
PUFA |
CHO |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet HM/HGI and HM/LGI | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female |
| g/d | kJ/d | g/d | ||||||||||||
| Study spread, high MUFA | 20 | 15 | 592 | 444 | 16.0 | 12.0 | 3.0 | 2.3 | 9.5 | 7.2 | 3.4 | 2.5 | 0 | 0 |
| Study oil, high MUFA | 11 | 6 | 407 | 222 | 11.0 | 6.0 | 1.7 | 0.9 | 8.4 | 4.6 | 1.0 | 0.5 | 0 | 0 |
| Cheese (half-fat) | 17 | 12 | 186 | 131 | 2.6 | 1.8 | 1.6 | 1.1 | 0.7 | 0.5 | 0.1 | 0.1 | 0.02 | 0.01 |
| Milk (skimmed) | 230 | 190 | 318 | 262 | 0.2 | 0.2 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 11.5 | 9.5 |
| CHO portion | −45 | −30 | −442 | −295 | −0.9 | −0.6 | −0.2 | −0.1 | −0.2 | −0.1 | −0.2 | −0.2 | −22.2 | −14.8 |
| Study snack (mean of 2) | 27 | 27 | 492 | 494 | 4.7 | 4.7 | 0.6 | 0.6 | 3.4 | 3.4 | 0.7 | 0.7 | 16.9 | 16.9 |
| Study mayonnaise | 20 | 12 | 592 | 355 | 16.0 | 9.6 | 2.3 | 1.4 | 12.5 | 7.5 | 1.2 | 0.7 | 0 | 0 |
| Intake less fats, milk, and cheese | 7558 | 5212 | 50.5 | 35.9 | 19.2 | 13.7 | 17.3 | 12.3 | 7.3 | 5.8 | 244.7 | 180.7 | ||
| Total | 9703 | 6825 | 100.1 | 69.6 | 28.3 | 20.0 | 51.6 | 35.4 | 13.5 | 10.1 | 250.9 | 192.3 | ||
| %E | 38.8 | 38.4 | 11.0 | 11.0 | 20.0 | 19.5 | 5.2 | 5.6 | 40.6 | 44.2 | ||||
TABLE 4.
Replacement of exchangeable sources of dietary fat with study foods: diets LF/HGI and LF/LGI
| Amount |
Energy |
Fat |
SF |
MUFA |
PUFA |
CHO |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet LF/HGI and LF/LGI | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female |
| g/d | kJ/d | g/d | ||||||||||||
| Study spread, low fat | 20 | 15 | 355 | 266 | 5.5 | 4.1 | 1.2 | 0.9 | 2.8 | 2.1 | 1.5 | 1.1 | 0.0 | 0.0 |
| Study oil | 4.5 | 4.5 | 166 | 166 | 4.5 | 4.5 | 0.5 | 0.5 | 2.5 | 2.5 | 1.5 | 1.5 | 0.0 | 0.0 |
| Cheese (half-fat) | 17 | 12 | 186 | 131 | 2.6 | 1.8 | 1.6 | 1.1 | 0.7 | 0.5 | 0.1 | 0.1 | 0.02 | 0.01 |
| Milk (skimmed) | 230 | 190 | 318 | 262 | 0.2 | 0.2 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 11.5 | 9.5 |
| Study snack (mean of 3) | 28 | 28 | 460 | 460 | 1.9 | 1.9 | 0.6 | 0.6 | 1.1 | 1.1 | 0.5 | 0.5 | 21.8 | 21.8 |
| CHO portion, e.g. slice bread | 45 | 30 | 442 | 295 | 0.9 | 0.6 | 0.2 | 0.1 | 0.2 | 0.1 | 0.2 | 0.2 | 22.2 | 14.8 |
| Intake less fats, milk, and cheese | 7558 | 5212 | 50.5 | 35.9 | 19.2 | 13.7 | 17.3 | 12.3 | 7.3 | 5.8 | 244.7 | 180.7 | ||
| Total | 9484 | 6792 | 66.1 | 49.0 | 23.4 | 17.0 | 24.6 | 18.6 | 11.1 | 9.2 | 300.2 | 226.8 | ||
| %E | 26.2 | 27.2 | 9.3 | 9.4 | 9.8 | 10.3 | 4.4 | 5.1 | 49.7 | 52.4 | ||||
TABLE 5.
Composition of fat sources used for intervention diets1
| Fatty acid composition |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study spread |
Baking/cooking product |
Study oil |
Mayonnaise |
||||||
| HS | HM | LF | HS | HM | HM | LF | HM | LF | |
| g/100 g | |||||||||
| Total FA | 80.0 | 80.0 | 27.4 | 99.5 | 99.0 | 100.0 | 100.0 | 79.1 | 29.8 |
| Total SF | 38.7 | 15.1 | 6.0 | 47.6 | 19.9 | 15.2 | 11.4 | 6.2 | 2.7 |
| 16:0 | 12.3 | 7.8 | 2.73 | 15.5 | 9.5 | 11.2 | 7.4 | 4.0 | 1.7 |
| 18:0 | 12.1 | 4.0 | 1.7 | 15.8 | 5.4 | 2.9 | 2.8 | 1.4 | 0.6 |
| Total cis-MUFA | 22.5 | 47.7 | 14.1 | 33.6 | 55.1 | 75.9 | 55.0 | 50.0 | 18.7 |
| 18:1 | 20.93 | 46.6 | 13.7 | 32.7 | 53.6 | 74.6 | 53.9 | 48.3 | 18.1 |
| Total cis-PUFA | 19.8 | 17.0 | 7.3 | 18.2 | 23.8 | 8.78 | 33.2 | 22.9 | 8.3 |
| 18:2(n-6) | 17.0 | 14.5 | 6.2 | 15.3 | 20.0 | 8.20 | 28.6 | 16.3 | 5.9 |
| 18:3(n-3) | 2.8 | 2.5 | 1.1 | 2.9 | 3.8 | 0.6 | 4.6 | 7.0 | 2.5 |
| Total trans-FA | 0.2 | 0.3 | 0.1 | 0.3 | 0.3 | 0.1 | 0.4 | 0 | 0 |
16:0, palmitic acid; 18:0, stearic acid; 18:1, oleic acid; 18:3(n-3) α-linolenic acid.
Strategy for achieving target CHO intakes
To maintain the energy content of the prescribed diet, changes in the amount of CHO were also necessary. The reference diet (HS/HGI) and the HM diets required participants to consume an extra ∼4%E from fat compared with the U.K. mean intake; therefore, CHO of equivalent %E was removed daily (Tables 2 and 3). The target for the LF diets required a 6%E reduction in fat compared with the mean U.K. intake; therefore, the energy from the fat removed from the diet was replaced by adding at least 1 extra CHO portion (Table 4).
An exchange system was used to manipulate GI and participants were provided with CHO foods either high or low in GI to substitute into their habitual diet in a similar manner to previous studies (11). Breakfast cereals were provided by Cereal Partners UK, Grampian, Weetabix Ltd., and Sainsbury's Supermarkets Ltd. The impact of these dietary exchanges was modeled on intakes of major contributors to CHO intake derived from the latest NDNS survey in men 35–49 y old (9) and published GI values (12). These data suggest that it is possible to decrease GI by ∼13 points in the LF/LGI diet and by ∼11 points in the HM/LGI diet. The lower decrease in the high-MUFA diet reflects the limitations imposed by a reduced CHO intake overall.
Appropriate foods to include in the exchange system were identified using GI data derived principally from independent testing undertaken at MRC HNR using the methodology previously described (13). In general, foods included in the exchange model fell within the standard definition of high- and low-GI products with values either >70 or <55.
Implementation of the diets
After standardized training, study dietitians or researchers gave one-on-one dietary advice at the beginning of both the run-in and the intervention diet. At the initial dietary instruction, the investigator assessed the habitual diet of the participant through discussion and reference to a previously completed diet diary and used this to tailor individual advice. Participants were provided with a booklet to supplement the verbal information, which included clear tables of foods to be substituted, target intakes, and advice on food choices when eating out. Asian men and women, a population group predisposed to greater metabolic risk, were a specific target group for RISCK and therefore a separate booklet was developed based on an Asian diet. Participants were advised to keep to the targets set without changing other components of the diet.
Due to the length of the study, specific advice was given for eating during holidays and special occasions (such as Christmas) and no outcome measurements were scheduled immediately after such events.
Dietary fat advice
High-SF diet.
For all participants during the run-in and those randomized to the HS/HGI intervention, 2 solid fats were provided: a spread and the other to use in all cooking and baking. Because there was no liquid oil, a salad dressing recipe was suggested using crème fraiche. Participants were asked to replace all dairy products with full-fat options and to use the minimum weekly target for cheese. A choice of 2 snacks (chocolate wafer biscuit or all-butter shortbread) was provided and advice given to replace 1 habitually eaten snack each day.
High-MUFA diets.
For participants receiving the HM diets, oil for cooking and salad dressings, study spread, and baking fat were provided. Participants were also asked to replace all dairy products with low-fat varieties. A choice of 2 snacks were provided (muesli bar containing nuts or high-MUFA crisps) to replace 1 snack each day. In addition, participants were asked to eat 1 tablespoon (15 mL) of the provided mayonnaise or 2 tablespoons (30 mL) of hazelnuts per day to increase MUFA intakes. They were also given mixed nuts to snack on during the day. Advice for holidays or eating out included selection of olives, avocadoes, and nuts over pastry-based snacks and crisps, vegetable-based sauces over cheese- or cream-based sauces, and selection of olive oil-based dressings, sauces, or dips.
LF diets.
Participants following the LF diets were asked to replace all dairy products with low-fat varieties, given advice on low-fat cooking and baking, and provided with a choice of 3 low-fat snacks (muesli bar containing dried fruit, jaffa cakes, or iced gems) to replace habitually eaten snacks such as chocolate or crisps. Participants were asked to make low-fat choices while on holiday or eating out.
Dietary CHO advice
All participants during the run-in diet and those assigned to the high-GI intervention diets were asked to avoid eating pasta, which has a low GI, and were supplied with a choice of high-GI staple foods. These included breakfast cereals, standard sliced whole-meal and white bread, potatoes (especially old potatoes), and Sainsbury's easy-cook basmati rice. Participants following the low-GI diets were asked to avoid eating potatoes and were given a choice of low- or relatively low-GI staples, including all types of pasta, lower GI breakfast cereals, and bread including Tesco Jumbo Porridge Oats and Multigrain Batch Bread. Additional lower GI alternatives, sweet potatoes, egg noodles, and crisp breads were also recommended.
Assessment of dietary intake
On 4 occasions (screening, end of run-in, 12 wk, and 24 wk), participants completed an unweighed 4-d diet diary for 3 wk and 1 weekend day. Portion sizes were estimated using published tables (14).
MRC HNR's in-house database of the nutrient composition of foods consumed in the U.K. diet, DIDO (Diet In, Data Out), was used to calculate specific macronutrient and micronutrient intake. This was based on McCance and Widddowson's ‘The Composition of Foods’ (15) with additional data obtained from manufacturers. GI values for CHO-containing foods have been incorporated into a newly developed database at MRC HNR, DINO (Diet In, Nutrients Out) for the analysis of dietary GI.
Assessment of plasma phospholipid fatty acid status
Fasted plasma phospholipid fatty acids were measured at the University of Reading. Fatty acids were solvent extracted and isolated using a sep-pac C18 column (Waters Associates) (16). The lipids were then transmethylated and quantified by GC using a CPSil 88 column (Chrompak) using a method adapted from Indu and Ghafoorunissa (17). Mean inter-assay and intra-assay CV were 7.2 and 3.2%, respectively.
Statistical analysis
For dietary data, a repeated-measures ANOVA adjusted for gender and baseline value was used. Between-diet differences were assessed by Tukey's multiple range test. For phospholipid fatty acids, an ANCOVA model was used. Transformations were used to stabilize the variance of the residuals where necessary. Once outcome measures had been transformed, outliers were defined according to their end of run-in and end of intervention (transformed) measures, as well as the difference between their (transformed) measures. In each case, outliers were defined as points >2.5× interquartile range (IQR) away from the median. Center and ethnicity together with the end of run-in measure and the minimization variables [baseline waist, age, log(HDL), and gender] were included in the model. A global test of between-diet differences (4 degrees of freedom) provided the main P-value. Only if this P-value was <0.05 were further comparisons made.
Results
Of the 720 randomized participants, 24% dropped out [105 before and 67 during or after attending the visit (wk 0) after run-in]. Of those who withdrew prior to the visit following the run-in period, approximately one-third (32/105) described the run-in HS/HGI diet as the primary or contributing factor compared with approximately one-sixth (9/67) of participants who attended the visit following the run-in. A similar number of participants dropped out in each of the dietary treatments after completing the run-in (HS/HGI, 12; HM/HGI, 12; HM/LGI, 14; LF/HGI, 16; and LF/LGI, 13).
Dietary records of habitual food intake were completed by 517 participants and 481 participants completed both the run-in and 24-wk diet diary. To assess dietary data quality, estimated energy requirements were calculated using the Institute of Medicine equations (18) based on a low active population. Reported habitual energy intakes were −15.7 ± 19.8% (mean ± SD) lower than estimated energy requirements. This discrepancy was of a similar magnitude during the run-in period (−13.8 ± 19.8%) but greater at the end of the intervention period (−20.1 ± 19.1%).
Participants' reported habitual intakes (including alcohol) were representative of the U.K. population (9) (Table 6). During the intervention period, there were substantial and significant differences in nutrient intakes between the groups that were consistent with dietary intervention targets and were maintained throughout the 24-wk intervention period (Table 7). Total fat intake from the LF diets was successfully reduced to 28%E compared with 39%E in the reference diet (P = 0.0001) and there were compensatory significant increases in CHO in the LF groups (P = 0.0001). In the high-MUFA and LF diets, SF intake was reduced to ≤10%E compared with 17%E in the reference diet (P = 0.0001). Dietary MUFA in the HM diets was higher (∼17%E) than both the reference (12%E) and LF (10%E) diets (P = 0.0001). There was an inadvertent increase in (n-6) PUFA intake in the high MUFA diets; this was partially due to a higher PUFA content of the mayonnaise than originally modeled and also to the increase in consumption of nuts in these 2 groups. The intake of (n-3) PUFA was similar across all groups (data not shown). Reported energy intake from the LF groups was slightly lower than by the other groups (P = 0.001). The intervention strategy produced a significant 9-point GI difference between the high- and low-GI diet groups, but nonstarch polysaccharides (NSP) intake did not differ between the HM/HGI and HM/LGI or the LF/HGI and LF/LGI groups.
TABLE 6.
Reported habitual dietary intakes of RISCK participants1
| Nutrient | Males | Females |
|---|---|---|
| n | 216 | 301 |
| Energy,2MJ/d | 9.7 ± 2.5 | 7.6 ± 1.7 |
| Fat, %E | 34.7 ± 6.4 | 35.8 ± 5.9 |
| SF, %E | 12.8 ± 3.3 | 13.1 ± 3.6 |
| MUFA, %E | 11.6 ± 2.7 | 11.7 ± 2.4 |
| (n-6) PUFA, %E | 5.4 ± 2.0 | 5.7 ± 2.0 |
| (n-3) PUFA, %E | 0.7 ± 0.3 | 0.8 ± 0.4 |
| CHO, %E | 44.3 ± 7.4 | 45.2 ± 7.4 |
| GI | 62.0 ± 3.9 | 61.1 ± 4.2 |
| NSP, g/d | 18.0 ± 6.9 | 15.8 ± 5.1 |
| Protein, %E | 16.2 ± 2.9 | 16.4 ± 3.0 |
Values are means ± SD.
% total energy including alcohol (%E).
TABLE 7.
Energy, fat, SF, MUFA, PUFA, CHO, NSP daily intakes, and GI during run-in, mid-intervention, and after 24 wk of dietary intervention along with planned target intakes1
| HS/HGI | HM/HGI | HM/LGI | LF/HGI | LF/LGI | P-value3 | |
|---|---|---|---|---|---|---|
| n | 85 | 111 | 116 | 115 | 121 | |
| Energy, MJ/d | ||||||
| Run-in | 8.35 ± 1.95 | 8.58 ± 1.97 | 8.66 ± 2.41 | 8.51 ± 2.14 | 8.78 ± 2.05 | |
| Mid | 8.30 ± 2.28 a | 7.98 ± 1.94 a | 8.51 ± 2.25a | 7.67 ± 2.33b | 8.04 ± 2.05 a | 0.001 |
| Final | 8.19 ± 2.17 a | 7.99 ± 2.01 a | 8.41 ± 2.19a | 7.66 ± 2.33b | 7.46 ± 1.75b | |
| Fat,2 %E | ||||||
| Run-in | 37.8 ± 5.3 | 38.9 ± 6.0 | 36.7 ± 5.1 | 37.9 ± 4.4 | 38.0 ± 5.5 | |
| Mid | 39.2 ± 5.7a | 36.3 ± 6.7ab | 34.4 ± 5.4b | 27.8 ± 5.7c | 27.2 ± 5.6c | <0.0001 |
| Final | 37.7 ± 5.5a | 36.5 ± 6.9ab | 34.5 ± 5.7b | 27.4 ± 6.5c | 26.1 ± 6.2c | |
| Target | 38 | 38 | 38 | 28 | 28 | |
| SF, %E | ||||||
| Run-in | 16.7 ± 2.9 | 16.9 ± 3.4 | 16.0 ± 2.8 | 16.4 ± 2.7 | 16.5 ± 3.1 | |
| Mid | 17.0 ± 3.0a | 9.6 ± 2.5b | 9.2 ± 2.6bc | 9.0 ± 2.6bc | 8.3 ± 2.3c | <0.0001 |
| Final | 16.2 ± 2.9a | 9.9 ± 2.5b | 9.0 ± 2.5bc | 9.0 ± 2.7bc | 8.2 ± 2.5c | |
| Target | 18 | 10 | 10 | 10 | 10 | |
| MUFA, %E | ||||||
| Run-in | 11.6 ± 2.1 | 11.9 ± 2.3 | 11.1 ± 2.0 | 11.7 ± 1.8 | 11.6 ± 2.1 | |
| Mid | 11.9 ± 2.3a | 16.7 ± 4.3b | 15.6 ± 3.7b | 10.1 ± 3.0c | 10.2 ± 2.6c | <0.0001 |
| Final | 11.7 ± 2.5a | 16.4 ± 4.7b | 15.7 ± 3.8b | 9.9 ± 3.1c | 9.6 ± 2.9c | |
| Target | 12 | 20 | 20 | 11 | 11 | |
| PUFA, %E | ||||||
| Run-in | 5.6 ± 1.5 | 5.9 ± 1.7 | 5.6 ± 1.5 | 5.8 ± 1.4 | 5.9 ± 2.2 | |
| Mid | 6.1 ± 1.5a | 6.7 ± 1.9a | 6.3 ± 1.5a | 5.5 ± 1.9b | 5.4 ± 1.5b | <0.0001 |
| Final | 5.8 ± 1.7a | 6.8 ± 1.9b | 6.6 ± 1.9b | 5.3 ± 1.7a | 5.1 ± 1.8a | |
| Target | 6 | 6 | 6 | 6 | 6 | |
| CHO, %E | ||||||
| Run-in | 42.8 ± 6.6 | 42.5 ± 6.8 | 43.7 ± 7.0 | 42.6 ± 5.9 | 43.2 ± 6.1 | |
| Mid | 40.9 ± 7.7a | 44.6 ± 8.1b | 45.6 ± 6.4b | 50.3 ± 8.2c | 51.7 ± 6.6c | <0.0001 |
| Final | 41.5 ± 7.4a | 44.6 ± 7.1b | 45.5 ± 7.1b | 50.2 ± 8.2c | 51.8 ± 7.9c | |
| Target | 45 | 45 | 45 | 55 | 55 | |
| GI | ||||||
| Run-in | 64.1 ± 4.0 | 63.4 ± 3.9 | 63.3 ± 3.4 | 63.7 ± 3.6 | 63.2 ± 3.3 | |
| Mid | 64.1 ± 3.5a | 63.7 ± 3.8a | 55.1 ± 3.8b | 64.1 ± 3.5a | 56.1 ± 3.6b | <0.0001 |
| Final | 64.6 ± 3.7a | 63.2 ± 3.7a | 55.0 ± 3.7b | 64.8 ± 3.8a | 56.0 ± 3.5b | |
| Target | 64 | 64 | 53 | 64 | 51 | |
| NSP, g/d | ||||||
| Run-in | 16.2 ± 4.8 | 17.2 ± 6.1 | 17.5 ± 6.0 | 17.8 ± 5.6 | 18.6 ± 6.0 | |
| Mid | 15.4 ± 4.1a | 17.2 ± 5.4ab | 19.1 ± 5.7bc | 18.9 ± 7.6bc | 20.3 ± 6.1c | <0.0001 |
| Final | 15.5 ± 5.0a | 17.6 ± 5.9ab | 19.3 ± 6.2b | 18.4 ± 6.7b | 18.8 ± 6.0b | |
Values are means ± SD. Means in a row with superscripts without a common letter differ, P < 0.05.
Includes alcohol.
Repeated-measures ANOVA adjusted for gender and baseline value.
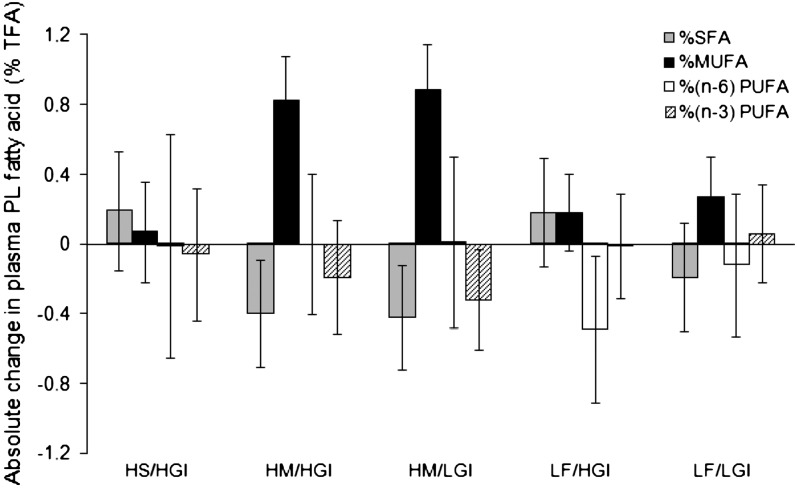
There were significant changes in the profile in the proportions of plasma phospholipid fatty acid classes over the 24 wk of intervention (Fig. 1). The changes in %SF and %MUFA differed between the diets (P ≤ 0.04). Changes for both HM diets were similar as were changes for the HS/HGI and both LF diets. After intervention, the combined HM groups had lower plasma phospholipid %SF than the combined LF groups (P ≤ 0.03) and higher %MUFA by 0.69% (P = 0.0001). The dietary interventions did not affect other fatty acid classes [(n-3) PUFA, (n-6) PUFA, and trans FA]. These data provide further evidence that dietary objectives for RISCK were met.
FIGURE 1 .
Changes in the profile of plasma phospholipid (PL) fatty acid classes [% total fatty acids (TFA)] from run-in to end of intervention (calculated on transformed scale but expressed as change from median value at run-in) (mean and 95% CI). The main P-values were: %SF, P = 0.039; %MUFA, P = 0.001; %(n-6) PUFA, P = 0.22; %(n- 3) PUFA, P = 0.62. The HM/HGI (n = 106) and HM/LGI (n = 103) groups combined had lower %SF than HS/HGI (n = 78) (P = 0.028) and LF/HGI (n = 102) and LF/LGI (n = 109) combined (P = 0.009) and higher %MUFA than HS/HGI (n = 82) (P = 0.0001) and LF/HGI (n = 110) and LF/LGI (n = 119) combined (P = 0.0001). Transformations used: %(n-6)PUFA cubed, %(n-3)PUFA log.
Discussion
To our knowledge, the RISCK study implemented changes to dietary fat and CHO on a scale not attempted in any other randomized controlled trial in free-living individuals outside of “supermarket” models (19).
The intervention strategy was successful in attaining 5 dietary regimens that were clearly characterized in terms of total fat and CHO intakes, the composition of fat (high vs. low SF and high vs. low MUFA) and the composition of CHO (high vs. low GI). Specific dietary targets during the run-in and within each dietary prescription during the intervention period were largely achieved. The increases in MUFA intake from the HM diets and the differential in dietary GI between the high and low groups were slightly smaller than modeled. There was a small reduction in energy intake in the LF groups. In each case, the changes achieved are likely to represent realistic intakes using a substitution strategy based on participants' existing dietary habits. Another study implementing dietary fat manipulation in a free-living pan-European population also reported achieving similar dietary fat targets as the current study using a food exchange model (20).
It proved difficult to increase MUFA intake to 20%E using available foodstuffs. Nuts were an important food but contributed to the unintentional increases in (n-6) PUFA. Study-specific testing of a range of CHO foods allowed selection of foods that demonstrated real differences in GI for the high and low interventions. The study sought to maximize the differences in GI through specific food substitutions rather than broader dietary changes, e.g. inclusion of pulses. While this strategy may have placed some limitations on the extent of the GI manipulation, it minimized differences between the dietary regimens in other compositional aspects of the diet. Importantly, the GI intervention did not lead to differences in fiber intake, which have confounded the interpretation of some other GI interventions (21,22). Population-wide increases in dietary MUFA and reduction in GI intake will require substantial reformulation of foodstuffs and new product innovation with MUFA-rich and low-GI options.
While few foods had to be avoided and a wide range of alternative foods were provided, some participants underestimated the impact of having to substitute foods across such a large proportion of their total diet and dropped out at an early stage citing difficulty with following the prescribed diet (SF/HGI) as a primary reason for withdrawing during the run-in period. This was a less common reason for dropping out of the study during the intervention period. Furthermore, a similar number of participants withdrew from each of the diet groups after the run-in, suggesting that each of the dietary prescriptions were equally acceptable.
The success of the RISCK strategy to implement such wide-ranging dietary changes was facilitated not only by the detailed modeling of each dietary prescription, but was also due to the practical aspects of delivering the intervention. The regular provision of specially formulated or selected foods ensured that participants had access to foods with the appropriate composition at no financial cost or added inconvenience. Regular visits to the study center helped to motivate the participants and find solutions to any problems in following the diets.
The staggering of participant recruitment meant that the intervention period of the study was conducted for over 25 mo. The logistical challenges (including delivery and storage arrangements) and costs of providing study foods were substantial. Vigilant recording of any changes in nutritional composition of the study foods added to the complexity of dietary coding.
This article describes a successful strategy for the design of 5 isoenergetic, experimental diets with specific targets for fat and CHO intakes and describes the process of implementation. It provides a comprehensive account of areas that need consideration in planning, executing, and evaluating the impact of dietary interventions.
Acknowledgments
We thank the following individuals from MRC Human Nutrition Research: Claire Lawrence, Edel Magee, and Kit Tsoi for research assistance; Darren Cole for database management; and Anna Gent, Celia Greenberg, and Caroline Stokes for coding and analysis of dietary data. We also thank Claire Howard, Namrata Dhopatkar, and Bushra Siddiqui from Imperial College, London and Samantha Bowen, L. Chen, and Robert Gray from Kings College, London for research assistance. In addition, we thank the following people from University of Reading: Katie Newens and Sean Lovegrove for research assistance and Ana Rodriguez-Mateos for sample analysis of plasma fatty acids.
Supported by funding from the United Kingdom Food Standards Agency (project no. NO2031).
Author disclosures: C. Moore, R. Gitau, L. Goff, F. J. Lewis, M. D. Griffin, M. D. Chatfield, S. A. Jebb, G. S. Frost, T. A. B. Sanders, B. A. Griffin, and J. A. Lovegrove, no conflicts of interest.
Abbreviations used: CHO, carbohydrate; %E, percent of energy; GI, glycemic index; HM/HGI, high-monounsaturated fat/high-glycemic index diet; HM/LGI, high-monounsaturated fat/low-glycemic index diet; HS/HGI, high-SF/high-glycemic index diet; LF, low fat; LF/HGI, low-fat/high-glycemic index diet; LF/LGI, low-fat/low-glycemic index diet; MRC HNR, Medical Research Council Human Nutrition Research; MUFA, monounsaturated fatty acid; NDNS, National Diet and Nutrition Survey; NSP, nonstarch polysaccharide; SF, saturated fat.
References
- 1.Tonkin R. The X Factor: obesity and the metabolic syndrome. Geneva: The Science and Public Affairs Forum; 2003.
- 2.Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. JAMA. 2002;288:2569–78. [DOI] [PubMed] [Google Scholar]
- 3.FAO/WHO. Diet, nutrition and the prevention of chronic disease. Geneva: WHO; 2003.
- 4.Miller GJ, Martin JC, Webster J, Wilkes H, Miller NE, Wilkinson WH, Meade TW. Association between dietary fat intake and plasma factor VII coagulant activity: a predictor of cardiovascular mortality. Atherosclerosis. 1986;60:269–77. [DOI] [PubMed] [Google Scholar]
- 5.Sanders TA, de Grassi T, Miller GJ, Humphries SE. Dietary oleic and palmitic acids and postprandial factor VII in middle-aged men heterozygous and homozygous for factor VII R353Q polymorphism. Am J Clin Nutr. 1999;69:220–5. [DOI] [PubMed] [Google Scholar]
- 6.Sanders TA, Oakley FR, Cooper JA, Miller GJ. Influence of a stearic acid-rich structured triacylglycerol on postprandial lipemia, factor VII concentrations, and fibrinolytic activity in healthy subjects. Am J Clin Nutr. 2001;73:715–21. [DOI] [PubMed] [Google Scholar]
- 7.Yaqoob P, Knapper JA, Webb DH, Williams CM, Newsholme EA, Calder PC. Effect of olive oil on immune function in middle-aged men. Am J Clin Nutr. 1998;67:129–35. [DOI] [PubMed] [Google Scholar]
- 8.Jebb S, Frost G, Griffin B, Lovegrove J, Moore C, Sanders T, Williams C. The RISCK study: testing the impact of the amount and type of dietary fat and carbohydrate on metabolic risk. Nutrition Bulletin. Oxford: Wiley-Blackwell, 2007.
- 9.Henderson L, Gregory J, Irving K. The National Diet and Nutrition Survey: adults age 19–64 years. London: TSO, 2003;2.
- 10.Williams CM, Francis-Knapper JA, Webb D, Brookes CA, Zampelas A, Tredger JA, Wright J, Meijer G, Calder PC, et al. Cholesterol reduction using manufactured foods high in monounsaturated fatty acids: a randomized crossover study. Br J Nutr. 1999;81:439–46. [PubMed] [Google Scholar]
- 11.Aston LM, Bluck L, Stokes CS, Jackson SJ, McKenna S, Jebb SA. Effect of a low glycaemic index diet on insulin sensitivity in overweight women. Int J Obes. 2007;31:S1.5. [Google Scholar]
- 12.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002;76:5–56. [DOI] [PubMed] [Google Scholar]
- 13.Aston LM, Gambell JM, Lee DM, Bryant SP, Jebb SA. Determination of the glycaemic index of various staple carbohydrate-rich foods in the UK diet. Eur J Clin Nutr. 2008;62:279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ministry of Agriculture. Food portion sizes. 2nd ed. London: HMSO; 1993.
- 15.McCance and Widdowson's The compositionof foods. 6th ed. London: The Royal Society of Chemistry, Cambridge and the Food Standards Agency; 2002.
- 16.Hamilton J, Comai K. Separation of neutral lipid, free fatty acid and phospholipid classes by normal phase HPLC. Lipids. 1988;23:1150–3. [DOI] [PubMed] [Google Scholar]
- 17.Indu M, Ghafoorunissa. n-3 fatty acids in Indian diets: comparison of the effects of precursor (alpha linolenic acid) vs product (long chain) n-3 polyunsaturated fatty acids. Nutr Res. 1992;12:569–82. [Google Scholar]
- 18.Institute of Medicine of the National Academies. Dietary reference intakes for energy, protein and amino acids. Washington, DC: National Academies Press; 2002.
- 19.Saris WH, Astrup A, Prentice AM, Zunft HJ, Formiguera X, Verboeket-van de Venne WP, Raben A, Poppitt SD, Seppelt B, et al. Randomized controlled trial of changes in dietary carbohydrate/fat ratio and simple vs complex carbohydrates on body weight and blood lipids: the CARMEN study. The Carbohydrate Ratio Management in European National diets. Int J Obes Relat Metab Disord. 2000;24:1310–8. [DOI] [PubMed] [Google Scholar]
- 20.Shaw DI, Tierney A, McCarthy S, Uprichard J, Vermunt S, Gulseth H, Drevon C, Blaak E, Saris W, et al. LIPGENE food exchange model for alteration of dietary fat quantity and quality, in free-living participants with the metabolic syndrome from eight European countries. Br J Nutr. 2009;101:750–9. [DOI] [PubMed] [Google Scholar]
- 21.Wolever TM, Mehling C. High-carbohydrate-low-glycaemic index dietary advice improves glucose disposition index in subjects with impaired glucose tolerance. Br J Nutr. 2002;87:477–87. [DOI] [PubMed] [Google Scholar]
- 22.Rizkalla SW, Taghrid L, Laromiguiere M, Huet D, Boillot J, Rigoir A, Elgrably F, Slama G. Improved plasma glucose control, whole-body glucose utilization, and lipid profile on a low-glycemic index diet in type 2 diabetic men: a randomized controlled trial. Diabetes Care. 2004;27:1866–72. [DOI] [PubMed] [Google Scholar]