Abstract
This essay is about the 1975 JEEM paper by Françoise Dieterlen-Lièvre (Dieterlen-Lièvre, 1975) and the studies that followed it, which indicated that the adult hematopoietic system in the avian embryo originates, not from the blood islands of the extraembryonic yolk sac as was then believed, but from the body of the embryo itself. Dieterlen-Lièvre’s 1975 paper created a paradigm shift in hematopoietic research, and provided a new and lasting focus on hematopoietic activity within the embryo body.
Introduction
Until the mid-1970s, developmental studies of the blood focused on the extraembryonic tissue, the yolk sac, as being the source of the adult hematopoietic system. In 1965, it was demonstrated, using twin and parabiosed (joined by their circulation) chick embryos, that circulating blood cells colonized the spleen and bone marrow during development (Moore and Owen, 1965). Later, in 1967, it was shown that transplanted yolk sac cells could populate the spleen and bone marrow of later-stage, irradiated, avian embryos (Moore and Owen, 1967). It is not difficult to imagine why the yolk sac was the focus of attention and thought to be the source of adult hematopoiesis. The fact that the yolk sac is the earliest tissue in the embryo that shows hematopoietic activity during development has been known to embryologists for a long time, and has, therefore, attracted particular scrutiny. As long ago as the early 1900s, Sabin described the morphological similarity that is evident between emerging blood cells and vascular cells in the chick yolk sac blood islands (Sabin, 1920). This gave rise to the concept of the hemangioblast (Murray, 1932), the common mesodermal precursor of the hematopoietic and endothelial lineages.
During the 1970s, the relevance of these findings to mammalian species was investigated in mouse models (reviewed by Dzierzak and Speck, 2008). Aided by in vitro and in vivo assays to identify hematopoietic progenitors and hematopoietic stem cells (HSCs), Moore and Metcalf performed a detailed investigation of the temporal and spatial appearance of hematopoietic cells in the mouse conceptus (Moore and Metcalf, 1970). For the first time, immature progenitors that form large hematopoietic colonies in the spleen (colony forming units-spleen, CFU-S), and cells that repopulate the hematopoietic system of adult irradiated recipients (HSCs), were detected in the yolk sac. Both cell types, CFU-S and HSCs, are associated with adult hematopoiesis. Additional experiments showed that the appearance of hematopoietic activity in explants of the body of the embryo depended on the presence of the yolk sac. Based on these, and other reports (Weissman et al., 1977), it was accepted that the mammalian adult hematopoietic system, as was suggested in the chick at that time, originated in the yolk sac, and that these cells migrate to and colonize the adult blood tissues. It was in this scientific context and atmosphere that the developmental studies by Dieterlen-Lièvre came into play.
The 1975 JEEM publication by Dieterlen-Lièvre on the intraembryonic origins of the avian adult hematopoietic system is a true classic (Dieterlen-Lièvre, 1975). The results of her chick-quail embryo grafting experiments initiated an important paradigm shift in the field. Her results demonstrated that, in the avian species, it is the embryo body, not the yolk sac, that is the true source of cells for the adult blood system. Yolk sac-derived cells exist only transiently and do not contribute to the adult blood system. Later findings in amphibian embryo grafting and cell-tracing experiments supported an independent, intraembryonic source of adult hematopoietic cells (Ciau-Uitz et al., 2000; Kau and Turpen, 1983; Maeno et al., 1985; Turpen et al., 1981). However, the intraembryonic origin of adult hematopoiesis continued to be a hotly debated topic. At the heart of the controversy was whether findings in non-mammalian vertebrate embryos could accurately reflect the developmental origins of the adult hematopoietic system in mammalian embryos.
It took almost 20 years before Dieterlen-Lièvre’s findings were fully appreciated for their widespread impact on studies concerning the developmental origins of adult hematopoiesis in a variety of metazoans, including mammals. Her elegant yolk sac chimera experiments and novel discoveries, as discussed in more detail below, inspired a new wave of investigation into the temporal and spatial origins of the adult hematopoietic system, particularly in the mammalian conceptus. On a personal note, her work had a profound impact on us and on others in the early 1990s, as we searched for and found regions of potent intraembryonic hematopoietic activity in mouse midgestation embryos (Godin et al., 1993; Medvinsky et al., 1993). Although an increasing amount of molecular data supported the idea that blood developmental mechanisms were conserved among various animal models, it was the reading of Dieterlen-Lièvre’s pioneering work in JEEM that inspired the careful re-examination of mouse hematopoietic development and that paved the way to developmental hematopoietic studies in other vertebrates, including in humans, frogs and zebrafish.
Yolk sac chimeras: the approach taken by Dieterlen-Lièvre
Unequivocal proof that the hematopoietic cells of the adult avian originate in the body of the embryo came from a new experimental approach in which a whole embryo body of one avian species was grafted onto the yolk sac of another species (Dieterlen-Lièvre, 1975; Martin, 1972).
In contrast to the intrauterine development of mammalian embryos, avian embryos are easily accessed and are amenable to manipulations in which cells, tissue rudiments or territories are exchanged between species to follow cell morphogenesis, migration and organogenesis during development (Le Douarin, 1973; LeDouarin and Jotereau, 1973). The flatness of the avian embryo as it lies on the surface of the spherical yolk sac facilitates its dissection, allowing it to be replaced with the embryo of another species in ovo. When such manipulations were carried out, it was noted that there was a rapid reconstitution and joining of blood vessels between the grafted embryo body and the host yolk sac, and that the free circulation of blood cells was established between the yolk sac and the body, without any disruptions to normal development in the resulting chimeras (Martin, 1972).
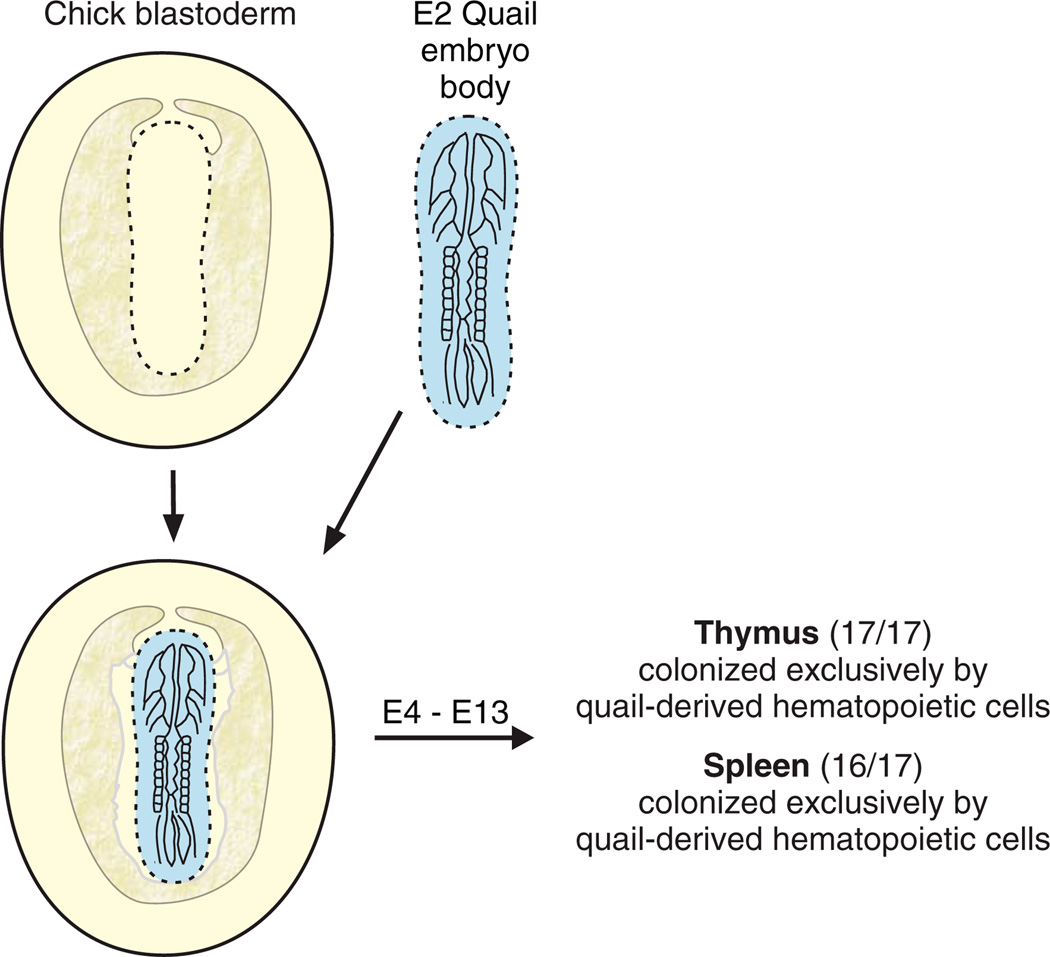
To examine the validity of the theory of the yolk sac origins of the adult blood lineage, Dieterlen-Lièvre established yolk sac chimeras by grafting a quail embryo body onto the extraembryonic area of a chick blastodisc (Fig. 1) before the establishment of a vascular connection between the embryo body and the area vasculosa (the area that contains the yolk sac blood islands). The prominent, identifiable nucleolus of quail cells (as compared to chick cells) was used as the biological marker. At the stage when hematopoietic cells begin to appear in the spleen and the thymus of chimeras, these tissues were examined for quail- and chick-specific nucleoli to investigate which embryonic territory the differentiated adult blood cells were derived from: the (quail) embryo body or the (chick) yolk sac. In the 17 yolk sac chimeras studied, the spleen and thymus contained exclusively quail cells, except in one case in which a few chick cells were observed amongst the quail cells (Dieterlen-Lièvre, 1975). Additional experiments verified that chick cells were capable of colonizing quail spleen rudiments in intracoelomic grafts. Thus, the interspecific avian chimeras proved that the body of the avian embryo generates the hematopoietic cells that seed the adult tissues.
Fig. 1. The avian yolk sac chimera experiment.
The experimental strategy for the generation and analysis of yolk sac chimeras. An embryonic day 2 (E2) quail embryo body (blue) is used to replace a chick embryo body on the chick blastoderm (presumptive yolk sac) before the circulation is established in the host or grafted embryo. After several days of in ovo development (E4–E13), the spleen and thymus tissues are examined for a natural marker that distinguishes quail cells from chick cells. Quail cells contain a characteristically large, irregular nucleus, with a large heterochromatic mass, whereas chick cells contain finely dispersed heterochromatin. The results show that the progeny of cells derived from the quail embryo body (and not the chick yolk sac) contribute to the hematopoietic cell population in these adult tissues.
This conclusion was later confirmed and advanced in studies that used homo-specific yolk sac chimeras generated from chick embryos of different sexes or from congenic chick strains that differed in histocompatibility markers (Beaupain et al., 1979; Lassila et al., 1978; Lassila et al., 1982; Martin et al., 1978). The combined results and conclusions of many such yolk sac chimera experiments are highlighted in Box 1.
Box 1. A summary of findings from avian yolk sac chimera experiments.
The first hematopoietic cells to emerge during avian development do so extraembryonically, from the yolk sac.
Slightly later in development, hematopoietic cells emerge from intraembryonic territories.
Some intraembryonically derived cells can be found in the yolk sac.
Intraembryonically derived hematopoietic cells are the permanent contributors to the adult hematopoietic system, whereas the extraembryonic yolk sac-derived hematopoietic cells become extinct by the hatching stage.
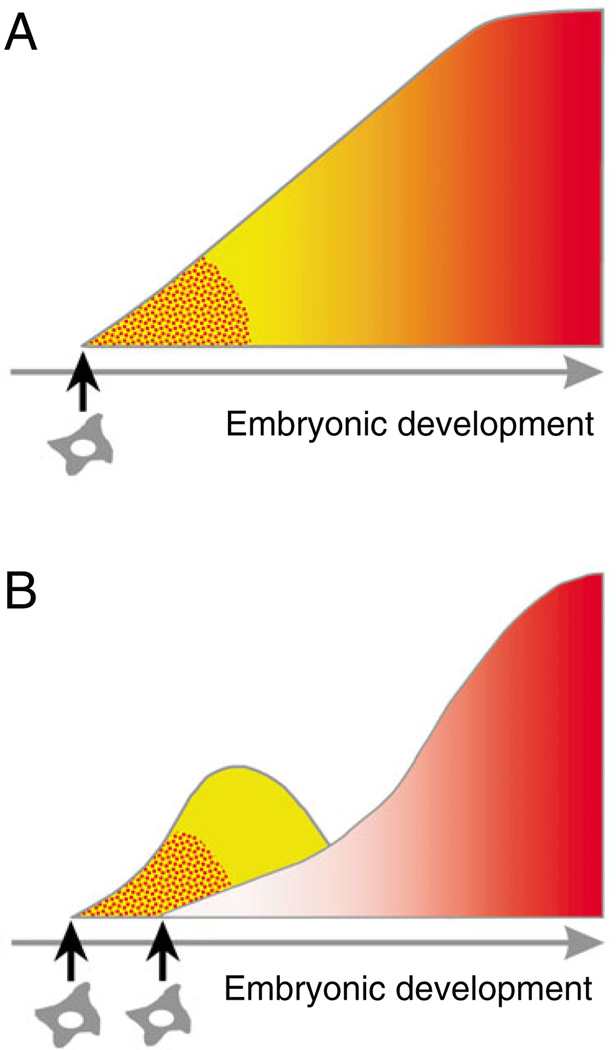
These results were in striking contrast to the generally held dogma of the yolk sac origins of hematopoiesis in mammals (Fig. 2A), and suggested at least two waves of hematopoietic cell generation (Fig. 2B): a first wave that consists of primitive transitory, short-lived blood cells from the yolk sac; and a second wave that originates from the embryo body and that generates the permanent, long-lived adult hematopoietic system.
Fig. 2. Principle scenarios for vertebrate hematopoietic development.
A schematic representation of hematopoietic system generation during embryonic development. (A) Prior to the avian yolk sac chimera experiments of Dieterlen-Lièvre, the hematopoietic system was thought to emerge once during development and to originate from a single cohort of mesenchymal/endothelial-type precursors or hemangioblasts (stellar-like cells). Primitive erythroid cells (red punctate areas) first appear and are followed by the appearance of myeloid or mixed erythroid-myeloid hematopoietic progenitors (yellow region transiting into the region of graded red). (B) In the JEEM publication, Dieterlen-Lièvre showed that two independent waves of hematopoietic generation exist during avian development: the transitory, embryonic hematopoietic cell hierarchy coming from the yolk sac (left curve containing red-punctate and yellow regions); and the adult definitive hematopoietic cell hierarchy coming from the body of the embryo (right curve containing graded red region). The hierarchies emerge independently from at least two distinct types of embryonic precursors (indicated by two stellar cells) and overlap in developmental time. This scenario became the prevailing model in the field of developmental hematopoiesis, and was followed by the finding in mammals that immature adult-type progenitors and HSCs develop with a significant delay in the conceptus (Medvinsky and Dzierzak, 1996; Medvinsky et al., 1993; Muller et al., 1994).
Other pivotal studies in avian developmental hematopoiesis
The findings of these avian chimera studies prompted Dieterlen-Lièvre to search for the source of hematopoietic cells in the body, leading her laboratory to focus on the diffuse hematopoietic foci in the dorsal aorta that had been described in vertebrate embryos decades earlier (Emmel, 1916; Jordan, 1916; Miller, 1913), but which had been neglected by the literature at that time. Her detailed anatomical descriptions of the microscopic hematopoietic cell clusters that are present on the ventral wall of the avian embryonic dorsal aorta (Dieterlen-Lièvre and Martin, 1981) were followed by studies in mouse and human embryos (Garcia-Porrero et al., 1995; Tavian et al., 1996) (reviewed by Jaffredo et al., 2005). This aortic region was found to contain multipotential hematopoietic progenitors at the time when hematopoietic cell clusters appear on its ventral aspect (Cormier and Dieterlen-Lièvre, 1988). The close association of hematopoietic and endothelial cells led to the speculation that either hemogenic endothelial cells exist on the ventral aspect of the aorta or that hemangioblasts are present in foci in the underlying para-aortic mesenchyme (Dieterlen-Lièvre and Martin, 1981).
To examine whether the hematopoietic cells in these clusters derive from endothelial cells, embryonic aortic endothelial cells were labelled with the lipophilic lipoprotein DiI-LDL, which was injected into the chick heart cavity during pre-hematopoietic embryonic stages (Jaffredo et al., 1998) to allow its uptake by the aortic endothelium. One day later, DiI-LDL-labelled intra-aortic hematopoietic clusters on the ventral aspect of the aorta were found, suggesting that they originated from the endothelium. The ability of endothelial cells to give rise to hematopoietic cells was also supported by culture experiments (Nishikawa et al., 1998).
The inclusion of the allantoic bud in the quail bodies that were grafted in the 1975 experiments (Dieterlen-Lièvre, 1975) raised the possibility that it might also give rise to hematopoietic cells. Indeed, later grafting experiments undertaken by Dieterlen-Lièvre identified another embryonic territory capable of hematopoietic cell generation, the allantois (Caprioli et al., 1998). The prevascularized quail allantoic bud was grafted into the coelom of a chick host. Quail-derived cells of both the hematopoietic and endothelial lineages were found in the bone marrow of the host, demonstrating that their precursors arose in the allantois. Hence, both the allantois and the embryo proper can contribute to the adult avian blood system.
From dogma to new sites of mammalian hematopoietic cell generation
Avian embryo in ovo grafting studies have greatly influenced developmental hematopoiesis research in other animal models. In the absence of the ability to grow mouse conceptuses ex utero for similar studies, short-term explant cultures of individual mouse embryonic tissues, together with in vitro and in vivo hematopoietic assays, have been used to investigate their hematopoietic potential. Early studies with mouse yolk sac and with whole embryo body explants (before the establishment of the circulation at E7–E8.5) have suggested that the embryo body hematopoietic activity is acquired through colonization from the yolk sac (Moore and Metcalf, 1970). However, the discovery of enriched hematopoietic activity within the avian embryonic dorsal aorta prompted a more precise spatial and temporal re-evaluation of this region in the mouse embryo.
In the early 1990s, several investigators probed for hematopoietic cell activity in the mouse embryonic region encompassing the dorsal aorta, which at E8 is known as the paraaortic splanchnopleura (PAS) and at E9 as the aorta-gonad-mesonephros region (AGM). Potent hematopoietic progenitors (Godin et al., 1993; Medvinsky et al., 1993) and HSCs capable of long-term multilineage reconstitution of adult irradiated recipients (Muller et al., 1994) were localized to this area. As shown in explant cultures, the PAS (at the pre-circulation stage) produces the first multipotent (lymphomyeloid) hematopoietic progenitors (Cumano et al., 1996). Importantly, at E10.5, the AGM region autonomously generates the first HSCs that are as potent as those found in the adult (Medvinsky and Dzierzak, 1996). The generation of these functionally complex hematopoietic progenitors and adult-type HSCs by the PAS/AGM precedes the appearance of such cells in the mouse yolk sac. HSCs localize to the aortic endothelium and to hematopoietic clusters (de Bruijn et al., 2002; North et al., 1999; North et al., 2002), and map to the ventral aspect of the dorsal aorta (Taoudi and Medvinsky, 2007). Similarly, the human PAS/AGM region autonomously generates potent hematopoietic progenitors prior to their appearance in the yolk sac (Tavian et al., 2001). Hematopoietic cell clusters are also found on the ventral aspect of the human aorta at this developmental time (Tavian et al., 1996).
Recently, the mouse allantois has, as in avian embryos, also been found to be a site of hematopoietic cell potential (Corbel et al., 2007; Zeigler et al., 2006). Hematopoietic progenitors and HSCs are found in the vitelline/umbilical arteries (de Bruijn et al., 2000) and in the chorio-allantois placenta (Alvarez-Silva et al., 2003; Gekas et al., 2005; Ottersbach and Dzierzak, 2005). Thus, hematopoiesis in the mouse conceptus closely parallels that of avian embryos, and it is likely that mouse AGM HSCs and their progeny provide (at least in part) the life-long adult hematopoietic system.
Unanswered questions
The controversy that surrounds whether adult mammalian hematopoiesis originates from the yolk sac persists to this day, despite a wealth of molecular, cellular and functional data from various animal models that indicate otherwise. Recent studies have shown that mouse embryos with defective circulation (Lux et al., 2008), or that are deficient for cell migration (Ghiaur et al., 2008), develop hematopoietic cells in the yolk sac but not in the embryo. Clearly, these experiments indicate that the yolk sac generates hematopoietic progenitors that may colonize the body of the embryo. However, the absence of hematopoietic progenitors in the body may not be indicative of the absence of HSCs. The midgestation lethality of these mutant embryos precluded direct HSC analysis, but one may hope that alternative approaches (Yoder et al., 1997) will shed light on whether the precursors of HSCs develop in the yolk sac.
In this context, Dieterlen-Lièvre’s avian embryo chimera experiments, which suggested that permanently functioning adult HSCs could be generated by the conceptus independently of a transient population of hematopoietic progenitors, have changed the landscape of the field of developmental hematopoiesis. Dieterlen-Lièvre’s avian yolk sac chimera data indicated that the body of the avian embryo is a ‘maternity ward’ for the generation of HSCs (Metcalf, 2008), making it very likely that the mouse AGM also plays this role. However, the mouse AGM might also have an additional function as a ‘finishing school’ for yolk sac hematopoietic progenitors, influencing them to become HSCs. Further insights into the role(s) of the mouse AGM (as well as the yolk sac and placenta) await the development of new experimental approaches and solutions.
In conclusion, avian embryos remain informative developmental models that have provided crucially important insights into mammalian development. The insights provided by Dieterlen-Lièvre’s avian yolk sac chimera experiments initiated a paradigm shift that continues to inform and to challenge research in the field of developmental hematopoiesis to this day.
Acknowledgments
We thank Thierry Jaffredo for his helpful comments on the text. Our work in this field is supported by the National Institutes of Health, Dutch BSIK Innovative and ZonMW VICI programs for E.D., and by the MRC and Leukaemia Research (UK) for A.M.
Contributor Information
Elaine Dzierzak, Email: e.dzierzak@erasmusmc.nl.
Alexander Medvinsky, Email: a.medvinsky@ed.ac.uk.
References
- Alvarez-Silva M, Belo-Diabangouaya P, Salaun J, Dieterlen-Lièvre F. Mouse placenta is a major hematopoietic organ. Development. 2003;130:5437–5444. doi: 10.1242/dev.00755. [DOI] [PubMed] [Google Scholar]
- Beaupain D, Martin C, Dieterlen-Lièvre F. Are developmental hemoglobin changes related to the origin of stem cells and site of erythropoiesis? Blood. 1979;53:212–225. [PubMed] [Google Scholar]
- Caprioli A, Jaffredo T, Gautier R, Dubourg C, Dieterlen-Lièvre F. Blood-borne seeding by hematopoietic and endothelial precursors from the allantois. Proc. Natl. Acad. Sci. USA. 1998;95:1641–1646. doi: 10.1073/pnas.95.4.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciau-Uitz A, Walmsley M, Patient R. Distinct origins of adult and embryonic blood in Xenopus. Cell. 2000;102:787–796. doi: 10.1016/s0092-8674(00)00067-2. [DOI] [PubMed] [Google Scholar]
- Corbel C, Salaun J, Belo-Diabangouaya P, Dieterlen-Lièvre F. Hematopoietic potential of the pre-fusion allantois. Dev. Biol. 2007;301:478–488. doi: 10.1016/j.ydbio.2006.08.069. [DOI] [PubMed] [Google Scholar]
- Cormier F, Dieterlen-Lièvre F. The wall of the chick embryo aorta harbours M-CFC, G-CFC, GM-CFC and BFU-E. Development. 1988;102:279–285. doi: 10.1242/dev.102.2.279. [DOI] [PubMed] [Google Scholar]
- Cumano A, Dieterlen-Lièvre F, Godin I. Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell. 1996;86:907–916. doi: 10.1016/s0092-8674(00)80166-x. [DOI] [PubMed] [Google Scholar]
- de Bruijn MF, Speck NA, Peeters MC, Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J. 2000;19:2465–2474. doi: 10.1093/emboj/19.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn MF, Ma X, Robin C, Ottersbach K, Sanchez MJ, Dzierzak E. Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity. 2002;16:673–683. doi: 10.1016/s1074-7613(02)00313-8. [DOI] [PubMed] [Google Scholar]
- Dieterlen-Lièvre F. On the origin of haemopoietic stem cells in the avian embryo: an experimental approach. J. Embryol. Exp. Morphol. 1975;33:607–619. [PubMed] [Google Scholar]
- Dieterlen-Lièvre F, Martin C. Diffuse intraembryonic hemopoiesis in normal and chimeric avian development. Dev. Biol. 1981;88:180–191. doi: 10.1016/0012-1606(81)90228-1. [DOI] [PubMed] [Google Scholar]
- Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat. Immunol. 2008;9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmel V. The cell clusters in the dorsal aorta of mammalian embryos. Am. J. Anat. 1916;19:401–412. [Google Scholar]
- Garcia-Porrero JA, Godin IE, Dieterlen-Lièvre F. Potential intraembryonic hemogenic sites at pre-liver stages in the mouse. Anat. Embryol. 1995;192:425–435. doi: 10.1007/BF00240375. [DOI] [PubMed] [Google Scholar]
- Gekas C, Dieterlen-Lièvre F, Orkin SH, Mikkola HK. The placenta is a niche for hematopoietic stem cells. Dev. Cell. 2005;8:365–375. doi: 10.1016/j.devcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Ghiaur G, Ferkowicz MJ, Milsom MD, Bailey J, Witte D, Cancelas JA, Yoder MC, Williams DA. Rac1 is essential for intraembryonic hematopoiesis and for the initial seeding of fetal liver with definitive hematopoietic progenitor cells. Blood. 2008;111:3313–3321. doi: 10.1182/blood-2007-08-110114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin IE, Garcia-Porrero JA, Coutinho A, Dieterlen-Lièvre F, Marcos MA. Para-aortic splanchnopleura from early mouse embryos contains B1a cell progenitors. Nature. 1993;364:67–70. doi: 10.1038/364067a0. [DOI] [PubMed] [Google Scholar]
- Jaffredo T, Gautier R, Eichmann A, Dieterlen-Lièvre F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development. 1998;125:4575–4583. doi: 10.1242/dev.125.22.4575. [DOI] [PubMed] [Google Scholar]
- Jaffredo T, Nottingham W, Liddiard K, Bollerot K, Pouget C, de Bruijn M. From hemangioblast to hematopoietic stem cell: an endothelial connection? Exp. Hematol. 2005;33:1029–1040. doi: 10.1016/j.exphem.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Jordan H. Evidence of hemogenic capacity of endothelium. Anat. Rec. 1916;10:417–420. [Google Scholar]
- Kau CL, Turpen JB. Dual contribution of embryonic ventral blood island and dorsal lateral plate mesoderm during ontogeny of hemopoietic cells in Xenopus laevis. J. Immunol. 1983;131:2262–2266. [PubMed] [Google Scholar]
- Lassila O, Eskola J, Toivanen P, Martin C, Dieterlen-Lièvre F. The origin of lymphoid stem cells studied in chick yolk sac-embryo chimaeras. Nature. 1978;272:353–354. doi: 10.1038/272353a0. [DOI] [PubMed] [Google Scholar]
- Lassila O, Martin C, Toivanen P, Dieterlen-Lièvre F. Erythropoiesis and lymphopoiesis in the chick yolk-sac-embryo chimeras: contribution of yolk sac and intraembryonic stem cells. Blood. 1982;59:377–381. [PubMed] [Google Scholar]
- Le Douarin N. A biological cell labeling technique and its use in experimental embryology. Dev. Biol. 1973;30:217–222. doi: 10.1016/0012-1606(73)90061-4. [DOI] [PubMed] [Google Scholar]
- LeDouarin NM, Jotereau FV. Origin and renewal of lymphocytes in avian embryo thymuses studied in interspecific combinations. Nat. New Biol. 1973;246:25–27. doi: 10.1038/newbio246025b0. [DOI] [PubMed] [Google Scholar]
- Lux CT, Yoshimoto M, McGrath K, Conway SJ, Palis J, Yoder MC. All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood. 2008;111:3435–3438. doi: 10.1182/blood-2007-08-107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno M, Tochinai S, Katagiri C. Differential participation of ventral and dorsolateral mesoderms in the hemopoiesis of Xenopus, as revealed in diploid-triploid or interspecific chimeras. Dev. Biol. 1985;110:503–508. doi: 10.1016/0012-1606(85)90108-3. [DOI] [PubMed] [Google Scholar]
- Martin C. Technique d’explantation in ovo de blastodermes d’embryons d’oiseaux. C R Seances Soc. Biol. Fil. 1972;116:283–285. [PubMed] [Google Scholar]
- Martin C, Beaupain D, Dieterlen-Lièvre F. Developmental relationships between vitelline and intra-embryonic haemopoiesis studied in avian ‘yolk sac chimaeras’. Cell Differ. 1978;7:115–130. doi: 10.1016/0045-6039(78)90012-x. [DOI] [PubMed] [Google Scholar]
- Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- Medvinsky AL, Samoylina NL, Muller AM, Dzierzak EA. An early pre-liver intraembryonic source of CFU-S in the developing mouse. Nature. 1993;364:64–67. doi: 10.1038/364064a0. [DOI] [PubMed] [Google Scholar]
- Metcalf D. AGM: maternity ward or finishing school? Blood. 2008;111:3305–3306. [Google Scholar]
- Miller AM. Histogenesis and morphogenesis of the thoracic duct in the chick: development of the blood cells and their passage to the blood stream via the thoracic duct. Am. J. Anat. 1913;15:131–198. [Google Scholar]
- Moore MA, Owen JJ. Chromosome marker studies on the development of the haemopoietic system in the chick embryo. Nature. 1965;208:956. doi: 10.1038/208956a0. [DOI] [PubMed] [Google Scholar]
- Moore MA, Owen JJ. Chromosome marker studies in the irradiated chick embryo. Nature. 1967;215:1081–1082. doi: 10.1038/2151081a0. [DOI] [PubMed] [Google Scholar]
- Moore MA, Metcalf D. Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br. J. Haematol. 1970;18:279–296. doi: 10.1111/j.1365-2141.1970.tb01443.x. [DOI] [PubMed] [Google Scholar]
- Muller AM, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1:291–301. doi: 10.1016/1074-7613(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Murray P. The development in vitro of the blood of the early chick embryo. Proc. R. Soc. London B Biol. Sci. 1932;11:497–521. [Google Scholar]
- Nishikawa S-I, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. Progressive lineage analysis by cell sorting and culture identifies FLK+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development. 1998;125:1747–1757. doi: 10.1242/dev.125.9.1747. [DOI] [PubMed] [Google Scholar]
- North T, Gu TL, Stacy T, Wang Q, Howard L, Binder M, Marin-Padilla M, Speck NA. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development. 1999;126:2563–2575. doi: 10.1242/dev.126.11.2563. [DOI] [PubMed] [Google Scholar]
- North TE, de Bruijn MF, Stacy T, Talebian L, Lind E, Robin C, Binder M, Dzierzak E, Speck NA. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16:661–672. doi: 10.1016/s1074-7613(02)00296-0. [DOI] [PubMed] [Google Scholar]
- Ottersbach K, Dzierzak E. The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev. Cell. 2005;8:377–387. doi: 10.1016/j.devcel.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Sabin F. Studies on the origin of blood vessels and of red blood corpuscles as seen in the living blastoderm of chicks during the second day of incubation. Contr. Embryol. 1920;9:215–262. [Google Scholar]
- Taoudi S, Medvinsky A. Functional identification of the hematopoietic stem cell niche in the ventral domain of the embryonic dorsal aorta. Proc. Natl. Acad. Sci. USA. 2007;104:9399–9403. doi: 10.1073/pnas.0700984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavian M, Coulombel L, Luton D, Clemente HS, Dieterlen-Lièvre F, Peault B. Aorta-associated CD34+ hematopoietic cells in the early human embryo. Blood. 1996;87:67–72. [PubMed] [Google Scholar]
- Tavian M, Robin C, Coulombel L, Peault B. The human embryo, but not its yolk sac, generates lympho-myeloid stem cells: mapping multipotent hematopoietic cell fate in intraembryonic mesoderm. Immunity. 2001;15:487–495. doi: 10.1016/s1074-7613(01)00193-5. [DOI] [PubMed] [Google Scholar]
- Turpen JB, Knudson CM, Hoefen PS. The early ontogeny of hematopoietic cells studied by grafting cytogenetically labeled tissue anlagen: localization of a prospective stem cell compartment. Dev. Biol. 1981;85:99–112. doi: 10.1016/0012-1606(81)90239-6. [DOI] [PubMed] [Google Scholar]
- Weissman IL, Baird S, Gardner RL, Papaioannou VE, Raschke W. Normal and neoplastic maturation of T-lineage lymphocytes. Cold Spring Harb. Symp. Quant. Biol. 1977;41:9–21. doi: 10.1101/sqb.1977.041.01.005. [DOI] [PubMed] [Google Scholar]
- Yoder MC, Hiatt K, Dutt P, Mukherjee P, Bodine DM, Orlic D. Characterization of definitive lymphohematopoietic stem cells in the day 9 murine yolk sac. Immunity. 1997;7:335–344. doi: 10.1016/s1074-7613(00)80355-6. [DOI] [PubMed] [Google Scholar]
- Zeigler BM, Sugiyama D, Chen M, Guo Y, Downs KM, Speck NA. The allantois and chorion, when isolated before circulation or chorio-allantoic fusion, have hematopoietic potential. Development. 2006;133:4183–4192. doi: 10.1242/dev.02596. [DOI] [PubMed] [Google Scholar]