Abstract
Background
Newborn screening (NBS) enables early treatment and some consider it a natural vehicle for genetic screening. Bioethicists argue for caution since families of carrier infants can develop psychosocial complications. This paper describes methods and feasibility of our statewide project for quality improvement of communication and psychosocial outcomes after NBS.
Methods
When NBS identifies carrier status for cystic fibrosis or sickle cell, we contact primary care providers (PCPs), answer questions, and invite them to rehearse informing the parents. Three months later we telephone parents, assess knowledge and psychosocial outcomes, provide counseling, and assist with self-referral to further resources. Afterwards, anonymous evaluation surveys are provided.
Results
Birthing facilities provided accurate PCP names for 73% of 817 infants meeting inclusion criteria; we identified PCPs for 21% more. We reached 47.3% of PCPs in time to invite a rehearsal; 60% of these accepted. We successfully called 50.2% of eligible parents; 61% recalled a PCP explanation and 48.5% evaluated the explanation favorably. Evaluations by parents with limited health literacy were less favorable.
Conclusion
It is feasible to follow parents for psychosocial outcomes after NBS. Preliminary data about communication is mixed, but further data will soon describe psychosocial outcomes, and investigate outcomes’ associations with communication.
Keywords: Newborn Screening, Genetic counseling, Genetic screening, Cystic fibrosis, Sickle cell trait, Provider-patient communication, Communication quality assurance
INTRODUCTION
This paper describes the methods, feasibility, and early experience of a statewide, multifaceted Quality Improvement project that is designed to assess and improve the quality of provider-parent communication after newborn screening (NBS) identifies heterozygous (“carrier”) status for cystic fibrosis (CF) or sickle cell hemoglobinopathy (SCH).
NBS is a population-scale public health program in which newborn infants’ blood specimens are applied to a special filter paper, dried, and tested at a centralized laboratory for a panel of genetic and metabolic diseases1. CF and SCH are included on NBS panels because the diseases’ risk of death and disability can be reduced if identified before becoming symptomatic 2–4. CF is a metabolic disease in which abnormal secretions lead to lung disease, nutritional problems, and dangerous losses of salt in sweat 2. SCH is a blood disorder in which a hemoglobin mutation (S) is associated with painful crises, life-threatening infections, and vasculopathy leading to problems like stroke 3.
Both CF and SCH are autosomal recessive conditions, and carrier infants are identified in far greater numbers than infants with the actual diseases. Infants with carrier status for CF and SCH do not develop the actual disease, but their children may develop the disease if the other parent is also a carrier. Unfortunately, many families of carrier infants develop psychosocial complications after NBS, ranging from clinical levels of parental anxiety and depression to impaired parent-child bonding and the vulnerable child syndrome 5–10. NBS programs have developed materials for education and support of families, but first conversations can be critical, and the quality of primary care providers’ (PCPs) communication about NBS has been criticized by parents and NBS officials 11,12. Psychosocial problems after carrier identification are cited by bioethicists and others as grounds for delaying or discontinuing some NBS activities 7–9,13,14.
To ensure the continuation of successful NBS programs and reduce harm from psychosocial complications, we developed the Wisconsin Project on Improvement of Communication Process and Outcomes after Newborn Screening (referred to in this paper as “the Project”). We adapted our methods from Quality Improvement techniques used for medical record review, simplified telephone follow-up, and patient tracking, so that the Project would be affordable and sustainable after research funding ended, and replicable by other NBS programs without major budget increases. Eventually, it is hoped that these types of methods may be useful for other genetic conditions, as well as for false positive results of metabolic screening tests.
The purpose of this paper is to describe the initial workings of the Project, ranging from feasibility of identifying NBS results and PCPs, to preliminary findings from evaluation surveys.
METHODS
At its core, the Project is designed to be a quality improvement effort by the NBS program of the Wisconsin State Laboratory of Hygiene and the Department of Health Services, with the Medical College of Wisconsin as a contracted agent. Methods and materials are approved by Institutional Review Boards at the Medical College of Wisconsin and University of Wisconsin, Madison.
Setting
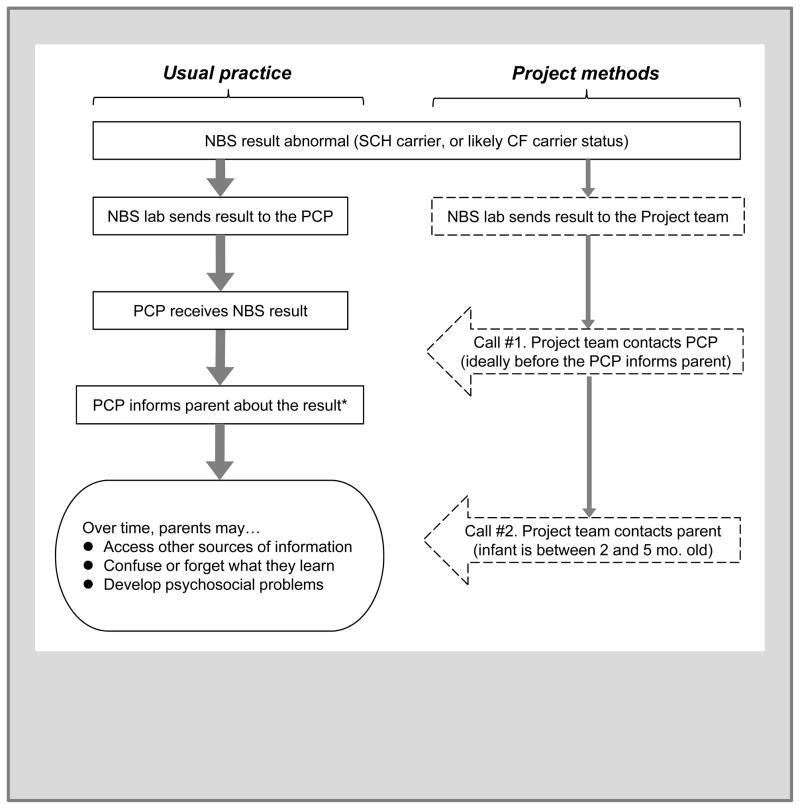
This subsection describes the usual practice for communication of NBS results when either CF or SCH are identified. These are outlined at the left of Figure 1 next to the Project methods at the right of Figure 1, and which will be described below
Figure 1.
Usual practice (left) and Project methods (right) after newborn screening identifies carrier status for sickle cell hemoglobinopathy or likely carrier status for cystic fibrosis
Abbreviations = NBS, newborn screening; SCH, sickle cell hemoglobinopathy; CF, cystic fibrosis; PCP, the infant’s primary care provider.
* Not shown: for infants with the likely CF carrier result, the PCP orders a sweat chloride test to verify that CF is not present.
First, the NBS laboratory communicates by telephone with the infant’s primary care provider (PCP) and subspecialists, in order to facilitate identification, treatment, and follow-up. The NBS laboratory obtains PCP contact information from the birthing facility’s specimen collection card. Anecdotal experience tells that the clinician listed on the NBS card is occasionally incorrect, and the baby’s full name may be less than informative (e.g. “Baby Boy Smith”). When the clinician’s name is not the PCP, the listed clinician is often expected to forward the results to the actual PCP or to take temporary responsibility for the infant. When the baby’s full name is incorrect, the clinician or the NBS laboratory must backtrack to the birthing facility to connect result with the correct infant and PCP.
Usual practice is somewhat different when NBS identifies heterozygous carrier status for CF and SCH, which occurs in far greater numbers than results indicating true CF or SCH. SCH carrier results (defined by the presence of fetal, adult, and sickle hemoglobin, or “FAS”) are mailed to the PCP because these results are not medically urgent. Note that NBS also identifies carriers for other hemoglobinopathies (e.g. Hemoglobin C, D, and E), but the Project is limited to hemoglobin S to focus its analyses on the most common conditions.
CF Carrier status in NBS is defined by a blood spot showing an elevated immunoreactive trypsinogen and a single CF-associated mutation, followed by a normal sweat chloride test. The sweat test is done because up to 2–5% of infants with a single mutation have an unmeasured second mutation that results in actual CF2. It has been recommended to have the sweat test before 8 weeks of age to have the benefit of early identification4, so the NBS laboratory faxes results to PCPs and tracks whether sweat tests have been done. The Project uses the term “likely CF carrier” for infants who had an elevated immunoreactive trypsinogen and a single CF-associated mutation, but who have not yet had a sweat chloride test.
Project design
The Project expands the standard NBS methods for telephone follow-up to serve the typical number of about 900 infants born each year in Wisconsin with SCH carrier status or likely CF carrier status. An initial telephone call is conducted with the infant’s PCP immediately after the abnormal NBS result is found. A second call is conducted with the infant’s parents when the infant is between 3 and 5 months old, allowing sufficient time for infants to have at least one well-child visit during which the NBS result could be discussed. Scripts for telephone calls are similar to those that might be used for purely clinical follow-up, but have some additional research questions embedded into them. After the PCP and parent calls, an anonymous evaluation survey is distributed. The survey’s questions on the survey will be described in the Results section as they are reported.
Participants
The main participants in the Project are the infants’ parents, although data are also collected about the infants and their PCPs. SCH carrier status is defined by the hemoglobin FAS result.
To reduce confounding effects of other factors that might cause potential anxiety or correlate with other psychosocial issues, we exclude infants when the NBS report (a) lists more than one abnormality, (b) states that the gestational age was less than 35 weeks, or (c) states that the calendar age at the time of specimen collection was greater than 180 days of age. We also exclude infants if we discover the infant required either (a) more than five days in a neonatal intensive care unit, (b) hospitalization after discharge from the nursery, or (c) evaluation for some other medical abnormality. During the PCP call we ask the PCP to identify parents do not speak English and other contraindications to contacting the family by asking the question “Can you think of any reason why it would not be appropriate to contact this family later this year?”
Prior to the parent call, a second exclusion criterion is implemented when we use NBS laboratory tracking data to exclude parents of infants who had non-normal sweat test results (i.e. which indicate the presence of CF, the disease).
Parents are offered a $20 gift certificate to more than 200 local or Internet merchants as a gratuity for their participation.
Procedures
Protocol for locating PCPs
The first goal of the Project is to ensure that the NBS laboratory report has reached the provider who has actual primary care responsibility for the infant. We begin by sending an introductory fax and a copy of the NBS result to the clinician listed by the birthing facility, using information from a directory maintained by the NBS laboratory. A Project caller then telephones the clinician’s office and asks if the clinician is the infant’s PCP. If the clinician does not know the infant or denies a PCP relationship, the Project caller attempts to find the PCP by asking the clinician for advice, and then by contacting the birthing facility or its medical record department. If these methods are not successful in finding the PCP, in a few days the Project team contacts the listed clinician again to see if baby has made an appointment. IRB stipulations disallow the Project team from contacting families directly.
Call #1: The PCP
When the PCP is reached, the Project caller asks if he or she has questions about the NBS result or its implications, and describes the Project goals and the parent call. If time is available, the Project caller invites the PCP to rehearse how he or she will inform the infant’s parent(s) about the result. We attempt to invite a rehearsal from every PCP contacted, but the Project callers exercise judgment in deferring the invitation if the PCP is hurried due to being contacted between patients. When the PCP does agree to rehearse, that portion of the call is audiotaped, transcribed, and de-identified for future analysis.
Protocol for locating parents
If neither the NBS laboratory report nor the PCP identify a reason for exclusion, the parents are mailed an initial contact letter when the infant is about 3 months old. The letter purposely does not mention the infant’s NBS result, in order to avoid confusion or distress for parents who have not heard their child’s results or may not fully understand the implications of the results. Also included is a “decline of contact” card to give the parents an opportunity to decline participation without becoming fully informed about the Project.
Call #2: The parent
Approximately ten days after the initial contact letter is mailed, a Project caller telephones the parents. Parents are asked if they recall our letter, and if they are interested in participating in completing the call, and if the present time is an appropriate time to do so. Parents are given the opportunity to decline continuing with our phone call if it is not a convenient time or if they are simply not interested. The Project caller follows a carefully designed script that weaves together components of informed consent, discussion about the screening result, open-ended survey questions and fixed answer questions from established scales to assess psychosocial outcomes such as parental anxiety and perceptions of the child’s health 15–18.
Parent callers have a clinical background, so they have the expertise to perceive emotional distress or confusion over the phone. If serious distress or confusion becomes evident, the Project caller has the option of bypassing the research questions, transitioning to a purely clinical intervention. Regardless of whether a parent completes the entire call, the conversation ends with a debriefing effort to ensure there is no lingering confusion, and assistance with self-referral to additional resources.
Analysis
Both the PCP and parent calls are audio-recorded, transcribed, de-identified, and abstracted for quantitative data. Descriptive data, including the majority of data for this paper, are stored in a Microsoft Access database (Redmond, Washington), and analyzed using JMP software (SAS Institute, Cary, North Carolina). A separate series of papers will report analysis of psychosocial data from the parent calls, and communication data from the PCP calls following our communication quality indicator approach. The communication quality indicators follow our previously published techniques for: jargon usage19,20, assessments of understanding21, organizing behaviors22, communication about potential emotions23 and inclusion of key content messages24,25.
RESULTS
During the first 14 months of the Project, 929 NBS results were sent to the Project team by the NBS laboratory; 709 showed SCH carrier status and 220 showed likely CF carrier status. In 141 of the 220 likely CF carrier results the ΔF508 mutation was seen (64.1%), while the other 79 infants had one of 18 other mutations from the 23 included on Wisconsin’s screening panel. Gender was evenly distributed (49.1% male).
Information included on the NBS laboratory report, gestation age and the presence of multiple conditions, was sufficient to exclude 112 infants (12.1%) without the need for a PCP call. The remaining 817 infants constitute the main sample for this analysis.
These 817 specimens had been submitted by 70 different birthing facilities and four home births. The median number of results listed for a facility was 36 (SD 26.1).
A total of 414 clinicians were listed by the birthing facilities for the 817 infants without exclusion criteria. The highest number of infants logged for a single PCP was 13.
Information about PCPs
Inaccuracy of PCP listing provided by the birthing facility
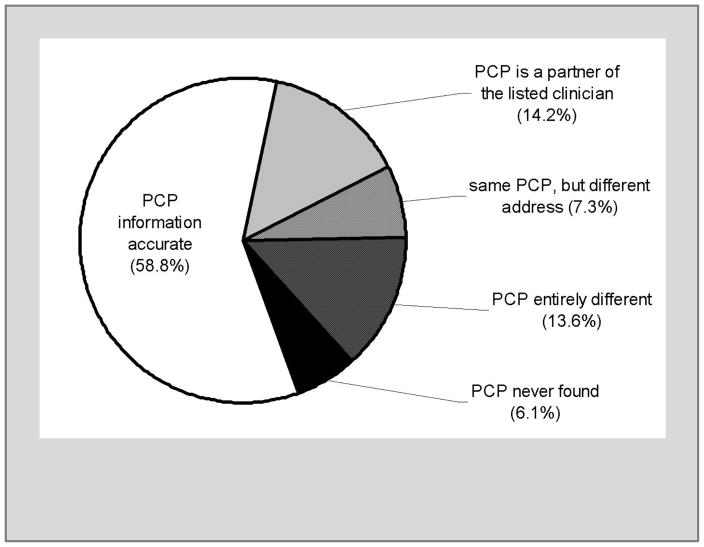
For 58.8% of infants the birthing facility listed the accurate PCP and the NBS laboratory had accurate contact information (Figure 2). For another 14.2% of infants, the birthing facility had listed the name of a clinical partner of the correct PCP, so the NBS laboratory’s contact information was accurate even if the responsible PCP had not been listed.
Figure 2.
Accuracy of PCP information provided by the birthing facility for SCH (sickle cell hemoglobinopathy) and CF (cystic fibrosis) carrier infants.
For the other 27% of infants, the information provided by the birthing facility was not sufficient for the NBS result to automatically reach the PCP. For 20.9% of the 817 infants we found the PCP by following the protocol described in the methods section. For 7.3% of the 817 infants the birthing facility had provided the correct PCP’s name but the PCP had changed locations recently enough that the NBS report was faxed or mailed to an old address. PCPs of infants with likely CF carrier results were more likely to have moved than PCPs of infants with SCH carrier status (χ2, p=0.03).
We were unable to identify a PCP for 50 infants with SCH carrier reports (6.1% of 817). In summary, our contact procedures were able to identify PCPs for 767 infants, or 93.9% of the 817 infants without exclusion criteria.
PCPs’ description of results communication
Of the 767 infants for whom we identified and reached a PCP in the first 14 months of the Project, in 41 cases (5.4%) the PCP reported that he or she had not received the NBS result fax.
For the other 672 infants, 130 PCPs reported already informing the parent in person (19.4%), 134 had already told the parent over the telephone or planned to do so that day (19.9%), 377 planned to tell the parent at the next scheduled appointment (56.1%), 3 planned to send a letter or an e-mail to the parent(s), and 16 PCPs had not decided how to inform the parent. Only 3 PCPs planned to schedule a special appointment to discuss the NBS result.
PCPs were more likely to wait until the next appointment if the infant had an SCH carrier result than a likely CF carrier result (73% vs. 43%, χ2, p < 0.001).
When we asked PCPs if they had questions about the NBS result or its implications, PCPs for 33 infants (4.9% of the 672) asked for an explanation. PCPs were more likely to request an explanation about likely CF carrier results than SCH carrier results (13.3% vs. 3.0%, χ2, p < 0.001).
Many PCPs were willing to rehearse telling the infant’s parent(s). Of the 414 individual PCPs identified in the first 14 months of the Project, we invited rehearsals from 196 PCPs (47.3%) who had not yet informed the parent(s). Of these, 118 agreed to rehearse (60.2%). Another 42 PCPs (21.4%) indicated willingness to rehearse for another infant but deferred rehearsal for the current infant because of time limitations. There were no significant differences by PCP gender or clinical specialty with regard to availability for invitation or agreement to rehearse.
The PCPs who rehearsed supplied some demographic information. The average number of years since graduation from training was 16.7 (SD 10.4 years), with a maximum of 44 years. The average number of months since the PCP last discussed genetic carrier status with a patient was 12.8 (SD 24.7 months).
Acceptability of the Project for PCPs
By the end of the 14-month period analyzed for this paper, we received 79 anonymous evaluations from the PCPs who rehearsed with us. We asked PCPs, “Was the information you obtained during the telephone call useful?” and gave them three options: 27/79 answered “very useful;” 44/79 answered “somewhat useful;” and 8/74 responded “not at all useful.” We asked PCPs, “Was the amount of time spent on the interview appropriate?” and gave them three options: 71/79 answered that the time was “just right,” 6/79 said that the time was “too long,” no one responded that the time spent was “too short,” 2 left the response choices blank. As shown in Table 1 slightly more than half of the PCP reported that being notified about the NBS result or having the opportunity to rehearse had influenced the PCP’s interaction with parents.
Table 1.
PCPs’ opinions about the influence of being called by the Project team
| Opinion | “Yes” | “Somewhat” | “No” | |||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | |
|
| ||||||
| “Being notified about the result influenced my interaction with… | ||||||
| … this parent.” | 14 | (17.9) | 29 | (37.2) | 35 | (44.9) |
|
| ||||||
| … all parents.” | 12 | (15.6) | 30 | (39) | 35 | (45.5) |
|
| ||||||
| “The rehearsal session influenced my interaction with… | ||||||
| … this parent.” | 13 | (17.6) | 23 | (31.1) | 38 | (51.4) |
|
| ||||||
| … all parents.” | 11 | (15.1) | 27 | (37) | 35 | (47.9) |
Information about the infants’ parents
Of the 767 infants for whom we identified and reached a PCP, we were told of contraindication to us contacting the parents for a follow-up call in 54 cases (7%), including 29 infants whose families did not speak English. Seventeen were excluded due to non-normal sweat test before the parental call.
The outcomes of our attempts to reach the remaining 696 infants are listed in Table 2 for infants with SCH carrier results and likely CF carrier results. Overall we were able to complete an call for 297 parents, or 50.4% of eligible parents. The infants’ average age at the time of the call was 107.5 days old.
Table 2.
Recruiting experience with parents
| Outcome of parent recruiting effort | Parents of infants with NBS results showing… | |||
|---|---|---|---|---|
| SCH carrier status | likely CF carrier status | |||
| n | (% for result) | n | (% for result) | |
| Unable to find reliable contact information | 102 | 19.0% | 5 | 3.1% |
| No response to voicemails | 183 | 34.1% | 46 | 28.8% |
| Parent reached by telephone but declined | 45 | 8.4% | 18 | 11.3% |
| Parent called | 206 | 37.4% | 91 | 56.9% |
| Total number of eligible infants | 536 | 160 | ||
Most of the called parents were mothers, but 8 fathers (2.7%) were called. The average age of parents called was 26.7 years (SD 6.6). The youngest person we called was a 14-year old mother; the oldest was a 46-year old mother. We asked most parents their ethnic background in an open-ended question, and 54% reported African American, 37% Caucasian, 4% Latino, and 5% reported a combination, such as African American and Latino.
Results of the 3-item health literacy screener identified 25 parents with the potential for a significant limitation in health literacy (9%). Another 83 parents (29.9%) answered the screening questions with intermediate-range answers consistent with occasional health literacy problems.
Parents’ description of communication with the PCPs
The parents of 38.5% of the SCH carrier infants did not recall an explanation from the PCP. All of the parents of likely CF carrier infants recalled an explanation except for one, despite that infant having gone through the sweat testing process, which includes meeting with a genetic counselor, prior to our phone call.
When asked how well the PCP had explained the result, 48.5% of parents responded “well” or “very well.” Responses were similar to a question about general satisfaction with the NBS experience. Parents were more likely to be satisfied if they remembered an explanation or if they evaluated the PCP’s explanation favorably (χ2, p < 0.01). There was no apparent difference in satisfaction of parents of likely CF carrier infants versus SCH carrier infants, but parents were more likely to evaluate PCP explanations unfavorably if their health literacy was marginal or inadequate (χ2, p = 0.04).
Acceptability of the Project for the parents
By the end of the 14-month period, we received 70 anonymous parent evaluations. When asked “Was the information you obtained during the telephone call useful?” 50 replied “very useful” (71.4%) and 17 replied “somewhat useful” (24.3%). Three respondents said the information was “not at all useful” (4.3%). When asked “Was the amount of time spent on the interview appropriate?” 63 said that the time was “just right” (90%) and 7 said it was “too long” (10%). No one responded that the call was “too short.”
Time and Labor involved
One of our main research questions at this point was the amount of time and labor that would be needed to do follow-up on communication processes and psychosocial outcomes in a typical sample of nearly 900 families per year. We had made initial personnel projections as part of our grant, but report our team’s composition and required time as Results because it answers our research question about feasibility.
To facilitate planning for similar programs in the future we have tracked time and expenses for clinical and research aspects of the Project. Not counting IRB-required activities necessary for research, we estimate that telephone calls to PCPs and related administrative needs have occupied half of each weekday for one staff person, or about 20 hours per week. Parent calls take longer, requiring almost 40 hours per week of staff time for calls and documentation. Because of the research and IRB needs for the Project, the call workload was spread out over several members of our lab’s team, including part of the time of a genetic counselor, 3 nurses, a coordinator, and the project director (a pediatrician).
DISCUSSION
Decades of experience have shown that NBS can effectively identify diseases before they become symptomatic, but also that NBS can be followed by serious psychosocial complications5–10. These complications and other communication problems are found across entire states, so we have developed a population-scale, Quality Improvement approach to addressing them.
We were pleased at the generally favorable reactions of PCPs and parents to our calls. Some of the busiest PCPs were annoyed to be telephoned directly, but our evaluation data and PCP calls found that most PCPs were either grateful or neutral. To improve acceptability for PCPs with large numbers of infants, we developed a protocol for relaying communications through office staff or fax machines. We were impressed by the number of PCPs who were willing to rehearse with us. We hope to eventually expand our service to parents who require English-language translation.
Our most troubling experience has been the difficulty of locating physicians willing to assume clinical responsibility for some carrier infants. This difficulty stands in sharp contrast to the urgency with which subspecialists and PCPs take action when presented with NBS reports for a life-threatening illness. For NBS to result in more good than harm, some of that urgency needs to be extended to parents of carrier infants. A casual attitude to carrier results may be partially to blame for the psychosocial complications seen in many previous studies.
The Project methods are elaborate in order to integrate into usual-practice NBS, but some limitations are inevitable. Some selection bias may be present despite our response rate and status as a Quality Improvement project. Due to IRB restrictions and NBS legislative rules about contacting parents directly, we have little or no reliable data about many of the parents that were not reachable via the two protocols described in the Methods section. In addition, the use of survey methods may be associated with the social desirability and Hawthorne effects. Further study may be needed to know whether it is reasonable to generalize our findings about infants with carrier status for CF and SCH to other types of carrier states and to false positive NBS results.
For the health care system to ensure that NBS and associated interventions consistently lead to more good than harm, clinicians need to assume responsibility and provide high-quality care for carrier and disease-affected infants. Our future reports will comment on the psychosocial data that we gathered indicating that parents do experience real psychosocial effects of poor communication about NBS results. The role of Communication Quality Assurance and centralized follow-up will be to support PCPs and parents as they deal with positive and false-alarm NBS results. We further hope that the Project and forthcoming papers will serve as a model for other population-scale efforts to improve the quality of communication in many other areas of health care.
Acknowledgments
The project is funded by NIH grants K01-HL072530 and R01-HL086691. The authors dedicate this paper in memory of our colleague Ronald H. Laessig PhD, a fierce advocate of newborn screening, laboratory quality improvement, and families everywhere.
References
- 1.Allen DB, Farrell PM. Newborn screening: principles and practice. Adv Pediatr. 1996;43:231–270. [PubMed] [Google Scholar]
- 2.Bobadilla JL, Farrell MH, Farrell PM. Applying CFTR molecular genetics to facilitate the diagnosis of cystic fibrosis through screening. Adv Pediatr. 2002;49:131–190. [PubMed] [Google Scholar]
- 3.Pass K, Harris K, Lorey F, Choi R, Kling S. Update: newborn screening for sickle cell disease--California, Illinois, and New York, 1998. MMWR. 2000;49(32):729–731. [PubMed] [Google Scholar]
- 4.Farrell PM, Kosorok MR, Rock MJ, et al. Early diagnosis of cystic fibrosis through neonatal screening prevents severe malnutrition and improves long-term growth. Wisconsin Cystic Fibrosis Neonatal Screening Study Group. Pediatrics. 2001 Jan;107(1):1–13. doi: 10.1542/peds.107.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Nelson RM, Botkin JR, Kodish ED, et al. Ethical issues with genetic testing in pediatrics. Pediatrics. 2001 Jun;107(6):1451–1455. doi: 10.1542/peds.107.6.1451. [DOI] [PubMed] [Google Scholar]
- 6.Markel H. The stigma of disease: implications of genetic screening. Am J Med. 1992;93(2):209–215. doi: 10.1016/0002-9343(92)90052-d. [DOI] [PubMed] [Google Scholar]
- 7.Fost N, Kaback NM. Why do sickle screening in children? Pediatrics. 1973 Apr;51(4):742–745. [PubMed] [Google Scholar]
- 8.Ross LF, Moon MR. Ethical issues in genetic testing of children. Arch Ped Adol Med. 2000;154(9):873–879. doi: 10.1001/archpedi.154.9.873. [DOI] [PubMed] [Google Scholar]
- 9.Hewlett J, Waisbren SE. A Review of the Psychosocial Effects of False-Positive Results on Parents and Current Communication Practices in Newborn Screening. Journal of Inherited Metabolic Disease. 2006 Oct;29(5):677–682. doi: 10.1007/s10545-006-0381-1. [DOI] [PubMed] [Google Scholar]
- 10.Tluczek A, Koscik RL, Farrell PM, Rock MJ. Psychosocial risk associated with newborn screening for cystic fibrosis: parents’ experience while awaiting the sweat-test appointment. Pediatrics. 2005;115(6):1692–1703. doi: 10.1542/peds.2004-0275. [DOI] [PubMed] [Google Scholar]
- 11.Ciske D, Haavisto A, Laxova A, Rock L, Farrell P. Genetic counseling and neonatal screening for cystic fibrosis: an assessment of the communication process. Pediatrics. 2001;107(4):699–705. doi: 10.1542/peds.107.4.699. [DOI] [PubMed] [Google Scholar]
- 12.Farrell MH, Certain L, Farrell PM. Genetic counseling and risk communication services of newborn screening programs. Arch Ped Adol Med. 2001;155(2):120–126. doi: 10.1001/archpedi.155.2.120. [DOI] [PubMed] [Google Scholar]
- 13.McNeil TF, Harty B, Thelin T, Aspegren-Jansson E, Sveger T. Identifying children at high somatic risk: long-term effects on mother- child interaction. Acta Psychiatr Scand. 1986 Dec;74(6):555–562. doi: 10.1111/j.1600-0447.1986.tb06284.x. [DOI] [PubMed] [Google Scholar]
- 14.Swerts A. Impact of genetic counseling and prenatal diagnosis for Down syndrome and neural tube defects. Birth Defects: Original Article Series. 1987;23(2):61–83. [PubMed] [Google Scholar]
- 15.Marteau TM, Bekker H. The Development of a Six-Item Short-Form of the State Scale of the Spielberger State-Trait Anxiety Inventory (Stai) British Journal of Clinical Psychology. 1992 Sep;31(Pt 3):301–306. doi: 10.1111/j.2044-8260.1992.tb00997.x. [DOI] [PubMed] [Google Scholar]
- 16.Morris NS, MacLean CD, Chew LD, Littenberg B. The Single Item Literacy Screener: evaluation of a brief instrument to identify limited reading ability. BMC Fam Pract. 2006;7:21. doi: 10.1186/1471-2296-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004 Sep;36(8):588–594. [PubMed] [Google Scholar]
- 18.Kerruish NJ, Settle K, Campbell-Stokes P, Taylor BJ. Vulnerable Baby Scale: Development and Piloting of a Questionnaire to Measure Maternal Perceptions of Their Baby’s Vulnerability. Journal of Paediatrics & Child Health. 2005 Aug;41(8):419–423. doi: 10.1111/j.1440-1754.2005.00658.x. [DOI] [PubMed] [Google Scholar]
- 19.Deuster L, Christopher S, Donovan J, Farrell M. A Method to Quantify Residents’ Jargon Use During Counseling of Standardized Patients About Cancer Screening. J Gen Intern Med. 2008;23(12):1947–1952. doi: 10.1007/s11606-008-0729-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farrell M, Deuster L, Donovan J, Christopher S. Pediatric residents’ use of jargon during counseling about newborn genetic screening results. Pediatrics. 2008 Aug;122(2):243–249. doi: 10.1542/peds.2007-2160. [DOI] [PubMed] [Google Scholar]
- 21.Farrell MH, Kuruvilla P. Assessment of parental understanding by pediatric residents during counseling after newborn genetic screening. Arch Pediatr Adolesc Med. 2008 Mar;162(3):199–204. doi: 10.1001/archpediatrics.2007.55. [DOI] [PubMed] [Google Scholar]
- 22.Christopher SA, Ahmad N, Bradford L, et al. A method to assess the organizing behaviors used in physicians’ counseling of parents. 2011. Submitted manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farrell MH, Speiser J, Deuster L, Christopher SA. Child Health Providers’ Precautionary Discussion of Emotions during Communication about Results of Newborn Genetic Screening. Arch Pediatr Adolesc Med. 2011 doi: 10.1001/archpediatrics.2011.696. Accepted manuscript. [DOI] [PubMed] [Google Scholar]
- 24.La Pean A, Farrell MH. Initially misleading communication of carrier results after newborn genetic screening. Pediatrics. 2005 Dec;116(6):1499–1505. doi: 10.1542/peds.2005-0449. [DOI] [PubMed] [Google Scholar]
- 25.Farrell MH, La Pean A, Ladouceur L. Content of communication by pediatric residents after newborn genetic screening. Pediatrics. 2005 Dec;116(6):1492–1498. doi: 10.1542/peds.2004-2611. [DOI] [PubMed] [Google Scholar]