Abstract
Background
Gays, lesbians, and bisexuals (i.e. nonheterosexuals) have been found to be at much greater risk for many psychiatric symptoms and disorders, including depression. This may be due in part to prejudice and discrimination experienced by nonheterosexuals, but studies controlling for minority stress, or performed in very socially liberal countries, suggest that other mechanisms must also play a role. Here we test the viability of common cause (shared genetic or environmental etiology) explanations of elevated depression rates in nonheterosexuals.
Method
A community-based sample of adult twins (N=9884 individuals) completed surveys investigating the genetics of psychiatric disorder, and were also asked about their sexual orientation. Large subsets of the sample were asked about adverse childhood experiences such as sexual abuse, physical abuse, and risky family environment, and also about number of older brothers, paternal and maternal age, and number of close friends. Data were analysed using the classical twin design.
Results
Nonheterosexual males and females had higher rates of lifetime depression than their heterosexual counterparts. Genetic factors accounted for 31% and 44% of variation in sexual orientation and depression, respectively. Bivariate analysis revealed that genetic factors accounted for a majority (60%) of the correlation between sexual orientation and depression. In addition, childhood sexual abuse and risky family environment were significant predictors of both sexual orientation and depression, further contributing to their correlation.
Conclusions
Nonheterosexual men and women had elevated rates of lifetime depression, partly due to shared etiological factors, although causality cannot be definitively resolved.
Keywords: sexual orientation, childhood abuse, depression, twins, genetics
Introduction
Several recent large-scale studies have indicated that gays, lesbians, and bisexuals (i.e., nonheterosexuals) are at elevated risk for many psychiatric symptoms and disorders (Fergusson et al., 1999, Cochran and Mays, 2000, Gilman et al., 2001, Sandfort et al., 2001, Meyer, 2003, Mills et al., 2004, King et al., 2008, Frisell et al., 2010, Bolton and Sareen, 2011, Chakraborty et al., 2011). For example, a recent meta-analysis revealed that, compared to heterosexuals, nonheterosexuals are at approximately twice the risk of major depressive disorder (depression) and anxiety disorders, deliberate self harm and attempted suicide (King et al., 2008). With nonheterosexuals comprising a substantial proportion of the population (≈3–10% depending on the definition used; Sell et al., 1995, Grulich et al., 2003, Zietsch et al., 2008) it is of considerable importance to understand the causes of their elevated psychiatric risk. These causes, though, remain unclear.
The dominant explanation is the “minority stress” hypothesis, whereby social prejudice and discrimination provoke mental health problems in nonheterosexuals (Meyer, 1995, Mays and Cochran, 2001, Meyer, 2003, Hatzenbuehler, 2009). Two studies found that controlling for reported levels of discrimination attenuated the relationship between sexual orientation and mental health (Mays and Cochran, 2001, Frisell et al., 2010). However, in both studies, even after controlling for levels of discrimination there remained a large effect, and other studies show the relationship between sexual orientation and mental health is just as strong in The Netherlands, where there has long been greater cultural acceptance of homosexuality than in other countries (Sandfort et al., 2001, Lewis, 2009). Thus, it seems likely that other mechanisms (Bailey, 1999) also contribute to the link between nonheterosexuality and psychiatric risk, but these are only now starting to be explored.
These other possible mechanisms may involve a common cause of both nonheterosexuality and psychiatric disorder. Frisell et al. (2010) found that controlling for familial confounding reduced or eliminated nonheterosexuals’ elevated psychiatric risk in a Swedish sample of twins, with or without adjustment for perceived discrimination or hate crime victimization. This suggests elevated risk in nonheterosexuals is influenced by common familial factors, but it is unclear if they have a genetic or shared-environmental basis. Twin and family studies generally indicate that genetic influences account for between a third and half of the variance in both sexual orientation (Pillard and Bailey, 1998, Kendler et al., 2000, Santtila et al., 2008, Zietsch et al., 2008, Langstrom et al., 2010) and depression (Sullivan et al., 2000, Kendler et al., 2001, Levinson, 2006), which we focus on in this study. These genetic influences may overlap; Zietsch et al. (2009) found that genetic correlation between sexual orientation and Neuroticism - a robust predictor of depression (Kendler et al., 1993b, Kendler et al., 2004) - was primarily responsible for elevated Neuroticism scores in both male and female nonheterosexuals. This finding provided some evidence for the existence of genetic factors that predispose to both nonheterosexuality and depression. On the other hand, potent nongenetic risk factors for depression include childhood sexual abuse and risky family environment (i.e. characterized by conflict and relationships that are cold, unsupportive, and neglectful; see Repetti et al.(2002)) (Felitti et al., 1998, Kendler et al., 2002, Kendler et al., 2004, Kendler et al., 2006, Fergusson et al., 2008) and nonheterosexuals are also more likely to experience childhood sexual abuse (Hughes et al., 2001, Tomeo et al., 2001, Balsam et al., 2005) and family instability (Fergusson et al., 1999) than are heterosexuals, according to self reports. Although most nonheterosexuals do not report childhood sexual abuse, the possibility remains that childhood experiences could influence individuals’ later sexual orientation as well as increasing their vulnerability to depression.
Here we investigate the viability of these genetic and environmental “common cause” explanations using two large community-based samples (N= 6233 and 3651 individuals) of identical and nonidentical twins, selected without reference to sexual orientation or depression. Individuals in both samples completed a detailed psychiatric assessment via phone interview, and also answered questions regarding their sexual orientation and childhood experiences. Using this genetically informative data we quantify the relative involvement of genetic influences, childhood sexual and physical abuse, and risky family environment in sexual orientation, lifetime depression, and their association. We also assess the contribution of other factors that have been thought to influence sexual orientation, including number of older brothers and parental ages, and test whether social connectedness (close friends) could mediate the relationship between sexual orientation and depression. This is the first study to investigate the link between sexual orientation, depression, and adverse childhood experiences in a genetically informative sample.
Methods
Participants
Participants were two community-based samples of identical (monozygotic; MZ) and nonidentical (dizygotic; DZ) twins drawn from the Australian Twin Registry (ATR), who participated in semi-structured telephone interviews primarily aimed at assessing links between psychiatric disorder and substance use. Sample 1 consisted of 6233 twin individuals aged between 23 and 39 years (M=29.9±2.5) interviewed between 1996 and 2000. Subjects were members of the young adult cohort of the ATR, a volunteer twin panel born between 1964 and 1971. Twins were registered with the ATR between 1980 and 1982 by their parents in response to approaches through school systems and mass media appeals. The response rate for this study was 77% (68% complete pairs). Fourteen percent of the twins contacted refused to collaborate with the study. Further details can be found elsewhere (Nelson et al., 2002, Knopik et al., 2004).
Sample 2 consisted of 3651 twin individuals aged between 27 and 37 years (M=31.8±2.5), interviewed between 2005 and 2009. Twins were already members of the ATR. The response rate for this study was 51%. This is lower than for study 1 because non-response after initial contact was not followed up as extensively, as changes to the protocol necessitated a two stage consent process whereby the ATR was required to obtain consent from twins to release their contact information while consent to participate in this specific study was obtained in a separate process. Written informed consent was obtained from all participants and data were obtained from a comprehensive assessment by trained interviewers.
The combined sample consisted of 9884 twins; 1133 female MZ pairs, 707 male MZ pairs, 861 female DZ pairs, 555 male DZ pairs, 946 opposite sex DZ pairs, and 1480 single twins whose co-twin did not participate. The zygosity of the twin pairs was determined based on their response to standard items about physical similarity, a procedure that has been found to have high (at least 95%) concurrence with DNA typing (Ooki et al., 1990) - the accuracy here would be even higher as the zygosity of many pairs was confirmed by further telephone queries and/or subsequent DNA testing.
Measures
For each dichotomous variable (those not listed under ‘Other variables’), the more common status (i.e. heterosexual, absence of depression and adverse childhood experiences) were used as the comparison group.
Sexual Orientation
Sexual orientation was assessed with the following question: ‘Do you have a sexual preference for males, females, or both?’ Previous studies (Fergusson et al., 1999, Zietsch et al., 2009) indicate that homosexuals and bisexuals are at similarly greater psychiatric risk compared with heterosexuals and have combined them into one nonheterosexual category.
Nonheterosexuals comprised 4.3% and 3.3% of the sample for males and females, respectively.
Lifetime depression
In both interviews, respondents completed identical assessments of depression episodes. In accordance with the DSM-IV criteria (American Psychiatric Association, 2000), respondents were coded as having had depression in their lifetime if they exhibited at least five of the nine DSM-IV symptoms of depression during the same 2-week period. Also in accordance to the DSM-IV, symptoms must have been present during the same two week period and must include at least symptoms 1) depressed mood, or 2) loss of interest or pleasure. However, our criteria do not comprise a full DSM-IV diagnosis of major depressive disorder in that exclusion criteria (i.e. symptoms being distinct from those of a mixed episode, causing clinically significant distress or impairment in functioning, and depression not induced by substance use or bereavement) were not used. There was substantially greater missingness if these criteria were included, with a concomitant cost in power. Individuals’ depression status was coded as missing if they had a missing value for either of symptoms 1) or 2) above, or when they answered yes to one or both of symptoms 1) and 2), but had missing values on more than three of the nine symptoms in total. The lifetime prevalence of depression was 24% and 37% for males and females, respectively. Depression rates were 43% and 45% for male homosexuals and bisexuals, and 53% and 70% for female homosexuals and bisexuals. These differences in depression rates in homosexuals and bisexuals were not significant (P=.08), and we thus combine them into a nonheterosexual category in order to achieve adequate power in genetic analyses.
Adverse childhood experiences
(Note that the childhood sexual and physical abuse items were only assessed in Sample 1, whereas risky childhood family environment and was available in Samples 1 and 2)
Risky childhood family environment was loosely based on factors identified in Repetti et al. (2002). It was operationalized to include those reporting at least one of the following: that between ages 6 and 13 1) they would often have had an unpleasant disagreement with one or both of the parents, 2) they were not at all close with their parents, 3) their parents were often fighting or arguing in front of respondent, 4) there was a lot of tension between the respondent’s parents in the household, or 5) one of the parents drank too much. These were the most extreme of several response options available for each item. The items are empirically linked (inter-item correlations (Cronbach’s alpha=.65, and all items were significantly correlated with each other).
Childhood sexual abuse was coded based on four sections of the interview regarding sexual abuse. Childhood sexual abuse was coded as present if any of the following were met: 1) the respondent reported having sexual contact (defined as ‘their touching your sexual parts, your touching their sexual parts, or sexual intercourse’) with an adult family member before the age of 14, or nonconsensual sexual contact with another child in the family; 2) a respondent reported being forced into sexual intercourse or any other form of sexual activity before the age of 14; 3) a respondent reported to have been raped, or sexually molested before the age of 14, or 4) a respondent reported to have had nonconsensual sex with someone 5 years or older before the age of 14. If none of the above were reported the childhood abuse variable was coded as not present, unless the respondent did not respond to any one of these items or failed to report the age at which the event occurred, in which case the variable was coded as missing.
Childhood sexual abuse took place at an average age of 8.7 years, well before the average age that sexual feelings developed (13.5 years; as assessed by an item in Sample 2 interviews only1). Around half of the participants that were sexually abused by either a family or non-family member participated in a follow-up interview, revealing that perpetrators were male in the cases of 94% and 98% of male and female victims, respectively.
Childhood parental physical abuse was considered to have occurred if a respondent reported at least one of the following: that, between the ages of 6 and 13, 1) they were often or sometimes hit with a belt or stick or something like that by their parents when they did something wrong, 2) their parents often or sometimes punished them so hard that it hurt the next day, or 3) the usual way in which their parents punished or disciplined them was physical and harsh (use weapon, punch, kick). These items were all significantly intercorrelated.
Previous research suggests that retrospective reports of adverse childhood experiences such as abuse and household dysfunction show good long-term test-retest reliability and little relation to mood states (Dube et al., 2004, Yancura and Aldwin, 2009), suggesting our participants probably had reasonably reliable recall of the above childhood factors.
Other variables
Variables previously linked to sexual orientation or otherwise relevant to our hypotheses were also assessed, in order to obtain the fullest etiological picture possible from the available data. These variables were number of older full brothers (Samples 1 and 2), paternal and maternal age (i.e. age of father and mother at birth; Samples 1 and 2), and number of close friends (Sample 2 only). Nonheterosexual orientation has been found to be associated with having more older brothers and having older parents (Hare and Moran, 1979, Blanchard, 2004); low social connectedness (for which number of close friends is a rough proxy) has been linked with depression (Baumeister and Leary, 1995) and could be relevant to the minority stress hypothesis if societal prejudice causes nonheterosexual individuals to be less socially connected on average.
All Sample 1 and some Sample 2 respondents were directly asked how many full biological older brothers they had. Most Sample 2 respondents were only asked about their total number of brothers - of these, those with zero brothers could be coded as having zero older brothers whereas those with a non-zero number of brothers were coded as missing. As such, the non-missing mean number of older brothers (see Table 1) is an underestimate of the true mean, though this only affects analyses involving this variable (i.e. reducing power by restricting the range).
Table 1.
Descriptive statistics. Prevalences and means of the variables used in this study, for heterosexuals, nonheterosexuals and the total sample.
| Heterosexuals | Nonheterosexuals | Total sample (N=up to 9,884) | ||||
|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | |
| Lifetime major depression | 24% | 36% | 44% | 60% | 24% | 37% |
| Childhood family dysfunction | 24% | 30% | 41% | 42% | 25% | 30% |
| Sexual abuse before the age of 14 | 4.2% | 11% | 12% | 24% | 4.5% | 11% |
| Childhood parental physical abuse | 40% | 27% | 40% | 38% | 40% | 27% |
| Number of older brothers* (Mean ± SD) | 0.72 (±1.1) | 0.62 (±.1.0) | 0.76 (±1.0) | 0.47 (0.8) | 0.72 (±1.1) | 0.62 (±1.0) |
| Age of father when born (Mean ± SD) | 31 (±6.2) | 31 (±6.1) | 31 (±6.6) | 30 (±6.0) | 31 (±6.2) | 31 (±6.1) |
| Age of mother when born (Mean ± SD) | 28 (±5.4) | 28 (5.3) | 28 (±5.8) | 27 (5.5) | 28 (±5.4) | 28 (±5.3) |
| Number of close friends (Mean ± SD) | 4.8 (±3.7) | 4.3 (±2.3) | 4.8 (±3.2) | 3.8 (±1.6) | 4.8 (±3.6) | 4.3 (±2.3) |
Mean is deflated due to non-random missingness, see Methods.
SD=standard deviation
Statistical Analyses
Statistical analyses employed maximum-likelihood modeling procedures using the statistical package Mx (Neale et al., 2006), which accounts for the non-independence of a twin pair. The dichotomous measures described above were analyzed in Mx as raw dichotomous data, where it is assumed that a normally distributed continuum of liability is cut in two at a certain threshold, yielding the two observed categories. In maximum-likelihood modeling, the goodness-of-fit of a model to the observed data is distributed as chi-square (χ2). By testing the change in chi-square (Δχ2) against the change in degrees of freedom (Δdf), we can test whether dropping model parameters, or constraining them to be equal, significantly worsens the model fit. In this way we can test hypotheses regarding those parameters.
The effects of childhood experiences and other variables of interest on sexual orientation and depression were tested by modeling them as covariate effects (within the genetic models described below, as per standard procedures in Mx) and then independently dropping each covariate from the model (separately for males and females) and comparing model fit.
Genetic modeling
This study uses the classical twin design, where variance in traits, and covariance between them, is partitioned into that due to genetic (A), shared environmental (nongenetic factors shared by twin pairs; C), and residual (E) sources. Nonadditive genetic effects can be modeled instead of C, but previous studies (Kendler et al., 1993a, Sullivan et al., 2000, Kendler et al., 2001) and our own preliminary modeling results found no significant nonadditive genetic effects so we do not model them here. Shared environmental factors may include shared home environment, parental style, and uterine environment. Residual variance includes environmental factors not shared by twin pairs (e.g. idiosyncratic experiences), stochastic biological effects, and measurement error. Trait variances are standardized to equal 1, so A, C, and E parameters equal the proportion of variance accounted for by each source, and A equals the heritability (h2) of the trait.
Partitioning of phenotypic variance into genetic, shared environmental and residual components can be achieved because MZ twins share all their genes, while DZ twins share on average only half of their segregating genes. Thus, A, C, and E influences predict different patterns of MZ and DZ twin correlations, and structural equation modeling is used to determine the combination of influences that best matches the observed data. Cross-twin cross-trait correlations allow us to partition covariance between traits into A, C, and E in the same way as we do for variance in a single trait. In this way we can calculate the genetic correlation, a measure of the overlap in the genetic variation underlying two traits. An assumption of the classical twin design is that trait-relevant environments are similar to the same extent in MZ and DZ twin pairs; tests of this assumption for sexual orientation (Kendler et al., 2000) and depression (Kendler et al., 1993c) suggest it is valid. Further details of the classical twin design can be found elsewhere (Neale and Cardon, 1992, Evans et al., 2002, Posthuma et al., 2003).
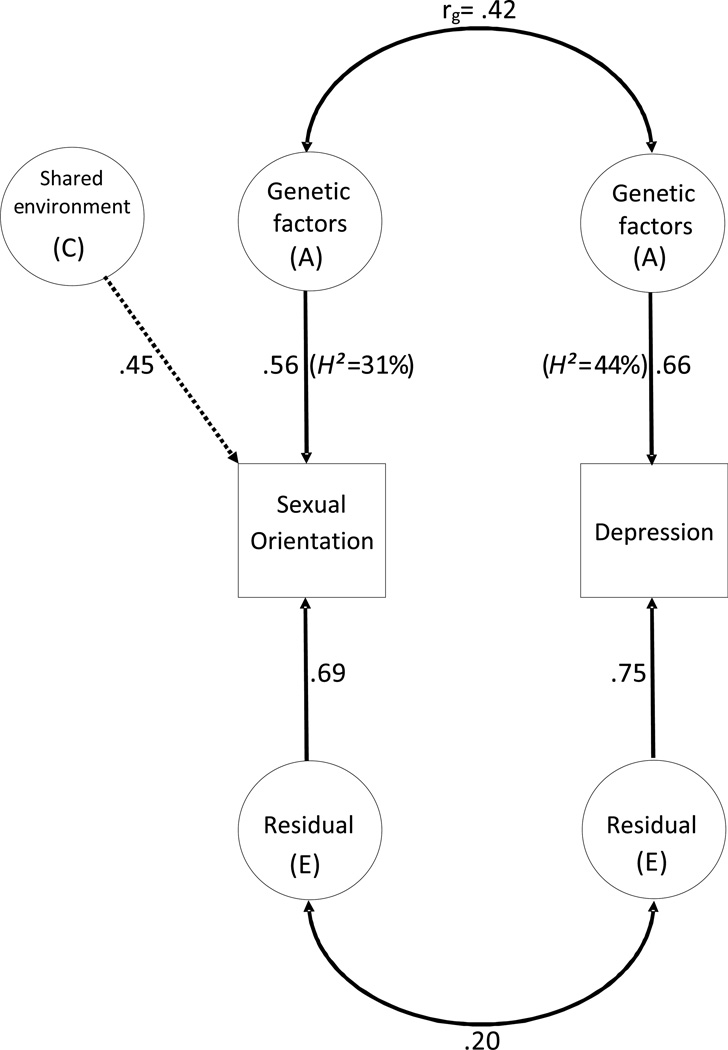
To test the significance (α = .05) of genetic and environmental influences on sexual orientation, depression, and their covariation, a bivariate Cholesky model was fitted. Sex and age effects were included as covariates. Genetic influences on each trait were tested by dropping the genetic path/s to each trait and comparing model fit. Similarly, genetic correlation was tested by dropping the genetic crosspath and residual correlation was tested by dropping the residual crosspath. Each test was performed independently, and compared to the base model. For ease of interpretation, the base model was transformed from a Cholesky form into a ‘correlated factors’ model as suggested by Loehlin (1996) (see Figure 1). This yields the proportion of variance in each trait accounted for by genes (h2), shared environment and residual factors, as well as genetic, shared environmental, and residual correlations.
Figure 1.
Correlated factors model for sexual orientation and lifetime depression. Dashed lines represent non-significant paths (P>.05). H2 (heritability) is the percentage of variance accounted for by genetic factors, and is the square of the corresponding path coefficient. Percentage of variance accounted for by the residual and shared environmental factors can also be calculated by squaring those path coefficients.
Results
Association of lifetime depression with sexual orientation
Nonheterosexuals had significantly elevated rates of lifetime depression compared with heterosexuals (males: OR, 2.8; 95% CI, 2.0–3.9; P<.001; females: OR, 2.7; 95% CI, 1.9–3.7; P<.001). Rates of depression in male and female heterosexuals and nonheterosexuals can be seen in Table 1. For males and females combined, the rates were 31% for heterosexuals and 52% for nonheterosexuals, corresponding to a tetrachoric correlation between sexual orientation and liability to depression of 0.26.
Heterosexuals with a nonheterosexual twin had a higher rate of depression (39%) than heterosexual pairs (31%) (Fisher’s exact 2-tailed test P=.015). This suggests that familial (genetic and/or family environmental) factors associated with both nonheterosexuality and depression contributed to the link between them (in accordance with Frisell et al (2010)) - these factors should not include minority-related stressors, since the heterosexual twin is not subject to these. Below we investigate what these shared risk factors might be.
Are there shared risk factors associated with sexual orientation and lifetime depression?
Factors associated with sexual orientation
The probandwise concordance rate (i.e. the probability that a twin is nonheterosexual given that his or her co-twin is nonheterosexual) was greater for MZ twin pairs (24%; 95% CI, 17–31%) than for DZ pairs (13%; 95% CI, 7–18%), suggesting a genetic component to sexual orientation, formally tested in ‘Genetic models…’ below.
We also tested the effect of adverse childhood experiences, and other variables thought to be associated with sexual orientation - the prevalence or means of each variable in heterosexuals and nonheterosexuals can be seen in Table 1, and the statistical tests are presented in Table 2. No significant effects were found for parental physical abuse, maternal or paternal age, or number of older brothers, so we did not consider these variables in subsequent analyses. However, in both males and females, significantly higher rates of nonheterosexuality were found in participants who experienced childhood sexual abuse and in those with a risky childhood family environment. We examined whether a particular item in the risky family environment variable was driving the effect, but each individual item was significantly associated with sexual orientation. Furthermore, nonheterosexual women tended to have fewer close friends than did heterosexual women, but there was no such effect in men.
Table 2.
Association between sexual orientation and adverse childhood experiences, family composition, and number of close friends.
| Association between sexual orientation and: |
Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Effect size | Δχ2 | P | N | Effect size | Δχ2 | P | |
| Risky family environment | 3666 | OR=2.1 (1.3–3.3) | 17.13 | <.001 | 5174 | OR=1.7 (1.1–2.7) | 11.00 | <.001 |
| Parental physical abuse | 2727 | OR=1.0 (0.6–1.8) | 0.01 | 0.93 | 3384 | OR=1.7 (1.0–3.2) | 5.67 | 0.02 |
| Sexual Abuse | 2723 | OR=2.9 (1.4–7.3) | 8.88 | 0.003 | 3380 | OR=2.4 (1.3–4.9) | 9.85 | 0.002 |
| Paternal age | 3543 | β=−.02 | 0.15 | 0.69 | 5156 | β=−.07 | 4.25 | 0.04 |
| Maternal age | 3697 | β=.02 | 0.49 | 0.49 | 5380 | β=−.06 | 3.72 | 0.05 |
| Number of older brothers | 3252 | β=.04 | 0.34 | 0.56 | 4405 | β=−.08 | 2.30 | 0.13 |
| Number of close friends | 1098 | β=.02 | 0.06 | 0.80 | 2139 | β=−.12 | 7.60 | 0.006 |
Beta coefficient obtained from biserial/polychoric correlations from PRELIS
Odds ratios (OR) are accompanied by estimated 95% confidence intervals (in brackets), taking twin relatedness into account.
Factors associated with lifetime depression
The probandwise concordance rate for depression was greater for MZ twin pairs (52%; 95% CI, 49–56%) than for DZ pairs (36%; 95% CI, 32–41%), suggesting a genetic component to depression, formally tested in ‘Genetic models…’ below.
For association with lifetime depression, we tested the same variables as for sexual orientation (Table 3). Significant effects were found in both sexes for childhood sexual abuse, risky childhood family environment, and parental physical abuse. There was also a significant negative relationship between number of close friends and depression in both males and females.
Table 3.
Association between lifetime depression and adverse childhood experiences, family composition, and number of close friends.
| Association between lifetime depression and: | Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Effect size | Δχ2 | P | Effect size | Δχ2 | P | ||||
| Risky family environment | 3342 | OR=1.8 (1.5–2.2) | 44.17 | <.001 | 4788 | OR=2.2 (2.0–2.4) | 135.39 | <.001 | |
| Parental physical abuse | 2442 | OR=1.5 (1.2–1.8) | 16.31 | <.001 | 3119 | OR=1.7 (1.5–1.9) | 37.04 | <.001 | |
| Sexual Abuse | 2430 | OR=2.3 (1.7–3.2) | 17.49 | <.001 | 3108 | OR=2.7 (2.3–3.1) | 69.44 | <.001 | |
| Paternal age | 3242 | β=−.01 | 0.29 | 0.59 | 4768 | β=.01 | 0.85 | 0.36 | |
| Maternal age | 3378 | β=−.03 | 1.69 | 0.19 | 4976 | β=−.02 | 0.83 | 0.36 | |
| Number of older brothers | 2939 | β=.05 | 2.75 | 0.10 | 4072 | β=.00 | 0.15 | 0.70 | |
| Number of close friends | 1048 | β=−.12 | 8.02 | 0.004 | 1978 | β=−.09 | 5.80 | 0.02 | |
Beta coefficient obtained from biserial/polychoric correlations from PRELIS
Odds ratios (OR) are accompanied by estimated 95% confidence intervals (in brackets), taking twin relatedness into account
Do shared etiological factors explain the relationship between sexual orientation and depression?
In Sample 1, where both childhood sexual abuse and risky childhood family environment are available, the correlation between sexual orientation and depression was 0.24 (very similar to r=0.26 in the full sample, see above). Accounting for the effects of a risky childhood family environment and sexual abuse separately reduced this correlation to 0.222 (7.7% reduction) and 0.220 (8.5% reduction), respectively, and to 0.208 (13.7% reduction) when combined. This suggests that the correlation between sexual orientation and depression can be partially explained by these adverse childhood experiences, but that by far most of the correlation remains unexplained. In women, we also assessed whether number of friends could account for any of the correlation between sexual orientation and depression, but this variable accounted for less than 2% of the correlation. Below we assess the role genetic factors might play.
Genetic models of the covariation between sexual orientation and depression
The heritability of sexual orientation and depression
Polychoric twin pair correlations are presented in Table 4. For both sexual orientation and depression, MZ twin correlations were higher than DZ twin correlations, suggesting genetic influences. Twin correlations were not significantly different for male versus female MZ pairs, nor for male versus female DZ pairs, suggesting no sex differences in the magnitude of genetic and environmental influences. We therefore equated male and female estimates of genetic and environmental influences in subsequent analyses. Furthermore, twin correlations for the opposite-sex DZ pairs were not significantly different than those for same-sex DZ pairs, suggesting the source of genetic influences are similar in males and females, and indicating that the heritability estimates of sexual orientation and depression are not being distorted by the opposite-sex DZ pairs.
Table 4.
Tetrachoric twin pair correlations (and 95% confidence intervals) between sexual orientation and lifetime depression by zygosity.
| Zygosity | Sexual orientation | Lifetime depression | ||||
|---|---|---|---|---|---|---|
| N pairs | Twin pair r | MZ vs DZ | N pairs | Twin pair r | MZ vs DZ | |
| MZ F | 1079 | .53 (.33–.70) | .52 (.37–.65) | 910 | .47 (.38–.55) | .46 (.38–.53) |
| MZ M | 633 | .50 (.25–.70) | 521 | .43 (.28–.56) | ||
| DZ F | 811 | .33 (.04–.57) | 696 | .16 (.04–.28) | ||
| DZ M | 503 | .27 (−.12–.60) | .35 (.17–.51) | 431 | .23 (.06–.39) | .17 (.09–.24) |
| DZ OS† | 866 | .43 (.12–.66) | 718 | .15 (.02–.27) | ||
F=female, M=male, OS =opposite-sex, MZ vs DZ = pooled MZ and DZ correlations (i.e. equated across sexes).
Genetic modeling showed that genetic factors accounted for 31% of variation in sexual orientation (Δχ21=13.1, P<.001) and 44% of variation in depression (Δχ22=133.1, P<.001). Shared environmental influences on sexual orientation were not significant (Δχ21=1.1, P=.29).
Bivariate genetic modelling of the relationship between sexual orientation and depression
Figure 1 shows the bivariate model of the association between sexual orientation and depression. The genetic correlation (rg=.42) between sexual orientation and depression was highly significant (Δχ21=11.6, P<.001), and there was a smaller corresponding residual (nongenetic) correlation of .20 (Δχ21=5.5, P=.02). See Figure 1 for a diagrammatic representation of these results.
Additionally, from these estimates it is possible, using standard path analysis, to estimate the extent to which the phenotypic correlation between sexual orientation and depression (r=.26; see above) could be attributed to genetic factors. This calculation is (.56*.42*.66)/(.56*.42*.66+.69*.20*.75), which indicates that genetic factors account for 60% (.155/.259) of the phenotypic correlation between sexual orientation and depression with the remaining 40% accounted for by correlated residual factors (i.e. E; e.g. nonshared environment).
Discussion
Firstly, we replicated previous findings that nonheterosexuals are at elevated risk of depression, an effect that we found in both men and women. To assess the viability of ‘common cause’ explanations of this effect, we initially determined what factors were significantly associated with both nonheterosexuality and depression. Genetic factors accounted for 31% and 44% of the variation in sexual orientation and depression, respectively, broadly consistent with the range of estimates from previous studies (Pillard and Bailey, 1998, Kendler et al., 2000, Sullivan et al., 2000, Kendler et al., 2001, Levinson, 2006, Santtila et al., 2008, Zietsch et al., 2008, Langstrom et al., 2010). Childhood experiences of sexual abuse and risky family environment were also significantly associated with both sexual orientation and depression. Paternal age, maternal age, and number of older brothers did not have significant effects on either sexual orientation or depression, and parental physical abuse was significantly associated with depression but not with sexual orientation. Heterosexual and nonheterosexual men had the same average number of close friends, so a lack of social connectedness in this form could not explain nonheterosexuals’ elevated depression risk; on the other hand, nonheterosexual women and depressed women both had significantly fewer close friends. - .
We then examined to what extent the factors associated with both sexual orientation and depression explain their association. A significant genetic correlation between sexual orientation and depression indicated an overlap in the genetic factors underlying the two variables. This overlapping genetic etiology accounted for most (60%) of the covariance between sexual orientation and depression. Further analysis found that childhood experiences of sexual abuse and risky family environment accounted for 8.5% and 7.7% of the covariance between sexual orientation and depression, respectively, whereas number of close friends accounted for less than 2% of this covariance in women. These results suggest that genetic factors, childhood sexual abuse, and risky family environment are all involved in the elevated rate of depression in nonheterosexuals.
It should be emphasized that these results do not imply that the minority stress hypothesis is false; only that other mechanisms may additionally contribute to the link between sexual orientation and depression. The elevated rates of depression in heterosexuals with a nonheterosexual co-twin suggest that familial factors not directly associated with nonheterosexuality also contribute to elevated depression rates in nonheterosexuals, consistent with the findings of Frisell et al. (2010). Some of these familial factors may be genetic - this is suggested by our finding that 60% of the correlation between sexual orientation and depression can be accounted for by genetic factors, and also by previous findings that elevated levels of Neuroticism and Psychoticism (trait markers for psychiatric vulnerability) in nonheterosexuals are primarily due to correlated genetic influences (Zietsch et al., 2009). Other familial factors that appear to influence both traits involve adverse childhood experiences such as childhood sexual abuse and risky family environment. In cross-sectional twin data with ideal properties, different causal hypotheses can be formally tested, but it is not possible here because the variables have similar heritabilities and are highly skewed (Duffy and Martin, 1994) - as such, interpretations should be cautious.
In both males and females, adverse childhood experiences elevated the likelihood of nonheterosexuality as well as MDD. Many studies have found an association of both childhood abuse and risky family environment with adult depression (Felitti et al., 1998, Nelson et al., 2002, Kendler et al., 2004, Kendler et al., 2006, Fergusson et al., 2008). Though the mechanisms involved are not yet entirely clear, there is mounting evidence that these associations are mediated by permanent effects of repeated stressors on HPA axis reactivity (Heim et al., 2008, Pariante and Lightman, 2008, Lupien et al., 2009, Romeo, 2010, Young and Korszun, 2010). In earlier studies, sexual orientation has also been linked with childhood sexual abuse (e.g. Cameron and Cameron, 1995, Paul et al., 2001, Tomeo et al., 2001, Balsam et al., 2005, Arreola et al., 2008; see Rothman et al 2011 for a review) and, in one study, with family instability (frequent change of parents and parent criminality; Fergusson et al., 1999). Those studies were smaller or selected based on sexual orientation, so the present findings in a large community-based sample provide robust support for those previous findings. It is not at all clear how adverse childhood experiences might affect adult sexual orientation, and indeed the prevailing scientific view is that sexual orientation is fixed before birth (Rahman, 2005, Swaab and Garcia-Falgueras, 2009). It is beyond the scope of this paper to speculate about possible explanations, but elsewhere the first author proposes a mechanism that could potentially explain both the link between childhood sexual abuse and adult nonheterosexuality and depression, as well as the genetic correlation between sexual orientation and depression (Zietsch, submitted).
A dominant biological theory of male sexual orientation is not supported by the present results. The maternal immune hypothesis proposes that some mothers are progressively immunized to male-specific antigens by each succeeding male fetus, lessening sexual differentiation of the brain in each succeeding male foetus (Blanchard, 2004). Firstly, the large opposite-sex twin pair correlation (r=0.43) for sexual orientation in our data suggests that the same familial (i.e. genetic and shared environmental) factors influence both male and female sexual orientation. Secondly, the ‘fraternal birth-order effect’ is central to the theory and has been replicated in numerous other studies (see Blanchard, 2004), but there was no such effect in our large dataset. There may be something about twin births or twin families that nullifies the relationship, though it should also be noted that there was no significant older brother effect in a recent, large (N>11,000), non-twin, probability sample of British young adults (Bogaert, 2010).
This study is not without substantial limitations. Firstly, modeling of the twin correlations suggested no C effects on depression, which appears to contradict the concurrent finding that childhood family-related experiences do affect depression. There are two explanations for this: firstly, a limitation of the classical twin design is that nonadditive genetic effects can mask C effects (i.e. cancel them out); secondly, C only includes factors which are shared within twin pairs - closeness or conflict with parents (items in ‘risky family environment’) may differ between co-twins, and indeed could index a genetic effect if tendency for closeness or conflict with parents has a genetic component. Certainly, various alternative causal explanations could play a role in the link between adverse childhood experiences and sexual orientation and depression, but these are unable to be resolved in our design. Other limitations relate to statistical power. Ideally, we would perform separate analyses for male and female homosexuals and bisexuals, since the relevant mechanisms could differ, but those subsamples were too small for this to be feasible. Notwithstanding, twin pair correlations were remarkably similar across male, female, and opposite-sex twins, suggesting that etiology in males and females has much in common. Another limitation is the lack of assessment of prejudice and discrimination experienced and of lifestyle factors (e.g. relationship history). Further, measurement of adverse childhood experiences was limited by the potential for bias and inaccuracy in retrospective self-reports, and our measurement of sexual orientation was limited by being based on a single item with only three possible categories – more of the population variation would be captured with measures assessing degrees of attraction to each sex, and distinguishing between sexual attraction, fantasy, behavior, and identification (Kinsey et al., 1948). Also, while this was a community-based sample not selected on any of the variables of interest, the fact that some eligible participants refused means that the possibility for non-response bias cannot be discounted.
It should be emphasized that these findings should not be interpreted so as to pathologize nonheterosexuality, any more than we should pathologize non-righthandedness, which is also associated with higher rates of psychiatric disorder (Elias et al., 2001, DeLisi et al., 2002). Research aiming to understand the link between sexual orientation and psychiatric disorder should not be stymied by groups that seek to misuse the findings to support an anti-gay agenda.
Overall, this study yielded several important findings from analysis of a large, genetically-informative, community-based sample with information about sexual orientation, lifetime MDD depression, family composition, close friends, and adverse childhood experiences including sexual abuse. Genetic factors predisposing to nonheterosexuality also predispose to depression, and certain adverse childhood experiences are also associated with both traits; these findings are consistent with common causes of nonheterosexuality and depression contributing to their covariation. However, uncertainty about the causal mechanisms involved in these associations generates many new questions and possibilities that cannot be addressed with our data. In particular, how genetic factors and adverse childhood experience might relate to adult sexual orientation is very unclear. Finally, we again emphasize that our findings regarding potential common causes do not suggest that other mechanisms (e.g. minority stress) are unimportant - a number of mechanisms probably contribute to the elevated psychiatric risk of nonheterosexuals, and a thorough understanding of the problem will require careful investigation of numerous possibilities.
Acknowledgements
We greatly thank the twins for their participation. Thanks also to Naomi Wray for statistical assistance. BPZ is supported by a UQ Postdoctoral Fellowship. KJHV is supported by an ANZ Trustees PhD scholarship in Medical Research. This study was supported in part by grants AA13446 (PI Nelson), AA07728, AA10249, AA11998, and AA13321 (PI Heath), and DA018267 (PI Lynskey) from the National Institutes of Health, Bethesda, Md.
Footnotes
Declaration of interest None
Although the age at abuse and age at first sexual feelings were assessed in different samples, there is no reason for these variables to differ markedly between the two samples.
The authors declare no conflict of interests.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorder, fourth edition, text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Arreola S, Neilands T, Pollack L, Paul J, Catania J. Childhood sexual experiences and adult health sequelae among gay and bisexual men: Defining childhood sexual abuse. Journal of Sex Research. 2008;45:246–252. doi: 10.1080/00224490802204431. [DOI] [PubMed] [Google Scholar]
- Bailey JM. Homosexuality and mental illness. Archives of General Psychiatry. 1999;56:883–884. doi: 10.1001/archpsyc.56.10.883. [DOI] [PubMed] [Google Scholar]
- Balsam KF, Rothblum ED, Beauchaine TP. Victimization over the life span: A comparison of lesbian, gay, bisexual, and heterosexual siblings. Journal of Consulting and Clinical Psychology. 2005;73:477–487. doi: 10.1037/0022-006X.73.3.477. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin. 1995;117:497–529. [PubMed] [Google Scholar]
- Blanchard R. Quantitative and theoretical analyses of the relation between older brothers and homosexuality in men. Journal of Theoretical Biology. 2004;230:173–187. doi: 10.1016/j.jtbi.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Bogaert AF. Physical development and sexual orientation in men and women: An analysis of NATSAL-2000. Archives of Sexual Behavior. 2010;39:110–116. doi: 10.1007/s10508-008-9398-x. [DOI] [PubMed] [Google Scholar]
- Bolton S, Sareen J. Sexual orientation and its relation to mental disorder and suicide attempts: Findings from a nationally representative sample. The Canadian Journal of Psychiatry. 2011;11:35–43. doi: 10.1177/070674371105600107. [DOI] [PubMed] [Google Scholar]
- Cameron P, Cameron K. Does incest cause homosexuality? sychological Reports. 1995;76:611–621. doi: 10.2466/pr0.1995.76.2.611. [DOI] [PubMed] [Google Scholar]
- Chakraborty A, McManus S, Brugha TS, Bebbington P, King M. Mental health of the non-heterosexual population of England. British Journal of Psychiatry. 2011;198:143–148. doi: 10.1192/bjp.bp.110.082271. [DOI] [PubMed] [Google Scholar]
- Cochran SD, Mays VM. Relation between psychiatric syndromes and behaviorally defined sexual orientation in a sample of the US population. American Journal of Epidemiology. 2000;151:516–523. doi: 10.1093/oxfordjournals.aje.a010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi LE, Svetina C, Razi K, Shields G, Wellman N, Crow TJ. Hand preference and hand skill in families with schizophrenia. Laterality. 2002;7:321–332. doi: 10.1080/13576500143000294. [DOI] [PubMed] [Google Scholar]
- Dube SR, Williamson DF, Thompson T, Felitti VJ, Anda RF. Assessing the reliability of retrospective reports of adverse childhood experiences among adult HMO members attending a primary care clinic. Child Abuse & Neglect. 2004;28:729–737. doi: 10.1016/j.chiabu.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Duffy DL, Martin NG. Inferring the direction of causation in cross-sectional twin data: Theoretical and empirical considerations. Genetic Epidemiology. 1994;11:483–502. doi: 10.1002/gepi.1370110606. [DOI] [PubMed] [Google Scholar]
- Elias LJ, Saucier DM, Guylee MJ. Handedness and depression in university students: A sex by handedness interaction. Brain and Cognition. 2001;46:125–129. doi: 10.1016/s0278-2626(01)80048-8. [DOI] [PubMed] [Google Scholar]
- Evans DM, Gillespie NA, Martin NG. Biometrical genetics. Biological Psychology. 2002;61:33–51. doi: 10.1016/s0301-0511(02)00051-0. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults - The adverse childhood experiences (ACE) study. American Journal of Preventive Medicine. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, Horwood LJ. Exposure to childhood sexual and physical abuse and adjustment in early adulthood. Child Abuse & Neglect. 2008;32:607–619. doi: 10.1016/j.chiabu.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Beautrais AL. Is sexual orientation related to mental health problems and suicidality in young people? Archives of General Psychiatry. 1999;56:876–880. doi: 10.1001/archpsyc.56.10.876. [DOI] [PubMed] [Google Scholar]
- Frisell T, Lichtenstein P, Rahman Q, Langstrom N. Psychiatric morbidity associated with same-sex sexual behaviour: influence of minority stress and familial factors. Psychological Medicine. 2010;40:315–324. doi: 10.1017/S0033291709005996. [DOI] [PubMed] [Google Scholar]
- Gilman SE, Cochran SD, Mays VM, Hughes M, Ostrow D, Kessler RC. Risk of psychiatric disorders among individuals reporting same-sex sexual partners in the National Comorbidity Survey. American Journal of Public Health. 2001;91:933–939. doi: 10.2105/ajph.91.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grulich AE, de Visser RO, Smith AMA, Rissel CE, Richters J. Sex in Australia: Homosexual experience and recent homosexual encounters. Australian and New Zealand Journal of Public Health. 2003;27:155–163. doi: 10.1111/j.1467-842x.2003.tb00803.x. [DOI] [PubMed] [Google Scholar]
- Hare EH, Moran PAP. Parental age and birth-order in homosexual patients: Replication of Slater's study. British Journal of Psychiatry. 1979;134:178–182. doi: 10.1192/bjp.134.2.178. [DOI] [PubMed] [Google Scholar]
- Hatzenbuehler ML. How does sexual minority stigma"get under the skin"? A psychological mediation framework. Psychological Bulletin. 2009;135:707–730. doi: 10.1037/a0016441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Hemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Hughes TL, Johnson T, Wilsnack SC. Sexual assault and alcohol abuse: A comparison of lesbians and heterosexual women. Journal of Substance Abuse. 2001;13:515–532. doi: 10.1016/s0899-3289(01)00095-5. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Neale MC, Prescott CA. Genetic risk factors for major depression in men and women: similar or different heritabilities and same or partly distinct genes? Psychological Medicine. 2001;31:605–616. doi: 10.1017/s0033291701003907. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for major depression in women. American Journal of Psychiatry. 2002;159:1133–1145. doi: 10.1176/appi.ajp.159.7.1133. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for major depression in men. American Journal of Psychiatry. 2006;163:115–124. doi: 10.1176/appi.ajp.163.1.115. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Prescott CA. Childhood sexual abuse, stressful life events and risk for major depression in women. Psychological Medicine. 2004;34:1475–1482. doi: 10.1017/s003329170400265x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. The lifetime history of major depression in women: Reliability of diagnosis and heritability. Archives of General Psychiatry. 1993a;50:863–870. doi: 10.1001/archpsyc.1993.01820230054003. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A longitudinal twin study of personality and major depression in women. Archives of General Psychiatry. 1993b;50:853–862. doi: 10.1001/archpsyc.1993.01820230023002. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A test of the equal-environment assumption in twin studies of psychiatric illness. Behavior Genetics. 1993c;23:21–27. doi: 10.1007/BF01067551. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, Gilman SE, Kessler RC. Sexual orientation in a US national sample of twin and nontwin sibling pairs. American Journal of Psychiatry. 2000;157:1843–1846. doi: 10.1176/appi.ajp.157.11.1843. [DOI] [PubMed] [Google Scholar]
- King M, Semlyen J, Tai SS, Killaspy H, Osborn D, Popelyuk D, Nazareth I. A systematic review of mental disorder, suicide, and deliberate self harm in lesbian, gay and bisexual people. BMC Psychiatry. 2008;8:70. doi: 10.1186/1471-244X-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey AC, Pomeroy WB, Martin CE. Sexual behavior in the human male. Philadelphia: W. B. Saunders; 1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Madden PAF, Bucholz KK, Slutske WS, Nelson EC, Statham D, Whitfield JB, Martin NG. Genetic effects on alcohol dependence risk: re-evaluating the importance of psychiatric and other heritable risk factors. Psychological Medicine. 2004;34:1519–1530. doi: 10.1017/s0033291704002922. [DOI] [PubMed] [Google Scholar]
- Langstrom N, Rahman Q, Carlstrom E, Lichtenstein P. Genetic and environmental effects on same-sex sexual behavior: A population study of twins in Sweden. Archives of Sexual Behavior. 2010;39:75–80. doi: 10.1007/s10508-008-9386-1. [DOI] [PubMed] [Google Scholar]
- Levinson DF. The genetics of depression: A review. Biological Psychiatry. 2006;60:84–92. doi: 10.1016/j.biopsych.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Lewis NM. Mental health in sexual minorities: Recent indicators, trends, and their relationships to place in North America and Europe. Health & Place. 2009;15:1029–1045. doi: 10.1016/j.healthplace.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Loehlin JC. The Cholesky approach: A cautionary note. Behavior Genetics. 1996;26:65–69. [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Mays VM, Cochran SD. Mental health correlates of perceived discrimination among lesbian, gay, bisexual adults in the United States. American Journal of Public Health. 2001;91:1869–1876. doi: 10.2105/ajph.91.11.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer IH. Minority stress and mental-health in gay men. Journal of Health and Social Behavior. 1995;36:38–56. [PubMed] [Google Scholar]
- Meyer IH. Prejudice, social stress, and mental health in lesbian, gay, bisexual populations: Conceptual issues and research evidence. Psychological Bulletin. 2003;129:674–697. doi: 10.1037/0033-2909.129.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills TC, Paul J, Stall R, Pollack L, Canchola J, Chang YJ, Moskowitz JT, Catania JA. Distress and depression in men who have sex with men: The urban men's health study. American Journal of Psychiatry. 2004;161:278–285. doi: 10.1176/appi.ajp.161.2.278. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical modeling. VCU Box 900126, Richmond, VA 23298: Department of Psychiatry; 2006. [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Boston: Kluwer; 1992. [Google Scholar]
- Nelson EC, Heath AC, Madden PAF, Cooper ML, Dinwiddie SH, Bucholz KK, Glowinski A, McLaughlin T, Dunne MP, Statham DJ, Martin NG. Association between self-reported childhood sexual abuse and adverse psychosocial outcomes - Results from a twin study. Archives of General Psychiatry. 2002;59:139–145. doi: 10.1001/archpsyc.59.2.139. [DOI] [PubMed] [Google Scholar]
- Ooki S, Yamada K, Asaka A, Hayakawa K. Zygosity diagnosis of twins by questionnaire. Acta Geneticae Medicae et Gemellologiae. 1990;39:109–115. doi: 10.1017/s0001566000005626. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends in Neurosciences. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Paul JP, Catania J, Pollack L, Stall R. Understanding childhood sexual abuse as a predictor of sexual risk-taking among men who have sex with men: The Urban Men's Health Study. Child Abuse & Neglect. 2001;25:557–584. doi: 10.1016/s0145-2134(01)00226-5. [DOI] [PubMed] [Google Scholar]
- Pillard RC, Bailey JM. Human sexual orientation has a heritable component. Human Biology. 1998;70:347–365. [PubMed] [Google Scholar]
- Posthuma D, Beem AL, de Geus EJC, van Baal GCM, von Hjelmborg JB, Lachine I, Boomsma DI. Theory and practice in quantitative genetics. Twin Research. 2003;6:361–376. doi: 10.1375/136905203770326367. [DOI] [PubMed] [Google Scholar]
- Rahman Q. The neurodevelopment of human sexual orientation. Neuroscience and Biobehavioral Reviews. 2005;29:1057–1066. doi: 10.1016/j.neubiorev.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–366. [PubMed] [Google Scholar]
- Romeo RD. Pubertal maturation and programming of hypothalamic-pituitary-adrenal reactivity. Frontiers in Neuroendocrinology. 2010;31:232–240. doi: 10.1016/j.yfrne.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Rothman EF, Exner D, Baughman AL. The prevalence of sexual assault against people who identify as, gay, lesbian, or bisexual in the United States: A systematic review. Trauma Violence & Abuse. 2011;12:55–66. doi: 10.1177/1524838010390707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandfort TGM, de Graaf R, Bijl RV, Schnabel P. Same-sex sexual behavior and psychiatric disorders: Findings from the Netherlands Mental Health Survey and Incidence Study (NEMESIS) Archives of General Psychiatry. 2001;58:85–91. doi: 10.1001/archpsyc.58.1.85. [DOI] [PubMed] [Google Scholar]
- Santtila P, Sandnabba NK, Harlaar N, Varjonen M, Alanko K, von der Pahlen B. Potential for homosexual response is prevalent and genetic. Biological Psychology. 2008;77:102–105. doi: 10.1016/j.biopsycho.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Sell RL, Wells JA, Wypij D. The prevalence of homosexual behavior and attraction in the United States, the United Kingdom and France: Results of national population-based samples. Archives of Sexual Behavior. 1995;24:235–248. doi: 10.1007/BF01541598. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: Review and meta-analysis. American Journal of Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Garcia-Falgueras A. Sexual differentiation of the human brain in relation to gender identity and sexual orientation. Functional Neurology. 2009;24:17–28. [PubMed] [Google Scholar]
- Tomeo ME, Templer DI, Anderson S, Kotler D. Comparative data of childhood and adolescence molestation in heterosexual and homosexual persons. Archives of Sexual Behavior. 2001;30:535–541. doi: 10.1023/a:1010243318426. [DOI] [PubMed] [Google Scholar]
- Yancura LA, Aldwin CM. Stability and Change in Retrospective Reports of Childhood Experiences Over a 5-Year Period: Findings from the Davis Longitudinal Study. Psychology and Aging. 2009;24:715–721. doi: 10.1037/a0016203. [DOI] [PubMed] [Google Scholar]
- Young E, Korszun A. Sex, trauma, stress hormones and depression. Molecular Psychiatry. 2010;15:23–28. doi: 10.1038/mp.2009.94. [DOI] [PubMed] [Google Scholar]
- Zietsch BP. Explanations for elevated psychiatric vulnerability in nonheterosexuals: Environmental stressors, genetics, and the HPA and HPG axes. In: Uehara T, editor. Psychiatric Disorders. Rijeka: InTech; (submitted). [Google Scholar]
- Zietsch BP, Morley KI, Shekar SN, Verweij KJH, Keller MC, Macgregor S, Wright MJ, Bailey JM, Martin NG. Genetic factors predisposing to homosexuality may increase mating success in heterosexuals. Evolution and Human Behavior. 2008;29:424–433. [Google Scholar]
- Zietsch BP, Verweij KJH, Bailey JM, Wright MJ, Martin NG. Sexual orientation and psychiatric vulnerability: a twin study of Neuroticism and Psychoticism. Archives of Sexual Behavior. 2009 doi: 10.1007/s10508-009-9508-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]