Abstract
We are studying the effectiveness of a semicircular canal prosthesis to improve postural control, perception of spatial orientation, and the VOR in rhesus monkeys with bilateral vestibular hypofunction. Balance is examined by measuring spontaneous sway of the body during quiet stance and postural responses evoked by head turns and rotation of the support surface; perception is measured with a task derived from the subjective visual vertical (SVV) test during static and dynamic rotation in the roll plane; and the angular VOR is measured during rotation about the roll, pitch, and yaw axes. After the normal responses are characterized, bilateral vestibular loss is induced with intratympanic gentamicin, and then multisite stimulating electrodes are chronically implanted into the ampullae of all three canals in one ear. The postural, perceptual, and VOR responses are then characterized in the ablated state, and then bilateral, chronic electrical stimulation is applied to the ampullary nerves using a prosthesis that senses angular head velocity in three-dimensions and uses this information to modulate the rate of current pulses provided by the implanted electrodes. We are currently characterizing two normal monkeys with these paradigms, and vestibular ablation and electrode implantation are planned for the near future. In one prior rhesus monkey tested with this approach, we found that a one-dimensional (posterior canal) prosthesis improved balance during head turns, perceived head orientation during roll tilts, and the VOR in the plane of the instrumented canal. We therefore predict that the more complete information provided by a three-dimensional prosthesis that modulates activity in bilaterally-paired canals will exceed the benefits provided by the one-dimensional, unilateral approach used in our preliminary studies.
I. Introduction
Clinical vestibular disorders are very common and debilitating, and often are unresponsive to the available modes of therapy. For these reasons, there is growing interest in the development of vestibular prosthetic implants, a new approach to treat vestibular symptomatology. We are developing and testing a vestibular prosthesis that senses head rotation and provides this information to the brain by electrically stimulating canal ampullary nerves. Our work in the past has focused on the vestibulo-ocular reflex (VOR) elicited by chronic electrical stimulation of lateral canal afferents in the squirrel monkey, and we have demonstrated that a one-dimensional prosthetic device elicits a compensatory VOR that reduces image motion on the retina during head rotations. To develop a clinically useful prosthesis, however, we must expand the prosthesis to three dimensions and must determine if the prosthesis can improve more complex, integrative behaviors such as balance and percepts of head orientation, in addition to reflexive eye movements, when normal vestibular cues are impaired or absent.
Patients and experimental animals with vestibular dysfunction demonstrate abnormal postural control, particularly when visual and proprioceptive cues are unreliable, and in response to head turns and rotation of the support surface. We are therefore investigating if a vestibular prosthesis that senses head rotation in three dimensions and stimulates all three canal ampullary nerves in one ear can improve postural control in vestibulopathic rhesus monkeys. We first characterize normal monkeys during quadrupedal stance on a balance platform when visual and somatosensory cues are modified, and during head rotations and tilts of the support surface. These measurements will be repeated after bilateral vestibular deficits are introduced with intra-tympanic (IT) gentamicin, and after the vestibular prosthesis is implemented in the vestibulopathic monkey. We predict that bilateral vestibular hypofunction will increase the amplitude of body sway when visual and somatosensory cues are minimized and in response to head turns and tilts of the support the support surface and that these postural measures will improve after rotational cues are re-introduced with the prosthesis.
There is extensive evidence that the rotational information derived from the canals and the graviceptive information derived from the otolith organs and other sensors are synthesized by the brain to generate relatively accurate percepts of head orientation and motion in normal subjects. In patients and animals with peripheral vestibular deficits, however, these perceptual estimates can be severely impaired. We are therefore investigating roll tilt psychophysics during static and dynamic roll tilts, and predict that bilateral vestibular deficits will result in less accurate perceptual estimates of head orientation in the roll plane. After three-dimensional canal rotational cues are chronically re-introduced with the canal prosthesis, we predict that the brain will learn to use this information to make more accurate perceptual estimates of head orientation in the roll plane.
Finally, the semicircular canals provide the sensory information that drives the angular VOR, and this response is markedly impaired in patients and experimental animals with bilateral vestibular loss. Our results from squirrel monkeys suggest that a compensatory angular VOR in one dimension can be produced by a canal prosthesis. We are therefore measuring the angular VOR during rotations in the yaw, roll, and pitch planes before and after bilateral canal cues are attenuated. After three-dimensional canal rotational cues are chronically reintroduced with a canal prosthesis, we predict that the brain will use this information to improve the three-dimensional angular VOR.s.
II. Methods
A. Prosthesis
Details of the prosthesis design and implementation have been previously published [1,2] and only briefly reviewed here. The prosthesis senses angular head velocity in one-dimension (our initial studies) or three-dimensions (our current studies), this information is high-pass filtered with a cut-off frequency of 0.03 Hz (to simulate the dynamics of the normal rhesus semicircular canals), and the filtered head velocity is used to modulate the rate of biphasic current pulses applied to the canal ampullary nerve. The baseline rate of electrical stimulation is chosen to be well above the normal tonic firing rate for canal afferents (usually 200 or 250 Hz) and the rate modulates upwards and downwards for head rotations in the plane of the instrumented canal(s) that normal increase or decrease the firing rate in the ampullary nerve. This modulation is based on angular head velocity and is calculated from a hyperbolic tangent function which is relatively linear over the central range of head velocities but saturates at larger velocities. With this approach we can provide bidirectional rotational cues with a unilateral implant.
B. Experimental Paradigm
The three behaviors described above are characterized in the normal monkey and again after vestibular function is ablated in both ears with aminoglycosides (referred to as the bilateral vestibular hypofunction, or BVH state). The behaviors are then re-measured during chronic electrical stimulation with the canal prosthesis to determine if they are improved by the prosthesis. The individual behaviors and associate tests are:
Posture: sway during quiet stance is measured with motion sensors on the animal’s head and trunk and with force sensors on the four footplates. The animals are tested in four conditions, with the footplates wide or narrow in the medial-lateral plane and with the support surface covered with thin rubber or thick foam. These sway measures are also made during voluntary head turns [3] between visual targets and during pseudorandom tilt of the support surface in the earth-horizontal roll plane. The motion is based on a pseudorandom ternary sequence (PRTS) [4] and covers a bandwidth of 0.05 to 2.5 Hz with peak-to-peak platform tilt ranging from 0.5 to 8 deg.
Perception: The monkeys are trained to align a light bar with the earth-vertical by rotating a small steering wheel. This task is based on the subjective visual vertical (SVV) test used in human patients and research. They perform this task during static tilt in the roll plane [5] and during dynamic sinusoidal rotation in the roll plane over a frequency range of 0.005 to 0.4 Hz. For dynamic testing the position of the light bar is continuously perturbed from the upright by filtered Gaussian noise and the response to the wheel turn is unstable since the light bar accelerates in the direction it is rotated.
VOR: In the monkey chronically stimulated with the one-dimensional prosthesis, the electrical stimulation was provided to the right posterior canal and the VOR was therefore measured during rotation about the roll and LARP (left anterior-right posterior) planes. For the current monkeys, which will obtain three-dimensional prosthesis, the VOR is measured about the roll, pitch, and yaw axes.
III. Results
A. Posture
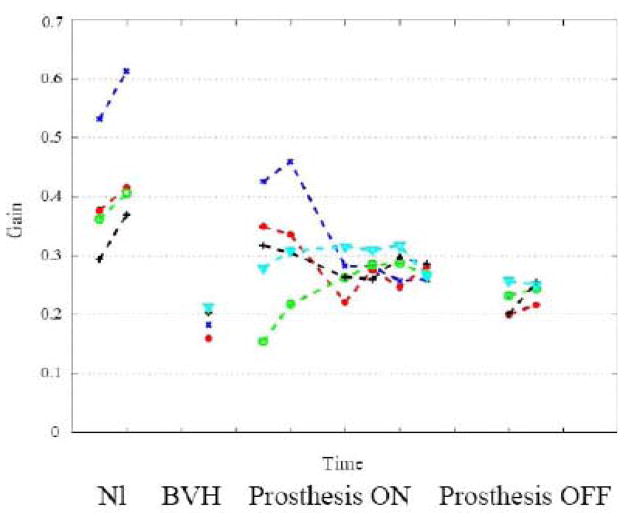
Sway during quiet stance in the normal monkey increased as the conditions were made more difficult, namely when the support surface width was narrowed and when the monkey stood on foam (which distorted somatosensory inputs form the legs) compared to the thin gum rubber (Figure 1, NL). After vestibular ablation (BVH state), sway increased, particular when standing on foam rubber (Figure 1). During chronic one-dimensional stimulation, the monkey was retested only on the most difficult condition and we found no reduction in sway. These results indicate that while we can characterize the vestibular contribution to postural control during quiet stance, one-dimensional canal stimulation does not appear adequate to improve postural stability.
Fig. 1.
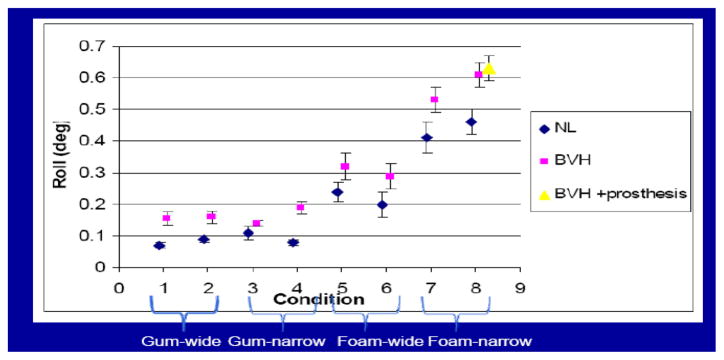
Root-mean-square of roll tilt of the trunk during quiet stance in the four conditions.
Sway induced by voluntary head turns was not measured in the normal monkey, only in the BVH and BVH + prosthetic stimulation states. We found that roll tilt of the trunk was reduced during head turns when prosthetic stimulation was provided, with the mean trunk tilt deceasing from 1.7 +/− 0.07 deg in the BVH state to 0.8 +/− 0.2 deg with the prosthesis.
Finally, trunk roll tilt during PRTS tilt of the platform has been performed only in normal monkeys to date, but shows similar patterns to findings in humans, with the animal tilting with the platform for lower amplitude tilts but then orientating more towards a gravitational upright position with larger tilts. Our presumption is that after ablation the animals, like vestibulopathic humans, will tilt with the platform even with large tilts, as the gravitational cue will be degraded, and will normalize in part during chronic prosthetic stimulation.
B. Perception
The monkeys accurately indicated the upright orientation during roll tilt in the normal state, but were about 50% less sensitive to head tilt after vestibular ablation (Figure 2). Chronic one-dimensional stimulation modestly improved the perception of upright, evidenced by a reduction in the SVV error (Figure 2). During dynamic roll tilt the monkeys could accurately estimate the earth vertical and had tilt gains (perceived tilt/head tilt) of between 0.8 and 0.9, which was relatively independent of frequency.
Fig. 2.
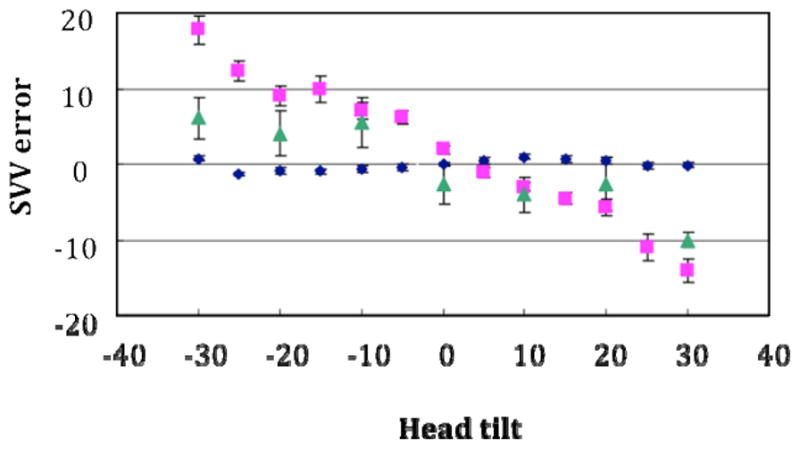
SVV error (e.g. deviation of the arrow from the true earth-vertical) during static head tilt in the roll plane. Head tilts towards the right-ear-down position are positive and SVV errors that are clockwise are negative. The results demonstrate that the SVV error was small in the normal monkey (blue diamonds), increased substantially in the BVH state (magenta square), and was reduced with prosthetic stimulation (green triangle).
C. Eye Movements
VOR gains were normal prior to ablation, decreased in the BVH state, and increased modestly with chronic one-dimensional prosthetic stimulation (Figure 3).
Fig. 3.
Vertical VOR measured during sinusoidal rotation about the LARP axis over a frequency range of 0.1 to 1.0 Hz.
IV. Conclusions
We have demonstrated that we can accurately assess posture, perception of head orientation, and the vestibulo-ocular reflex in rhesus monkeys in the normal state and can characterize the deficits in these behaviors that are produced by bilateral vestibular ablation. Preliminary studies with a one-dimensional prosthesis demonstrate that postural stability during head turns, perceived head orientation during roll tilts, and the VOR can all be improved with a canal prosthesis. Current work is focused on characterizing posture, perception, and eye movements with more complex and definitive paradigms and testing the ability of a three-dimensional prosthesis to improve these behavioral parameters.
Acknowledgments
This work was supported by the NIH National Institute of Deafness and other Communications Grant DC008362 and by the CLONS Project of the European Commission under Grant EU FP7 ICT 225929.
Contributor Information
Richard F. Lewis, Email: richard_lewis@meei.harvard.edu, Jenks Vestibular Physiology Laboratory (JVPL) at the Massachusetts Eye and Ear Infirmary (MEEI) and the Department of Otology and Laryngology at the Harvard Medical School (HMS), Boston MA 02114 USA (phone: 617-573-3620; fax: 617-573-5596
Csilla Haburcakova, Email: csilla_haburcakova@meei.harvard.edu, JVPL at the MEEI, Boston MA 02114 USA.
Wangsong Gong, Email: wangsong_gong@meei.harvard.edu, JVPL at the MEEI, Boston MA 02114 USA.
Daniel Lee, Email: daniel_lee@meei.harvard.edu, Department of Otology and Laryngology at the HMS, Boston MA 02114 USA.
Conrad Wall, III, Email: cwall@mit.edu, Director of the Jenks Vestibular Diagnostic Laboratory at the MEEI and a member of the Department of Otology and Laryngology at the HMS, Boston MA 02114 USA.
Lara Thompson, Email: lthomps@mit.edu, JVPL at the MEEI and a doctoral student in the Harvard-MIT Program in Speech and Hearing Bioscience and Technology, Boston MA 02114 USA.
Daniel M. Merfeld, Email: dan_merfeld@meei.harvard.edu, Daniel M. Merfeld is the Director of the JVPL at the MEEI and a member of the Department of Otology and Laryngology at the HMS, Boston MA 02114 USA.
References
- 1.Gong W, Merfeld DM. Prototype neural semicircular canal prosthesis using patterned electrical stimulation. Ann Biomed Eng. 2000;28:572–581. doi: 10.1114/1.293. [DOI] [PubMed] [Google Scholar]
- 2.Gong W, Merfeld DM. System design and performance of a unilateral horizontal semicircular canal prosthesis. IEEE Trans Biomed Eng. 2002;49:175–181. doi: 10.1109/10.979358. [DOI] [PubMed] [Google Scholar]
- 3.Stapley PJ, Ting LH, Kuifu C, Everaert DG, Machpherson JM. Bilateral vestibular loss leads to active destabilization of balance during voluntary head turns. J Neurophysiol. 2006;95:3783–3797. doi: 10.1152/jn.00034.2006. [DOI] [PubMed] [Google Scholar]
- 4.Peterka RJ. Sensorimotor integration in human posture control. J Neurophysiol. 2002;88:1097–1118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- 5.Lewis RF, Haburcakova C, Merfeld DM. Roll tilt psychophysics in rhesus monkeys during vestibular and visual stimulation. J Neurophysiol. 2008;100:140–153. doi: 10.1152/jn.01012.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]