SUMMARY
The aims of this study were to: (i) assess gender differences of objective sleep patterns in a general population sample; (ii) evaluate the effects of menopause and hormone treatment (HT) on the sleep of the same cohort; and (iii) examine gender differences in sleep resilience towards external stressors. The participants were (i) 1324 subjects without sleep complaints, recruited from the general population of Central Pennsylvania that spent one night in the sleep laboratory and (ii) 66 young, healthy volunteers whose sleep was disturbed during night four by an external stressor, i.e. 24-h blood drawing (average of nights 2 and 3 versus night 4). Women compared with men in the general population sample had significantly higher percentage of sleep time, lower percentage of stage 1, and higher percentage of slow wave sleep. Also, menopause, in the absence of HT, was associated with prolonged sleep latency and decreased deep sleep. Finally, young, healthy women compared with men experienced less sleep disturbance because of blood draws as indicated by a significantly smaller change in per cent sleep time, and percentage of stage 1 sleep. These findings suggest that women without sleep complaints sleep objectively better across age than men and the sleep of young women is more resistant to external stressors. Also, gonadal hormones exert a beneficial effect on women’s sleep. This gender dimorphism in sleep regulation may have been to protect women from the demands of infant and child care, and in part, might contribute to women’s lower cardiovascular risks and greater longevity.
Keywords: gender, menopause, sleep
INTRODUCTION
Several studies have established that the prevalence of two of the most common sleep disorders is sexually dimorphic. For example, women are at an increased risk for insomnia compared with men. In contrast, women have a lower risk for sleep-disordered breathing (SDB) than men (Bixler et al., 2003; Lugaresi et al., 1993; Shapiro and Dement, 1993). This difference is larger during premenopausal years (8 : 1), whereas it decreases considerably after menopause (1.6 : 1). Hormones and psychopathology have been postulated to be the main factors that affect the differing gender risks for SDB and insomnia, respectively. Furthermore, women appear to be more resilient to the effects of sleep loss-induced elevation of circulating inflammatory cytokines, and this might contribute to their increased longevity compared with men (Vgontzas et al., 2004).
Studies on the effect of gender on the sleep patterns in humans are limited. Previous reports were based on small samples of select populations (Bliwise, 2000; Roehrs et al., 2006), with two recent publications based on the general population of the Sleep Heart Health Study (SHHS) (Redline et al., 2004; Walsleben et al., 2004). The latter two studies demonstrated that women, in general, sleep better than men. However, one limitation of this cohort is that it did not include a wide age range, primarily focusing on middle-aged and older individuals. Furthermore, there have been two studies that reported no effects of menopause and hormone treatment (HT) on objective sleep patterns (Shahar et al., 2003; Young et al., 2003). This is in contrast to the frequent subjective complaints of disturbed sleep by women entering the menopause phase of life (Kravitz et al., 2003; Kuh et al., 1997; Matthews et al., 1990; Young et al., 2003).
In several of our projects that include 24-h serial blood sampling it has become evident, consistent with published reports (Adam, 1982; Jarrett et al., 1984; Kerkhofs et al., 1989; Vitiello et al., 1996), that this procedure is associated with some sleep disturbance. However, the possibility of a sexual dimorphic effect in young healthy men and women has not been assessed adequately.
Thus, the objectives of this report are: (i) to assess the gender differences of objective sleep patterns in a large cohort of the normally sleeping general population (Penn State Cohort) over a wide age range (20–88 years); (ii) to evaluate the objective sleep patterns associated with menopause and HT in the same cohort; and (iii) to examine potential gender differences in terms of sleep disturbance because of an external stressor, i.e. blood drawing through an intravenous catheter in young, healthy men and women.
METHODS
Study I: Normal sleep in a general random sample: gender and age effects
The data presented here were collected as part of a two-phase protocol for the assessment of the prevalence of sleep apnea in adult men and women. The data for men and women were collected separately in two identical protocols and have previously been reported (Bixler et al., 1998, 2001).
Our analysis was based on a two-stage random sample of men and women. In the first phase, 16 583 men and women, ranging in age from 20 to 100 years, were interviewed over the telephone with an overall response rate of 73.9%.
In the second phase of this study, a subsample of 1741 men and women randomly selected from those subjects previously interviewed by telephone were studied in the sleep laboratory for a single night with an overall response rate for this phase of 66.7%. We contrasted those subjects who were recorded in the laboratory with those who were selected but not recorded in terms of age, body mass index (BMI) and prevalence of sleep disorders. There were no significant differences between these two groups on any of these variables. Each subject selected for laboratory evaluation completed a comprehensive sleep history and physical examination.
In order to evaluate sleep and sleep stages in normal sleepers, from the sample of 1741 men and women we excluded subjects with sleep disorders. Specifically, we excluded those with a complaint of excessive daytime sleepiness, insomnia and those with an apnea/hypopnea index (A/HI) ≥5 and obesity-hypoventilation syndrome. The presence of insomnia was based on a complaint of ‘insomnia’ with a duration of at least 1 year. The presence of excessive daytime sleepiness was established based on a moderate or severe rating on the following question: ‘Do you feel drowsy or sleepy most of the day but manage to stay awake?’ This resulted in a sample of 1324 subjects, 609 men and 715 women, with a mean age of 48.0 ± 13.6 (range = 20–88) and an average BMI of 26.9 ± 5.1 (range = 18.0–68.0) (Table 1).
Table 1.
Demographic and sleep characteristics of the Penn State Cohort (study I)
| Men (n = 609) | Women (n = 715) | |
|---|---|---|
| Age | 49.1 ± 13.8 | 47.0 ± 13.3 |
| BMI | 26.6 ± 4.0 | 27.1 ± 5.9 |
| TIB (min) | 475.4 ± 0.7 | 477.0 ± 0.6 |
| SL (min) | 31.7 ± 1.3 | 32.7 ± 1.2 |
| WTASO (min) | 92.2 ± 2.2 | 77.9 ± 1.9 |
| %ST | 74.0 ± 0.6 | 76.8 ± 0.5* |
| %Stage 1 | 14.1 ± 0.3 | 5.4 ± 0.2* |
| %Stage 2 | 68.2 ± 0.3 | 71.7 ± 0.3 |
| %SWS | 3.0 ± 0.3 | 5.0 ± 0.2* |
| %REM | 16.7 ± 0.3 | 17.9 ± 0.3 |
| REM latency (min) | 104.9 ± 2.5 | 138.3 ± 3.0* |
| A/HI | 0.6 ± 0.05 | 0.3 ± 0.03* |
BMI, body mass index; SL, sleep latency; WTASO, wake time after sleep onset; ST, sleep time; SWS, slow wave sleep; REM, rapid eye movement; TIB, time in bed; A/HI, apnea/hypopnea index.
P < 0.05.
Study II: Effects of menopause and HT on objective sleep
In the same cohort, we evaluated the effects of menopause and HT on objective sleep. For analysis purposes, we grouped the women into premenopausal (n = 400, mean ± SD age 38.2 ± 7.0), postmenopausal with HT (n = 120, mean ± SD age 56.3 ± 9.4), and postmenopausal without HT (n = 196, mean ± SD age 59.2 ± 11.6). Menopausal status was assessed by self-reported menstrual history, history of hysterectomy and oophorectomy, and use of HT. Menopause was defined as a history of amenorrhoea for at least 12 months or complete hysterectomy performed 6 or more months previously. In order to use the men in our sample as controls, we established two groups based on the fact that most women become menopausal between the ages of 45 and 55. For comparison to premenopausal women, we selected men younger than 55 years of age (n = 332, mean ± SD age 39.9 ± 9.3). For comparison to postmenopausal women, we selected men older than 45 years of age (n = 277, mean ± SD age 60.0 ± 9.9).
Study III: Effects of blood draw on night-time sleep in young men and women
In study III, we included 66, young, healthy, normal sleepers (32 men and 34 women) of similar age (mean age ± SD, 23.5 ± 3.7, range 19–34 for men; 24.4 ± 3.3, range 19–31 for women) and BMI (mean BMI ± SD, 24.1 ± 2.3, range 20.8–29.3 for men; 23.5 ± 2.6, range 18.3–29.3 for women), who participated in research protocols that included four consecutive nights in the sleep laboratory and a 24-h blood draw. They were recruited from the community and from the medical and technical staff and students of the Milton S. Hershey Medical Center. They were in good general health, not exercising excessively, had no sleep complaints or circadian abnormalities, were not taking any medications and were screened in the sleep laboratory for SDB, nocturnal myoclonus or other primary sleep disorders. Also, a battery of clinical tests, including full blood count, urinalysis, thyroid indices and electro-cardiogram, were negative for abnormal findings.
Each subject participated in a four consecutive-night sleep laboratory protocol: night 1 served as an adaptation night, nights 2 and 3 served as baseline sleep measures, and a fourth (catheter) night was associated with 24-h blood sampling. Blood sampling was performed serially every 30 min. An indwelling catheter was inserted in the antecubital vein about 30 min before the first blood sample. The catheter was kept patent with small amounts of heparin. During the sleep recording period, blood was collected outside the subjects’ room through a perforation in the wall, via extra tubing, in order to decrease sleep disturbance from the blood sampling technique. During the day, blood samples were taken in the Clinical Research Center of the University Hospital of the Milton S. Hershey Medical Center.
These studies were reviewed and approved by the Institutional Review Board and each subject signed a written consent form.
Sleep (polysomnographic) recordings
Sleep laboratory recordings were carried out in a sound-attenuated, light- and temperature-controlled room with a comfortable bedroom-like atmosphere. During this evaluation, each subject was monitored continuously for 8 h (c. 22:30–06:30 hours). The sleep schedule in the sleep laboratory was similar to the subjects’ normal sleep schedule. The polysomnographic recording included electroencephalogram, electrooculogram and electromyogram, using standard clinical polygraphs (Grass Instrument Co., Model 78d & e; West Warwick, RI, USA). The sleep records were scored independently of any knowledge of the experimental condition according to standardized criteria (Rechtschaffen and Kales, 1968). Sleep parameters assessed from the sleep recordings included sleep latency (SL); wake time after sleep onset (WTASO); total wake time which is the sum of SL and WTASO; %ST which is total sleep time as percentage of time in bed; and percentage of the various sleep stages [rapid eye movement (REM), 1, 2 and stages 3 and 4 combined (slow wave sleep, SWS)] which is calculated as the minutes in each stage as the percentage of total sleep time.
Statistical analyses
For comparison between genders over age, the multiple regression analysis was performed to adjust for potential confounding factors. Specifically, we controlled for BMI, menopause and HT, hypertension, diabetes, depression (current treatment or suicidal thoughts or attempts), smoking (current use of any type of tobacco product), alcohol (more than 2 alcoholic drinks per day) and race. Because approximately 58% of the men and 39% of the women had no SWS (P < 0.001), logistic regression analysis was performed for those without SWS. All analyses using the population dataset were adjusted for sampling weight (Bixler et al., 2001).
To assess the influence of menopause and HT on objective sleep measures, the analysis between groups used the General Linear Model controlling for age, BMI, hypertension, diabetes, depression, smoking, alcohol and race. Separate models were assessed for pre- and postmenopausal women. Because women tend to become menopausal between the ages of 45 and 55 years, we randomly selected two subsets of men within this age range in order to not use men twice in the two analyses. The post hoc comparisons among the three groups within the postmenopausal model were adjusted using the Bonferroni correction.
In the study for the assessment of the effects of blood draw on night-time sleep, we first compared differences in sleep parameters between baseline (average of nights 2 and 3) and night 4, for the entire group as well as within each gender group, using paired two-tailed Student’s t-test. Based on the Kolmogorov–Smirnov test for normality of distributions, when data were not normally distributed (baseline SL in the entire sample, as well as baseline SL within the men’s sample), comparisons were made using the non-parametric Wilcoxon signed-ranks tests. Secondly, we compared the differences of the change of sleep variables as a result of the blood draw (night 4 minus the average of nights 2 and 3) between men and women after adjusting for baseline sleep values, by using the ancova. We corrected for multiple comparisons by using the Bonferroni post hoc test.
RESULTS
Study I: Normal sleep in a general random sample: gender and age effects
Women compared with men had overall a significantly higher per cent sleep time, higher per cent slow wave, lower percentage of stage 1 sleep and longer REM latency (all P < 0.01). There were no significant differences between the two genders in terms of SL or stage 2 sleep.
In the multiple regression analysis, the percentage of sleep time was negatively associated with increasing age in both genders (Fig. 1). A linear decrease was observed in men and this trend was significant (P < 0.001). The decrease in the women’s group was quadratic (P = 0.007): per cent sleep time declined at a non-significant rate until the age of 50, whereas there was a significant drop thereafter. There were no significant differences between men and women in terms of amount of sleep between the ages of 20–49 years. In the decades of 50–69, women had higher percentage of sleep time (the predicted difference at age 50 is 2.5%, P = 0.025; and at age 60 is 2.2%, P = 0.077), whereas sleep time was similar between the two genders between the ages of 70 and 79 years (Fig. 1).
Figure 1.
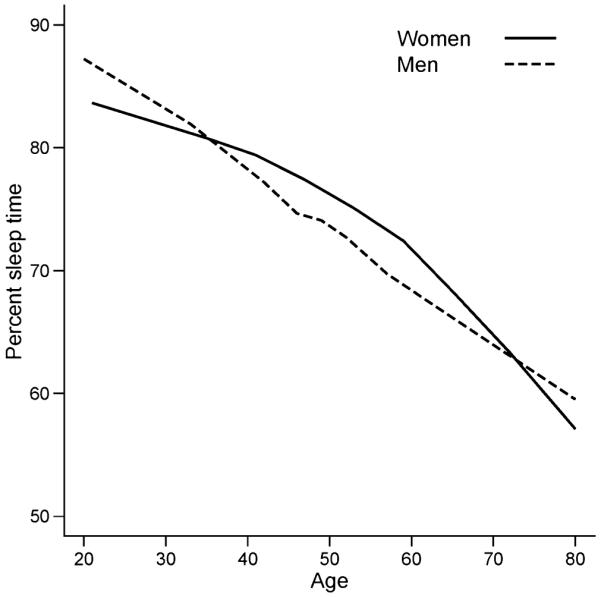
Age-specific prevalence of %sleep time in men and women across a wide age range, with no complaints of disturbed sleep.
Percentage of stage 1 sleep (log stage 1) was lower in women compared with men across the entire age spectrum (P < 0.0001). It was positively, linearly and significantly associated with age in both gender groups (Fig. 2). The per cent SWS was significantly higher in women compared with men across all ages (Fig. 2). The odds of having no SWS increased by 43% for men (P = 0.03), and decreased by 63% for postmenopausal women receiving HT (P < 0.001). There was no difference in per cent REM sleep between the genders (P < 0.51). REM latency (transformed to its square root) was longer in women compared with men across all ages (all P < 0.05) and quadratic models for age were observed for both genders (P = 0.02 for men and P = 0.001 for women, respectively).
Figure 2.
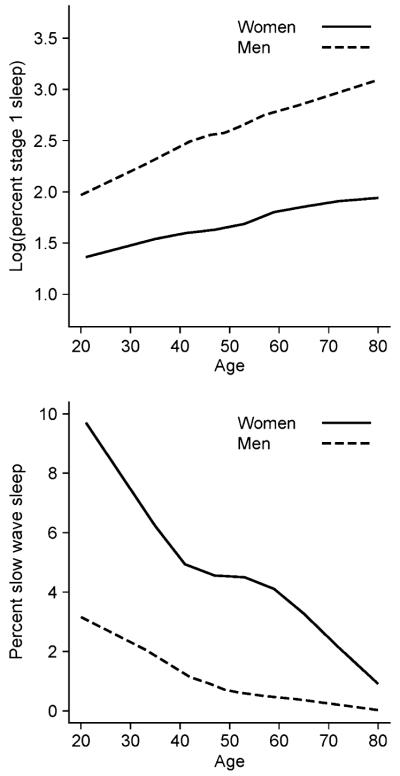
Per cent stage 1 sleep (log transformed) (top) and per cent slow wave sleep (bottom) of men and women, aged 20–88 years, in the Penn State Cohort in men and women who had no sleep complaints.
Study II: Effects of menopause and HT on objective sleep
The multiple regression analysis assessing the association between age and %ST suggested that menopause may be a contributing factor to some of the differences observed (Fig. 1). Specifically, in this analysis, a linear reduction in %ST was associated with increasing age in men. However, in women the association between %ST and age was quadratic, i.e. there was a non-significant decrease of %ST associated with ages <50, followed by a significance decrease associated with ages >50. As most women become menopausal between 45 and 55 years, we examined whether menopause might explain the change of the association between %ST with age at the age of 50. Further, %ST was significantly higher in women in the decade of 50–59, lost its significant in the decade of 60–69, and became similar between the two genders after age 70. Given that most women on HT use hormones during the first 5–10 years after menopause, we examined whether the use of HT may explain the change of the association of %ST between the two genders from mid-life to late-life.
Menopause appears to have a negative effect on SL and SWS, i.e. postmenopausal women without HT had a longer SL and less SWS than women on HT and compared with men. There was no significant difference for SL between premenopausal women and men (30.1 ± 2.0 min versus 29.9 ± 2.2 min, P = 0.944), and in postmenopausal women with HT compared with men (28.8 ± 3.1 min versus 32.8 ± 2.1 min, P = 0.882). Postmenopausal women without HT, however, had a significantly longer SL (42.1 ± 2.5 min) compared with men (P = 0.017) and compared with postmenopausal women with HT (P = 0.002). For SWS, there was no difference between premenopausal women and men (5.1 ± 0.5% versus 4.9 ± 0.5%, P = 0.782). Postmenopausal women with HT compared with men had significantly more SWS (5.3 ± 0.5% versus 1.1 ± 0.3%, P < 0.001) as did postmenopausal women without HT (3.9 ± 0.4%, P < 0.001), although women without HT had less SWS than those taking HT (P = 0.049).
Study III: Effects of blood draw on night-time sleep in young men and women
Sleep on catheter night versus baseline nights
No statistically significant differences were found between men and women with regard to any of the sleep variables at baseline. SL and percentage of WTASO significantly increased in all the subjects on the catheter night (both P < 0.001), such that the percentage of total sleep time significantly decreased (P < 0.001) (Table 2). There was a significant increase of stage 1 (P < 0.001) and a decrease of stage 2 sleep only in men (P < 0.001), while SWS and REM sleep were unaffected.
Table 2.
Night-time sleep patterns at baseline versus catheter nights in young, healthy men and women (study III)
| Baseline nights | Catheter night | P-value | |
|---|---|---|---|
| Entire sample (n = 66) | |||
| SL (1st epoch of stage 2, min) | 20.3 ± 2.0 | 27.2 ± 2.5 | 0.025* |
| %WTASO | 6.3 ± 0.5 | 14.0 ± 1.0 | <0.001 |
| %ST | 90.0 ± 0.7 | 81.4 ± 1.1 | <0.001 |
| %Stage 1 | 4.3 ± 0.3 | 5.9 ± 0.4 | <0.001 |
| %Stage 2 | 59.7 ± 0.8 | 58.0 ± 0.9 | NS |
| %SWS | 13.6 ± 0.7 | 14.0 ± 0.8 | NS |
| %REM | 22.4 ± 0.6 | 22.0 ± 0.7 | NS |
| REM latency (min) | 87.6 ± 3.4 | 78.3 ± 3.7 | 0.028* |
| TIB | 480.6 ± 0.7 | 478.3 ± 2.8 | NS |
| Men (n = 32) | |||
| SL (1st epoch of stage 2, min) | 17.4 ± 3.0 | 29.0 ± 3.9 | 0.005* |
| %WTASO | 6.2 ± 0.6 | 16.3 ± 1.6 | <0.001 |
| %ST | 90.6 ± 0.8 | 78.6 ± 1.4 | <0.001 |
| %Stage 1 | 4.4 ± 0.4 | 6.9 ± 0.6 | <0.001 |
| %Stage 2 | 60.6 ± 1.2 | 57.1 ± 1.5 | 0.003 |
| %SWS | 12.2 ± 1.1 | 12.6 ± 1.2 | NS |
| %REM | 22.9 ± 0.7 | 23.4 ± 1.0 | NS |
| REM latency (min) | 92.6 ± 5.5 | 77.1 ± 4.4 | 0.06 |
| TIB | 480.8 ± 0.2 | 473.0 ± 5.3 | NS |
| Women (n = 34) | |||
| SL (1st epoch of stage 2, min) | 23.1 ± 2.7 | 25.6 ± 3.2 | NS |
| %WTASO | 6.4 ± 0.8 | 11.8 ± 1.3 | <0.003 |
| %ST | 89.5 ± 1.0 | 84.0 ± 1.5 | <0.002 |
| %Stage 1 | 4.2 ± 0.6 | 5.0 ± 0.6 | <0.066 |
| %Stage 2 | 58.9 ± 1.2 | 58.9 ± 1.2 | NS |
| %SWS | 15.0 ± 1.0 | 15.4 ± 1.1 | NS |
| %REM | 21.9 ± 1.0 | 20.8 ± 1.1 | NS |
| REM latency (min) | 82.9 ± 3.9 | 79.4 ± 5.9 | NS |
| TIB | 480.5 ± 1.3 | 483.4 ± 1.8 | NS |
Data represent mean ± SEM. Baseline is the average of nights 2 and 3.
SL, sleep latency; WTASO, wake time after sleep onset; ST, sleep time; SWS, slow wave sleep; REM, rapid eye movement; TIB, time in bed.
P-value derived from non-parametric Wilcoxon signed-ranks test.
Comparison of catheter-induced sleep disturbance between men and women
When examining differences between men and women, both groups had increased WTASO and increased %ST, while only for the men was the increased stage 1 sleep significant. Men also had reduced stage 2 sleep (Table 2).
Men compared with women on catheter night (Table 3) demonstrated a significantly larger change in %ST (P < 0.01), and percentage of stage 1 sleep (P < 0.01).
Table 3.
Comparison of the differences of the change in sleep variables as a result of blood draw (night 4 minus mean values of nights 2 and 3) in young, healthy men versus women (study III)
| Men (n = 32) | Women (n = 34) | P-value | |
|---|---|---|---|
| SL (1st epoch of stage 2, min) | 10.84 ± 2.9 | 3.2 ± 2.8 | 0.28 |
| %WTASO | 9.99 ± 1.4 | 5.49 ± 1.3 | 0.12 |
| %ST | −11.76 ± 1.3 | −5.72 ± 1.3 | <0.01 |
| %Stage 1 | 2.46 ± 0.3 | 0.80 ± 0.3 | <0.01 |
| %Stage 2 | −3.16 ± 1.1 | −0.28 ± 1.0 | 0.28 |
| %SWS | 0.11 ± 0.8 | 0.63 ± 0.8 | 0.81 |
| %REM | 0.64 ± 0.7 | −1.22 ± 0.7 | 0.27 |
| REM latency (min) | −12.42 ± 5.0 | −6.42 ± 4.9 | 0.81 |
Data represent mean ± SEM.
SL, sleep latency; WTASO, wake time after sleep onset; ST, sleep time; SWS, slow wave sleep; REM, rapid eye movement.
DISCUSSION
Our study based on a large general random sample of normal sleepers of a wide age range, showed that (i) women sleep objectively more and better than men and (ii) menopause is associated with prolonged SL and decreased SWS, whereas HT appears to protect women from these unfavourable changes. Furthermore, our interventional study showed that young, healthy women are more resilient to externally induced sleep disturbance than young, healthy men.
In our general population sample, women compared with men had overall significantly higher sleep efficiency, lower percentage of stage 1 sleep, higher percentage of SWS and longer REM latency. These findings are consistent with the results of two previous studies based on the SHHS cohort, a prospective cohort study aimed at investigating the relations between SDB and cardiovascular disease. Redline et al. (2004) showed that women had a better quantity (total sleep time) and quality (SWS and REM sleep) of sleep than men. Similarly, Walsleben et al. (2004) found that women compared with men had a lower mean percentage of stage 1 and stage 2 sleep, a higher percentage of SWS and a longer REM latency.
Redline et al. (2004) limited the analysis from 6443 to 2685 subjects, after excluding subjects with a poor quality of sleep study, symptoms of sleep disturbance, medication or alcohol use, severe chronic medical illness and treatment for sleep apnea. Walsleben et al. focused on a very select sample of only 470 subjects, after excluding subjects with sleep disorders and chronic illnesses, i.e. sleep apnea, too lean or overweight (BMI < 16 or >30), history of cardiovascular or respiratory problems in the last 12 months or self-reported apnea, reports of frequent sleep disturbance, use of cigarettes, caffeine or alcohol, and use of psychotropic medications including sleeping pills. In our study, we included 1324 subjects from a general random sample in Central Pennsylvania of 1743, after excluding subjects with sleep complaints or evidence of SDB. It is of interest to note that including or excluding patients with sleep or other chronic disorders did not affect the gender differences reported in the three studies. Thus, these studies combined suggest that women with or without sleep complaints or physical illnesses sleep objectively better than men.
Compared to the previous studies, our sample was the only one to include young individuals aged between 20 and 40 years. In our 20–40 years subgroup, women still showed evidence of better sleep, as they had less stage 1 and more SWS than men, even though sleep efficiency and REM sleep were similar in both genders. Thus, it appears that women sleep better than men during the entire age spectrum in the controlled environment of the sleep laboratory setting.
Slow wave sleep was lower in our study compared to the other two studies. This may possibly be reflecting the difference in study environment, because home-based polysomnography possibly is associated with a smaller adaptation first night effect and/or differences in scoring SWS between paper and digital records.
Our study showed that the objective sleep of postmenopausal women without HT was worse than that of men matched for age, and postmenopausal women on HT. Specifically, in the absence of HT, menopause was associated with prolonged SL and decreased SWS. These objective findings are consistent with the complaint of poor sleep in some postmenopausal women (Kravitz et al., 2003; Kuh et al.,1997; Matthews et al., 1990; Young et al., 2003), as well as the symptomatic relief reported by those on HT (Hays et al., 2003; Scharf et al., 1997; Schiff et al., 1979; Thomson and Oswald, 1977). These data are also consistent with early epidemiological findings from a large sample of men and women from the Republic of San Marino (n = 6000) where there was a greater increase of prevalence of insomnia in women compared with men beginning in middle age (Lugaresi et al., 1983). Given that there was no increase of depression/anxiety in this age group of women, the authors attributed the increased prevalence of insomnia to physiological changes associated with menopause. This view is consistent with physiological data which showed that the injection of corticotropin-releasing hormone during the night sleep resulted in an increased sleep disturbance in middle-aged, normal sleepers compared with young, healthy, normal sleepers (Vgontzas et al., 2001). Assuming that similar deteriorating physiological mechanisms compounded by the changes of menopause are present in women, these changes may also be a contributing factor for the increasing prevalence of insomnia reported in the older samples.
Our findings, however, are inconsistent with the results of two other major studies in general population samples. In the Wisconsin Sleep Cohort, postmenopausal women had more deep sleep and significantly longer total sleep time than premenopausal women (Young et al., 2003). Also, in the SHHS, women older than 50 and on HT slept worse, e.g. %stage 1 sleep, per cent deep sleep, than women not on HT (Shahar et al., 2003). Although there is no apparent explanation for these inconsistent findings, there are some differences in the methodology employed in these three studies. Both the Wisconsin Sleep and SHHS Cohorts used an ad lib design in the sleep laboratory in contrast to ours that was fixed, i.e. an 8-h recording. This might have an effect on variables, such as per cent sleep time and per cent sleep stage. Also, both these studies, in contrast to ours, included subjects with sleep complaints or SDB. In addition, in the SHHS, menopausal status was unknown, whereas in the Wisconsin Cohort, objective sleep was based, for some women, on multiple recordings and within each woman, menopausal status and sleep measures varied from recording to recording. Furthermore, in our study, we used men matched for age as a control group, whereas this was not the case in the other two cohorts. Finally, all three studies were based on a single night recording that may not reflect actual habitual sleep patterns of the subjects, whereas our study was additionally limited in that it did not include assessment of perimenopausal status. Given all these differences and limitations, the question of the effect of menopause and HT on objective sleep remains open to further investigation.
In our group of young, healthy, non-obese adults, men compared with women showed a higher vulnerability of their objective sleep to an external stressor as indicated by a larger change of sleep efficiency, and percentage of stage 1 sleep as a result of the blood draw procedure. Although most earlier studies have also reported a negative impact of blood sampling procedures on sleep in terms of duration and efficiency (Adam, 1982; Jarrett et al., 1984; Moe et al., 2001; Vitiello et al., 1996), the issue of possible gender differences has not been assessed adequately. Vitiello et al. (1996) in a group of elderly men and women (aged 69.1 ± 0.6 years) reported that catheterization and periodic blood sampling was associated with significant sleep disturbance that was not different between the two genders. However, Moe et al. (2001), in a study of postmenopausal elderly women, reported much greater sleep disruption for women on no HT than women on HT. These studies suggested that sleep of postmenopausal women versus age-matched men is equally vulnerable to external sleep disturbance, while the use of hormone therapy protected women from this risk.
Our study clearly demonstrates that young, healthy, premenopausal women are more resilient to the effects of an external disturbing factor than age-matched men. The findings of our study on premenopausal women, as well as those of Moe et al. on postmenopausal women with and without HT, suggest that gonadal hormones may most likely be a major factor contributing to the more resilient response of women to external stressors.
The superiority of the objective sleep of women versus men appears to contrast with the finding that women have a higher incidence of sleep complaints, e.g. insomnia. There are several explanations of this seeming paradox. First, insomnia is strongly associated with depression and anxiety, conditions that are generally more prevalent in women (Benca et al., 1992; Bixler et al., 2002; Healey et al., 1981; Kales and Kales, 1984; Lindberg et al., 1997; Ohayon and Roth, 2003). Secondly, the female/male ratio for insomnia is lower, i.e. 1.3–1.5 than the female/male ratio for depression/anxiety, i.e. 2 : 1 (Bixler et al., 2002; Kales and Kales, 1984; Leger et al., 2000; Weyerer and Dilling, 1991). The relatively lower prevalence of insomnia compared with that of depression and anxiety in women than in men suggests that the stronger sleep homeostatic mechanisms in women, as indicated by the objective sleep data, provide some protection from the sleep disturbing effects of depression/anxiety.
Another possibility is that women sleep better objectively because in a controlled environment such as the sleep laboratory they are relieved from the demands of children and household. However, this is not supported by the facts that (i) even in home recordings women sleep better than men; (ii) elderly women with probably little or no childcare responsibilities sleep better than men and (iii) women are more resilient to sleep disturbance even after they have been acclimatized to the sleep laboratory environment for several consecutive nights, which would have eliminated the ‘positive’ adaptation effect to the new environments.
In conclusion, our data suggest that women sleep better compared with men across age and that the sleep of young women is more resistant to external stressors than young men. It is also possible that women’s sleep becomes worse during menopause because of the withdrawal of oestrogen, however, this issue remains open to further investigation. This marked sexual dimorphism in sleep may be to protect women from the profound demands of infant and childcare for the most of mankind’s history. In an earlier study (Vgontzas et al., 2004), we showed that women also did better than men in terms of the effects of sleep restriction on inflammation markers, maximum cortisol values and sleep consolidation during the nights of restricted sleep. Collectively, these data suggest that healthy women appear not only to sleep better, but also cope better with sleep loss or externally induced sleep disturbance, which, in part, might contribute to women’s lower cardiovascular risks and greater longevity.
ACKNOWLEDGEMENTS
This study was supported in part by NIH Grants R01 HL40916, R01 HL51931 and R01 HL64415.
REFERENCES
- Adam K. Sleep is changed by blood sampling through an indwelling venous catheter. Sleep. 1982;5:154–158. [PubMed] [Google Scholar]
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: a meta-analysis. Arch. Gen. Psychiatry. 1992;49:651–668. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- Bixler E, Vgontzas A, Lin HM, Ten Have T, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep disordered breathing in women: effects of gender. Am. J. Respir. Crit. Care Med. 2001;163:608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- Bixler EO, Vgontzas AN, Lin HM, Vela-Bueno A, Kales A. Insomnia in central Pennsylvania. J. Psychosom. Res. 2002;53:89–592. doi: 10.1016/s0022-3999(02)00450-6. [DOI] [PubMed] [Google Scholar]
- Bixler EO, Vgontzas AN, Lin HM, Vela-Bueno A, Kales A. Women and sleep-related disorders. Eur. Respir. Med. 2003;25:24–218. [Google Scholar]
- Bixler E, Vgontzas A, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am. J. Respir. Crit. Care Med. 1998;157:144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- Bliwise DL. Normal aging. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 3rd edn W.B. Saunders; Philadelphia: 2000. pp. 26–42. [Google Scholar]
- Hays J, Ockene J, Brunner R, Kotchen JM, Manson JE, Patterson RE, Aragaki AK, Shumaker SA, Brzyski RG, LaCroix AZ, Granek IA, Valanis BG, Women’s Health Initiative Investigators Effects of estrogen plus progestin on health-related quality of life. N. Engl. J. Med. 2003;348:1839–1854. doi: 10.1056/NEJMoa030311. [DOI] [PubMed] [Google Scholar]
- Healey ES, Kales A, Monroe LJ, Bixler EO, Chamberlin K, Soldatos CR. Onset of insomnia: role of life-stress events. Psychosom. Med. 1981;43:439–451. doi: 10.1097/00006842-198110000-00007. [DOI] [PubMed] [Google Scholar]
- Jarrett DB, Greenhouse JB, Thompson SB, McEachran A, Coble P, Kupfer DJ. Effect of nocturnal intravenous cannulation upon sleep – EEG measures. Biol. Psychiatry. 1984;19:1537–1550. [PubMed] [Google Scholar]
- Kales A, Kales JD. Evaluation and Treatment of Insomnia. Oxford University Press; New York: 1984. [Google Scholar]
- Kerkhofs M, Linkowski P, Mendlewicz J. Effects of intravenous catheter on sleep in healthy men and in depressed patients. Sleep. 1989;12:113–119. [PubMed] [Google Scholar]
- Kravitz HM, Ganz P, Bromberger J, Powell L, Sutton-Tyrrell K, Meyer P. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19–28. doi: 10.1097/00042192-200310010-00005. [DOI] [PubMed] [Google Scholar]
- Kuh DL, Wadsworth M, Hardy R. Women’s health in midlife: the influence of the menopause, social factors and health in earlier life. Br. J. Obstet. Gynaecol. 1997;104:923–933. doi: 10.1111/j.1471-0528.1997.tb14352.x. [DOI] [PubMed] [Google Scholar]
- Leger D, Guilleminault C, Dreyfus JP, Delahaye C, Paillard M. Prevalence of insomnia in a survey of 12,778 adults in France. J. Sleep. Res. 2000;9:35–42. doi: 10.1046/j.1365-2869.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- Lindberg E, Janson C, Gislason T, Bjornsson E, Hetta J, Boman G. Sleep disturbances in a young adult population: can gender differences be explained by differences in psychological status? Sleep. 1997;20:381–387. doi: 10.1093/sleep/20.6.381. [DOI] [PubMed] [Google Scholar]
- Lugaresi E, Cirignotta F, Coccagna C, Piana C. Some epidemiological data on snoring and cardiocirculatory disturbances. Sleep. 1993;5:403–408. doi: 10.1093/sleep/3.3-4.221. [DOI] [PubMed] [Google Scholar]
- Lugaresi E, Cirignotta F, Zucconi M, Mondini S, Lenzi PL, Coccagna G. Good and poor sleepers: an epidemiological survey of the San Marino population. In: Guilleminault C, Lugaresi E, editors. Sleep/Wake Disorders: Natural History, Epidemiology, and Long-Term Evolution. Raven Press; New York: 1983. pp. 1–12. [Google Scholar]
- Matthews KA, Wing RR, Kuller LH. Influences of natural menopause on psychological characteristics and symptoms of middle-aged healthy women. J. Consult. Clin. Psychol. 1990;58:345–351. doi: 10.1037//0022-006x.58.3.345. [DOI] [PubMed] [Google Scholar]
- Moe KE, Larsen LH, Vitiello MV, Prinz PN. Estrogen replacement therapy moderates the sleep disruption associated with nocturnal blood sampling. Sleep. 2001;24:886–894. doi: 10.1093/sleep/24.8.886. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. J. Psychiatr. Res. 2003;37:9–15. doi: 10.1016/s0022-3956(02)00052-3. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. U.S. Government Printing Office; Washington, D.C.: 1968. NIMH Publication 204. [Google Scholar]
- Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity and sleep disordered breathing on sleep architecture. Arch. Intern. Med. 2004;164:406–418. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Kapke A, Roth T, Breslau N. Sex differences in the polysomnographic sleep of young adults: a community-based study. Sleep Med. 2006;7:49–53. doi: 10.1016/j.sleep.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Scharf MB, McDannold MD, Stover R, Zaretsky N, Berkowitz DV. Effects of estrogen replacement therapy on rates of cyclic alternating patterns of hot-flush events during sleep in postmenopausal women: a pilot study. Clin. Ther. 1997;19:304–311. doi: 10.1016/s0149-2918(97)80118-x. [DOI] [PubMed] [Google Scholar]
- Schiff I, Regestein Q, Tulchinsky D, Ryan KJ. Effects of estrogens on sleep and psychological state of hypogonadal women. JAMA. 1979;242:2405–2407. [PubMed] [Google Scholar]
- Shahar E, Redline S, Young T, Boland LL, Baldwin CM, Nieto FJ, O’Connor GT, Rapoport DM, Robbins JA. Hormone replacement therapy and sleep-disordered breathing. Am. J. Respir. Crit. Care Med. 2003;167:1186–1192. doi: 10.1164/rccm.200210-1238OC. [DOI] [PubMed] [Google Scholar]
- Shapiro C, Dement W. ABC of sleep disorders: impact and epidemiology of sleep disorders. BMJ. 1993;306:1604–1607. doi: 10.1136/bmj.306.6892.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson J, Oswald I. Effect of oestrogen on the sleep, mood, and anxiety of menopausal women. Br. Med. J. 1977;2:1317–1319. doi: 10.1136/bmj.2.6098.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Bixler EO, Wittman AM, Zachman K, Lin HM, Vela-Bueno A, Kales A, Chrousos GP. Middle-aged men show higher sensitivity of sleep to the arousing effects of corticotropin-releasing hormone than young men: clinical implications. J. Clin. Endocrinol. Metab. 2001;86:1489–1495. doi: 10.1210/jcem.86.4.7370. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J. Clin. Endocrinol. Metab. 2004;89:2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- Vitiello MV, Larsen LH, Moe KE, Borson S, Schwartz RS, Prinz PN. Objective sleep quality of healthy older men and women is differentially disturbed by nighttime periodic blood sampling via indwelling catheter. Sleep. 1996;19:304–311. doi: 10.1093/sleep/19.4.304. [DOI] [PubMed] [Google Scholar]
- Walsleben JA, Kapur VK, Newman AB, Shahar E, Bootzin RR, Rosenberg CE, O’Connor G, Nieto FJ. Sleep and reported daytime sleepiness in normal subjects: the Sleep Heart Health Study. Sleep. 2004;27:293–298. doi: 10.1093/sleep/27.2.293. [DOI] [PubMed] [Google Scholar]
- Weyerer S, Dilling H. Prevalence and treatment of insomnia in the community: results from the Upper Bavarian Field Study. Sleep. 1991;14:392–398. [PubMed] [Google Scholar]
- Young T, Rabago D, Zgierska A, Austin D, Finn L. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep. 2003;26:667–672. doi: 10.1093/sleep/26.6.667. [DOI] [PubMed] [Google Scholar]


