Abstract
MicroRNA (miRNA) are a class of non-coding RNA that suppress gene expression by degradation or translational inhibition of target RNA. Several miRNA have been shown to target oncogenes and recently miRNA-125b was shown to translationally and transcriptionally inhibit the p53 gene. Here, we show that an additional isomer of miRNA-125 (miRNA-125a) translationally arrests mRNA of the p53 tumor suppressor gene. The basis of this activity is the high degree of sequence homology between the seed sequence of miR-125a and the 3′-UTR of p53. Our findings add miRNA-125a to the growing list of miRNA with oncogenic targets.
Keywords: MicroRNA, p53, RNA interference, Tumor suppressor, Apoptosis, DNA damage
1. Introduction
MicroRNA (miRNA) are a class of non-coding RNA that have emerged as important regulators of gene expression. Although identified nearly 25 years ago in the experimental organism Caenorhabditis elegans, their biologic importance has only become apparent recently [1–3]. Presently, some 721 miRNAs (Sanger miRbase; Release 14.0) have been identified in humans but the list is likely a vast underestimate according to recent bioinformatic investigations [4]. miRNA mediate posttranscriptional gene control by either direct degradation or translational inhibition depending upon the extent of sequence homology between miRNA and target RNA [5,6].
The role of miRNA in human health and disease is under active investigation. For example, extensive expression profiling of a large number of primary human neoplasms has revealed unique miRNA signatures associated with diseased tissue compared to surrounding healthy margins [7,8]. These miRNA species, termed oncomirs, are thought to regulate a number of basic cellular processes involved in neoplastic transformation and the clinical relevance of these malignancy associated expression patterns is becoming increasingly apparent. Distinct miRNA profiles predict the natural history of leukemia and lung cancer [9–11]. While detailed information is rapidly emerging regarding neoplasm-specific miRNA expression patterns, relatively little is known about their actual gene targets.
Altered expression of miRNA-125b has been demonstrated in a number of malignancies including prostate, breast and pancreatic cancer [12,13]. In humans, two isoforms of miRNA-125 exist: miR-125a and miR-125b1/b2 encoded in chromosomes 19, 11 and 21, respectively. Validated targets of miRNA-125b include ERBB2/3 and BAK1 but numerous reports indicate that a single miRNA may have hundreds if not thousands of targets [14,15]. Recent reports have identified p53 and several of its upstream regulators as a target of miRNA-125b [16]. Here, we extend these findings and report that miRNA-125a also targets the p53 protein. Unlike miRNA-125a, miRNA-125b appears to directly reduce p53 protein levels by translationally arresting p53 mRNA by binding to homologous regions of the p53 3′-UTR. In contrast to miRNA-125b, ectopic expression of miRNA-125a has no effect upon the transcriptional status of the p53 gene as mRNA levels are unchanged. In further experimentation, we show that the p53-spe-cific effects of miRNA-125a are independent of known negative regulators of p53 such as Pirh2 and COP1. Finally, miRNA-125b expression is associated with classic phenotypic and genotypic sequelae of reduced p53 expression including altered sensitivity to genotoxic agents and reduced expression of p53 related transcriptional targets such as miRNA-34a/b/c. Our findings add miRNA-125a to the growing list of small RNA molecules that may target p53 thus adding a further layer of complexity to the regulation of the “guardian of the genome”.
2. Materials and methods
2.1. Cell culture, transfection and molecular constructs
HEK 293T, HepG2 and MCF-7 cell lines were obtained from American Type Culture Collection (ATCC). Cells were maintained in growth media consisting of Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% Fetal Bovine Serum. HEK 293T cells and MCF-7 cells were transfected with plasmid DNA by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instruction. HepG2 cells were transfected by using HepG2 transfection kit (Altogen Biosystems) according to the manufacturer's instructions.
Human miRNA-125a precursor was amplified from genomic DNA by PCR (sense: 5′-cgggatccggaccctgggaggaagag-3′; antisense: 5′-cggaattccagaggtcaggtttcagttggt-3′) and cloned into RNAi-Ready pSIREN-DNR (BD Biosciences Clontech) expression vector. Mature miRNA-125a expression was confirmed by Northern blot. Mutant miRNA-125a plasmid was created by using QuikChange Site-Directed Mutagenesis Kit (Stratagene) (sense: 5′-gttgccagtctctaggtgcgtg agaccctttaacctg-3′; antisense: 5′-caggttaaagggtctcacgcacctagagact ggcaac-3′).
p53 3′-UTR was amplified by PCR (sense: 5′-ggactagtcag aagggcctgactcagactga-3′; antisense: 5′-aaggaaaaaagcggccgcctctggg aggctgagacaggtg-3′) and cloned into the psiCHECK2 dual-luciferase reporter plasmid (Promega).
2.2. Dual-luciferase reporter assays
Luciferase reporter assays were performed using the psiCHECK2 dual-luciferase reporter system. HEK 293T cells were co-transfected with psiCHECK2 plasmids and miRNA-125a expression vector or empty vector. Firefly and Renilla luciferase activities were quantified using the Dual-Luciferase Reporter Assay System (Promega) and Renilla luciferase activity was normalized to firefly luciferase activity.
2.3. Western blot
Total cellular protein (20 μg) was separated by electrophoresis in SDS-polyacrylamide gels before transfer to PVDF membrane (Bio-Rad). The following primary antibodies were used: anti-p53 (Cell Signaling), anti-p21 (BD Pharmingen), Pirh2 (Santa Cruz Biotechnology), COP1 (Santa Cruz Biotechnology). Signals were visualized by chemiluminescence using the ECL-Plus reagent (GE Healthcare).
2.4. Northern blot
Twenty-five micrograms total RNA was loaded on 15% acrylamide gel and transferred onto Hybond N+ membrane (Ambion). miRNA-125a probe (5′-tccctgagaccctttaacctgtgacctgtctc-3′) was labeled by using Biotin RNA labeling kit (Roche). Hybridization was carried out at 42 °C for 12 h and signal was detected by BrightStar BioDetect kit (Ambion).
2.5. RT-PCR quantification
Total RNA was extracted by TRIZOL and 1 μg RNA was used for cDNA synthesis using MMLV reverse transcriptase (New England Biolabs) with random primers. PCR analysis was performed by RT2 Real-Time™ SYBR Green PCR master mix (SuperArray) according to the manufacturer's protocol using the Eppendorf realplex2 Mastercycler machine (Eppendorf). Results were normalized by beta-actin (sense: 5′-gcc agg tca tca cca ttg-3′; antisense: 5′-gga agg aag gc tgg aag a-3′). Primers using for miRNA-34a, 34b and 34c primary/precursor quantification are as follows: pri-miRNA-34a (sense: 5′-ccc cac att tcc ttc tta tca ac-3′; antisense: 5′-cccca-catttccttcttatcaac-3′); pre-miRNA-34a (sense: 5′-cag tgt ctt agc tgg ttg ttg tgag-3′; antisense: 5′-gccagtatacttgctgattgcttc-3′); pri-miRNA-34b (sense: 5′-gcg tcc ctc gg tga aatgg-3′; antisense: 5′-cgcttctcaggcatcttctctc-3′); pre-miRNA-34b (sense: 5′-ggcagtgt-cattagctgattgtactg-3′; antisense: 5′-gggcagtggacttagtgattgtaac-3′); pri-miRNA-34c (sense: 5′-gcctgcctgtcacaacgtg-3′; antisense: 5′-gcacaggcagctcatttggac-3′); pre-miRNA-34c (sense: 5′-aggcagtg-tagttagctgattgc-3′; antisense: 5′-ggccgtgtggttagtgattg-3′).
TaqMan® microRNA assays (Applied Biosystems) that include RT primers and TaqMan probes were used to quantify the levels of mature miRNA All PCR reactions were run in triplicate.
2.6. Measurement of cell viability and proliferation
HepG2 cells were transfected with an empty vector (EV), p53 siRNA and miRNA-125a. After overnight incubation, the cells were treated with or without 10 μM arsenic trioxide (ATO, Sigma) for 48 h. Cell viability and proliferation were measured with an MTT Cell Proliferation Assay kit (ATCC) and BrdU Proliferation Assay kit (CalBiochem) following the manufacturer's instructions, respectively.
3. Results and discussion
3.1. miRNA-125a targets the 3′-UTR ofp53
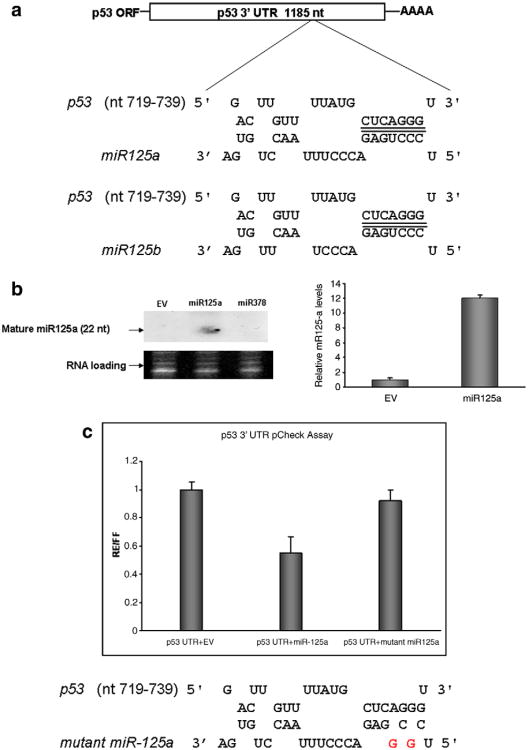
Using a recently developed pattern based miR target identification algorithm (Rna22), we found that the 3′-UTR of p53 harbors a sequence motif that is identical to the seed sequence (nucleotides 2–7 from the 5′-end) of miRNa-125a (Fig. 1a) [17]. Both isoforms 125a and 125b share perfect sequence homology in this seed sequence but vary towards the 3′-end of the mature miR molecule (Fig. 1a). We constructed a miRNA-125a expression vector by inserting a 365 nt long fragment of pri-miRNA-125a into a generic CMV promoter driven plasmid. Northern blot and Real Time PCR for mature miR-125a confirmed ectopic over-expression in vector transfected cells compared to relevant controls which included an empty vector or one encoding an irrelevant miRNA (Fig. 1b).
Fig. 1.
miR-125 targets the 3′-UTRof p53. (a) The p53-miRNA-125a/b interactome: both isomers of miR-125 (125a, 125b) share homology in their seed sequence (5′ nt 2–7) with the 3′-UTR of p53. (b) Ectopic miRNA-125a expression vector. HEK 293T cells were transfected with miRNA-125a expression vector or control vectors that harbored either irrelevant DNA construct encoding miRNA-378 or no insert (EV). Levels of mature miRNA-125a were visualized by Northern blot (left panel) and quantified by Real Time PCR (right panel). Both assays revealed that wild-type HEK 293T cells express low levels of miRNA-125a that were increased by ∼12-fold upon transfection of miRNA-125a as quantified by Real Time PCR in three independent experiments (results are reported as mean values ±S.D.). (c) Regulation of the p53 3′-UTR by miRNA-125a. Activity of miRNA-125a on the 3′-UTRof p53 was initially assessed by luciferase based reporter assays. The p53 3′-UTR was incorporated into the firefly luciferase gene and run off a single promoter. A constitutively expressed Renilla gene served to normalize transfections. All constructs were introduced into HEK 293T cells with miRNA-125a or an empty control vector (EV) and luminescence was measured at 48 h. miRNA-125a reduced luciferase levels by 40%. The sequence specificity of miRNA-125a/p53 3′-UTR interaction was probed by mutating the seed sequence of miRNA-125a in positions 2 and 4. In comparison to wild-type miRNA-125a, the mutated version had negligible effect (<5%) on the p53 3′-UTR as quantified by levels of luciferase reduction after transfection. Data are presented as mean fold reduction ± S.D. All experiments were performed in triplicate.
To determine whether miR-125a targets the 3′-UTR of p53, PCR was used to amplify the 1185 nt long 3′-UTR of p53 from human genomic DNA and the resulting amplicon was inserted into a luciferase reporter vector. Transfection of miRNA-125a and luciferase vectors into HEK 293T cells led to the 40% reduction in normalized luciferase values compared to relevant controls including an empty miRNA expression plasmid (Fig. 1c). The stringency of miRNA-125a sequence homology was next tested by creating miRNA-125a variants with nucleotide mutations at 5′ positions 2 and 4 of the seed sequence. When these constructs were introduced into cells along with the p53 3′-UTR luciferase system, activity was abrogated compared to transfections involving wild-type miRNA-125a (Fig. 1c).
3.2. miRNA-125a translationally arrests p53 mRNA
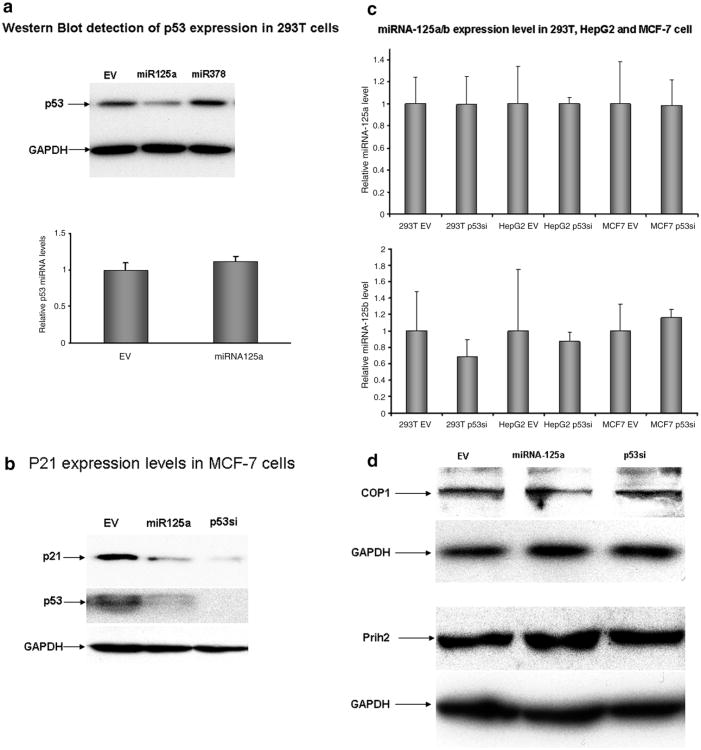
To determine whether ectopic expression of miRNA-125a decreases p53 protein levels, we transfected HEK 293T cells with an miRNA-125a expression vector. As seen in Fig. 2a, p53 protein was reduced in miRNA-125a transfected cells but not in cells that had been transfected by an empty vector or one encoding an irrelevant miRNA. Recent reports indicate that numerous potential interactions may exist between various miRNA species and the p53 network. For example, a bioinformatics based approach identified 115 miRNA, including miRNA-125b, that are predicted to target upstream or downstream regulators of p53 [18]. Interestingly, laboratory based investigation revealed that miRNA-125b did in fact share sequence homology and functionally target six genes that are upstream regulators of p53 [16]. Indeed, miRNA-125b expression was associated with decreased p53 mRNA levels. Thus, ectopic expression of miRNA-125b decreases p53 levels not only by translational inhibition but also by directly targeting p53 transcriptional regulators. To determine whether a similar effect was associated with miRNA-125a, we quantified p53 mRNA levels in cells transfected with miRNA-125a or control expression vectors. As seen in Fig. 2a, ectopic expression of miRNA-125a had no effect on p53 mRNA levels as quantified by Real Time PCR thus supporting translational inhibition as the primary mode of gene silencing. Similar results were observed when the experiment was repeated in MCF-7 cells (data not shown). miRNA-125a proved almost as efficacious as specific siRNA against p53 in reducing protein levels (Fig. 2b). As expected, decreased levels of p53 by miRNA-125a or siRNA targeting p53 was associated with decreased levels of p21, a downstream target of p53 (Fig. 2b). We next asked whether the p53 protein itself was involved in the biogenesis of miRNA-125b. RNAi mediated depletion of p53 had no effect upon endogenous mature miRNA-125a levels in the three cell lines examined. A similar result was also apparent for miRNA-125b (Fig. 2c). Given that levels of p53 are influenced my negative regulators such as Prih2 and COP1, we quantified levels of these proteins in cells over expressing miRNA-125a. As seen in Fig. 2d, miRNA-125a over-expression did not alter Prih2 or COP1 levels, as assessed by Western blot.
Fig. 2.
miRNA-125a reduces p53 protein levels by translational arrest of p53 mRNA. (a) Ectopic expression of miRNA-125a reduces p53 protein expression in HEK 293T cells by translational arrest. Cells were transfected with expression vectors for miRNA-125 or an irrelevant construct encoding miRNA-378 and p53 protein levels were quantified by Western blot. GAPDH levels were used to normalize protein input (upper panel). p53 protein reduction by miRNA-125a was not associated with reduction in p53 mRNA levels as quantified by Real Time PCR in cells that had been treated with an empty vector or one encoding miRNA-125a. Data are presented as mean fold reduction ± S.D. All experiments were performed in triplicate. (b) Efficacy of miRNA-125a approaches that of p53 short interfering RNA (siRNA). p53 protein levels were quantified by Western blot in a breast cancer cell line (MCF-7) that had been transfected with either p53 specific siRNA or miRNA-125a. Both agents reduced protein levels of both p53 and downstream protein p21. GAPDH levels were used to normalize protein input. (c) The effect of p53 levels themselves on miRNA-125a was examined in three cell lines. Reduction of p53 protein levels by siRNA had no effect upon levels of endogenously expressed miRNA-125a (upper panel) or miRNA-125b (lower panel) when compared to cells that had been treated with a control empty vector (EV). (d) Several proteins including COP1 and Prih2 are known to negatively regulate p53 protein levels. We found that ectopic expression of miRNA-125a had no effect on levels of either protein, as assessed by Western blot.
3.3. p53-Specific genetic and phenotypic sequelae of miRNA-125a over-expression
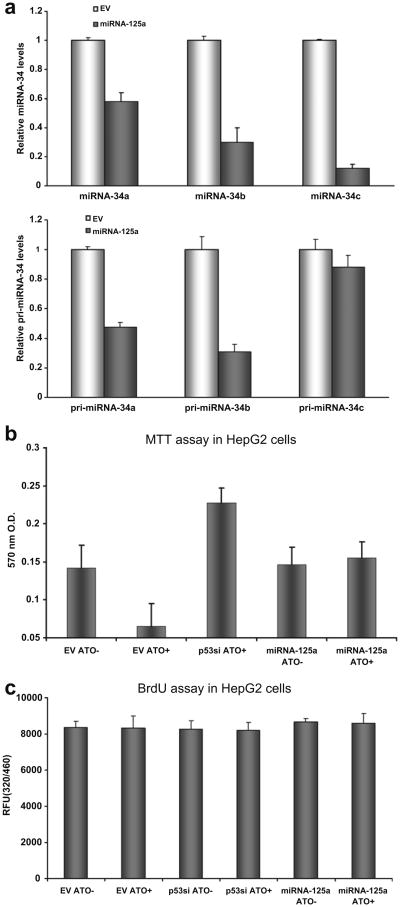
The p53 protein is involved in a myriad processes central to cellular health. We next used a variety of experimental conditions to quantify the effect of miRNA-125a expression on the phenotypic and genotypic sequelae of p53 down modulation. The transcriptional activity of p53 has been well characterized vis a vis its regulation of cell cycle checkpoints. A number of recent investigations have revealed that p53 induces the transcription of the miRNA-34 family, comprised of miR-34a, -34b and -34c. To determine whether a similar regulatory circuit could be enforced by miRNA-125a over-expression, we quantified the levels of miRNA-34a/b/c by stem-loop quantitative reverse transcription-PCR (RT-PCR) in control cells or those that over expressed miRNA-125a. As seen in Fig. 3a, levels of miRNA-34a/b/c were reduced by ectopic expression of miRNA-125a, albeit at different levels with the most dramatic reduction seen for miRNA-34c. As expected, a concomitant decrease in pri-miRNA-34a/b/c levels was also observed upon ectopic expression of miRNA-125a.
Fig. 3.
p53-specific genotypic and phenotypic sequelae of miR-125a expression. (a) p53-specific genotypic sequelae of miRNA-125a expression. miRNA-34a/b/c have been established as transcriptional targets of p53. Levels of all three miRNAs were reduced by 20–80% in cells over expressing miRNA-125a as quantified by Real Time PCR of mature (upper panel) and pri-miRNA levels (lower panel). Data are presented as mean fold reduction ± S.D. compared to cells treated with an empty vector (EV). All experiments were performed in triplicate. (b) p53-specific phenotypic sequelae of miRNA-125a expression. Over-expression of miRNA-125a protects cells from cell death induced by arsenic trioxide. Cells were treated with arsenic trioxide and their viability was quantified by MTT assay. As expected, EV treated cells could not withstand the toxicity of arsenic trioxide. Pre-treatment of cells with siRNA targeting p53 or miRNA-125a preserved viability. All experiments were performed in triplicate and the average and standard deviation of viable cells is shown. (c) The response to arsenic trioxide was assessed by BrdU assay in cells with p53 knockdown or in cells treated with an irrelevant empty vector or one encoding miRNA-125a. Arsenic trioxide or its absence was not related to any significant differences in cellular proliferative capacity in any of the six conditions tested.
Genotoxic stressors such as irradiation or drugs such as arsenic trioxide lead to apoptosis in a p53 dependent manner. For example, treatment of HepG2 cells with arsenic trioxide leads to a marked decrease in cellular survival as quantified by MTT assay. Using this system, we first probed the impact of miRNA-125a on the cellular response to arsenic trioxide. Lipid transfection of miRNA-125a or short interfering RNA targeting p53 protected arsenic trioxide cells from cell death in contrast to cells that had been treated with control constructs (Fig. 3b). These data were further bolstered by the parallel performance of a BrdU proliferation assay. We could detect no difference in the extent of cellular proliferation in cells with ectopic expression of miRNA-125a compared to relevant controls (Fig. 3c). Thus, our data in sum suggest that miRNA-125a can decrease p53 protein in human cells and that this reduction is associated with p53-specific phenotypic and genotypic sequelae. Interestingly, miR-125a/b is one of the handful of miRs that are conserved in flies, worms and mammals, similar to miRNA-34, thus suggesting that miRNA/p53 interactions are evolved processes [19].
As mentioned, a number of miRNAs have been implicated in the natural history of malignant disease and have the potential of serving as clinically useful biomarkers [9]. More recently, miRNA expression patterns have been associated with response rates to chemotherapy of colon cancer where high miRNA-21 expression levels were associated with poor clinical response to fluorouracil based adjuvant therapy [20]. Recently, levels of miRNA-125 have been reported to be upregulated in the majority of rectal cancer and associated with a poorer response to multi-modal therapy including pre-operative capecitabine, surgery and radiotherapy [21]. Our results suggest that miRNA-125a may impact response to treatments that rely upon functional p53 expression and merits further study, especially with respect to the cellular cues that lead to the transcriptional activation of the miRNA-125a locus. Such investigations may reveal upstream regulators of a locus that directly impacts p53 levels.
Acknowledgments
This work was supported by NIH 1P20RR025179.
References
- 1.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 2.Esquela-Kerscher A, Slack FJ. Oncomirs – microRNAs with a role in cancer. Nat Rev Cancer. 2006;6(4):259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 4.Bentwich I, et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet. 2005;37(7):766–770. doi: 10.1038/ng1590. [DOI] [PubMed] [Google Scholar]
- 5.Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431(7006):343–349. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- 6.Pillai RS, Bhattacharyya SN, Filipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol. 2007;17(3):118–126. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Iorio MV, et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007;67(18):8699–8707. doi: 10.1158/0008-5472.CAN-07-1936. [DOI] [PubMed] [Google Scholar]
- 8.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 9.Garzon R, et al. MicroRNA signatures associated with cytogenetics and prognosis in acute myeloid leukemia. Blood. 2008;111(6):3183–3189. doi: 10.1182/blood-2007-07-098749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yanaihara N, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9(3):189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 11.Volinia S, et al. AmicroRNA expression signature ofhuman solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103(7):2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi XB, et al. An androgen-regulated miRNA suppresses Bak1 expression and induces androgen-independent growth of prostate cancer cells. Proc Natl Acad Sci USA. 2007;104(50):19983–19988. doi: 10.1073/pnas.0706641104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomson JM, et al. Extensive post-transcriptional regulation of microRNAs and its implications for cancer. Genes Dev. 2006;20(16):2202–2207. doi: 10.1101/gad.1444406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee Y, et al. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23(20):4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selbach M, et al. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455(7209):58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 16.Le MT, et al. MicroRNA-125b is a novel negative regulator of p53. Genes Dev. 2009;23(7):862–876. doi: 10.1101/gad.1767609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miranda KC, et al. A pattern-based method for the identification of microRNA binding sites and their corresponding heteroduplexes. Cell. 2006;126(6):1203–1217. doi: 10.1016/j.cell.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 18.Sinha AU, et al. Dissecting microregulation of a master regulatory network. BMC Genom. 2008;9:88. doi: 10.1186/1471-2164-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruby JG, et al. Large-scale sequencing reveals 21U-RNAs and additional microRNAs and endogenous siRNAs in C. elegans. Cell. 2006;127(6):1193–1207. doi: 10.1016/j.cell.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 20.Schetter AJ, et al. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA. 2008;299(4):425–436. doi: 10.1001/jama.299.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Svoboda M, et al. Micro-RNAs miR125b and miR137 are frequently upregulated in response to capecitabine chemoradiotherapy of rectal cancer. Int J Oncol. 2008;33(3):541–547. [PubMed] [Google Scholar]