Abstract
Understanding individual differences in the variability of fibromyalgia pain can help elucidate etiological mechanisms and treatment targets. Past research has shown that spatial extent of pain, negative mood, and aftersensation (pain ratings taken after experimental induction of pain) accounts for 40 to 50% of the variance in clinical pain. Poor sleep is hypothesized to have a reciprocal relationship with pain, and over 75% of individuals with fibromyalgia report disturbed sleep. We hypothesized that measures of sleep would increase the predictive ability of the clinical pain model. Measures of usual pain, spatial extent of pain, negative mood, and pain aftersensation were taken from 74 adults with fibromyalgia. Objective (actigraph) and subjective (diary) measures of sleep duration and nightly wake time were also obtained from the participants over 14 days. Hierarchical regression indicated that greater spatial extent (R2 = .26), higher aftersensation ratings (R2 = .06), and higher negative mood (R2 = .04) accounted for 36% of the variance in clinical pain (average of 14 daily pain ratings). None of the sleep variables were significant predictors of clinical pain. Results replicate previous research and suggest that spatial extent of pain, pain aftersensation, and negative mood play important roles in clinical pain, but sleep disturbance did not aid in its prediction.
Keywords: Fibromyalgia, clinical pain, sleep, insomnia, central sensitization
Fibromyalgia syndrome (FM) is defined by chronic, widespread musculoskeletal pain across the 4 body quadrants, and the presence of at least 11 of 18 painful tender points.51 Clinical pain intensity can be conceptualized as a rating of overall bodily pain, and has strong associations with health care usage and quality of life. Due to the complex interplay between physical, psychological, and social factors involved with this condition, clinical pain can be variable across FM patients and is difficult to predict.
In an effort to identify predictors of clinical pain and thereby elucidate potential mechanisms and treatment targets, we44,45 found that negative mood, painful aftersensation ratings taken after experimental induction of second pain (using a temporal summation of second pain protocol), and a measure of spatial extent of pain (the sum of local pain areas) were significant predictors of clinical pain in FM patients, and together accounted for 40 to 50% of its variance. The results suggest that psychophysical and psychological variables are relevant to FM clinical pain. Temporal summation of second pain, or windup, refers to an increased perception of second pain evoked by repetitive noxious stimuli at constant intensities, and has been found to be mediated by central nervous system processes.36 Thus, windup and its aftersensations are thought to be proxy measures of a centrally mediated hypersensitivity to pain stimuli (central sensitization), a mechanism hypothesized to underlie FM.52 Relative to normal controls, FM patients show enhanced windup as well as prolonged and enhanced aftersensation following repetitive stimulation with thermal heat.38,43,46 Along with widespread pain and allodynia, these psychophysical studies suggest that central sensitization may play an important role in the FM pain experience.
Other subjective health complaints (fatigue, stiffness, abdominal pain, etc) represent core syndromal symptoms of FM, and are often considered by clinicians when makinga diagnosis. Chief among these complaints are those of poor sleep. In subjective assessments of sleep quality, over 75% of individuals with FM report disturbed and nonrestorative sleep.29,50 Objective findings from polysomnographic studies demonstrate that FM patients have abnormal sleep architecture, including an increased sleep onset latency,21 an increased number of nighttime arousals,7 reduced amounts of restorative stage 3/4 sleep,7 and greater alpha wave intrusion.31 Poor sleep may have a reciprocal relationship with pain, as there is evidence to suggest that it is both a consequence of32 and a causal or maintenance mechanism1,6,30 for chronic pain conditions. Additionally, sleep duration has been shown to predict clinical pain in healthy adults13 and adults with insomnia,12 and sleep disruption has been predictive of next day clinical pain in individuals with a pain condition.1,40
The cognitive activation theoryofstress16 offers a theoretical framework to understand our predictive model of FM clinical pain. The theory proposes that through chronic arousal, stress, and lack of restorative sleep, there are changes to the functioning of the HPA-axis and central nervous system that lead to an increased sensitivity to stimulation, particularly pain. Thus, sleep disturbance (in addition to central sensitization, negative mood, and peripheral nociceptive input) may be playing an important role in the maintenance of FM chronic pain. The current study built upon our44,45 predictive model of clinical pain in FM (negative mood, aftersensation ratings taken after a windup protocol, and sum of local pain areas) by examining the role of sleep. We hypothesized that we would replicate our previous results, and that the addition of sleep (measured subjectively and objectively) would significantly increase the predictive ability of the model. Specifically, we hypothesized that increased total wake time and decreased total sleep time would predict higher clinical pain.
Methods
Participants
Written informed consent was obtained from all participants before evaluation, and the University of Florida Institutional Review Board reviewed and approved all procedures described in this report. Adults with FM (N = 74) were recruited to participate in a cognitive behavioral treatment trial for pain and insomnia. Details of the intervention are beyond the scope of this report as it examined pretreatment, baseline data only. Subjects were recruited by television and radio advertising around the Gainesville, Florida area. Participants meeting inclusion criteria were those ≥18 years of age who were able to read and understand English and currently suffering from FM. Subjects were excluded from the study if they were unable to provide informed consent, did not endorse at least 11 sites as painful during tender point testing, or did not report pain in all 4 body quadrants. To maximize the generalizeability of findings, comorbid medical or psychiatric conditions were not exclusion factors for this pretreatment analysis. Medication use was also allowed; however, participants were asked to remain stable on all medications during the study period, and refrain from changing their existing regimen or initiating a new medication.
Procedures
The study observation period lasted 14 days. Participants provided ratings of clinical pain intensity each day in the evening, and completed a sleep diary each day in the morning describing their previous night's sleep. They also wore an actigraph continuously throughout the 14 days, from which objective measures of sleep were derived. Office-based assessments were performed at 3 time points over the 14 days. On day 1, participants provided a measure of spatial extent of pain (shading areas on a body diagram). On day 7, participants underwent quantitative sensory testing—experimental induction of second pain (aftersensation). On day 14, participants completed self-report measures of negative mood, and were asked to make their mood ratings based on the previous 14 days, corresponding to the observation period.
Measures
Clinical Pain Intensity
Participants provided ratings of current clinical pain intensity once a day in the evening for 14 days. Ratings were made using a visual analogue scale anchored with “no pain sensation” on the left side, and “most intense pain imaginable” on the right side. The visual analogue scale was instantiated on paper as a 10-centimeter horizontal line with the 2 anchors, and participants indicated their pain level by marking a spot on the line. To obtain a numerical score for this rating, a ruler was used to measure the distance (in centimeters, to the tenth decimal) between the mark and the left end of the line. This value was then multiplied by 10 to rescale it to a 0 to 100 range. The 14 daily values were averaged to get 1 rating of clinical pain per person. The term clinical pain intensity is used to distinguish it from experimental pain intensity, measured in this study as ratings of painful aftersensation.
Tender Point Testing
On day 1 of the 14-day observation period, tender point sensitivity was assessed at 9 bilaterally paired sites as specified by the American College of Rheumatology criteria for FM.51 The evaluation was done by trained investigators using a Wagner Force One FDIX Dolorimeter (Wagner Instruments, Greenwich, CT). The rubber tip (7/ 16-inch diameter) of the Dolorimeter was placed at each tender point site and pressure was applied with a force of 1 kg/second until the participant indicated pain, or until 4 kg of force had been reached. The tender point was considered positive if pain was indicated at ≤4 kg of force. A total number of positive tender points was calculated for each person, and only those who endorsed ≥11 positive tender points were included in the analysis.
Sum of Local Pain Areas
Participants indicated the location of their current pain by shading the corresponding areas on a diagram depicting the front and back of a human body (Fig 1)44 on day 1 of the observation period. The diagrams were divided into 48 areas, indicated by letters and numbers in the figure. If any part of an individual body area was shaded, it was scored as 1 (painful). An unshaded area was scored as 0 (not painful). A sum of local pain areas was calculated for each subject.
Figure 1.
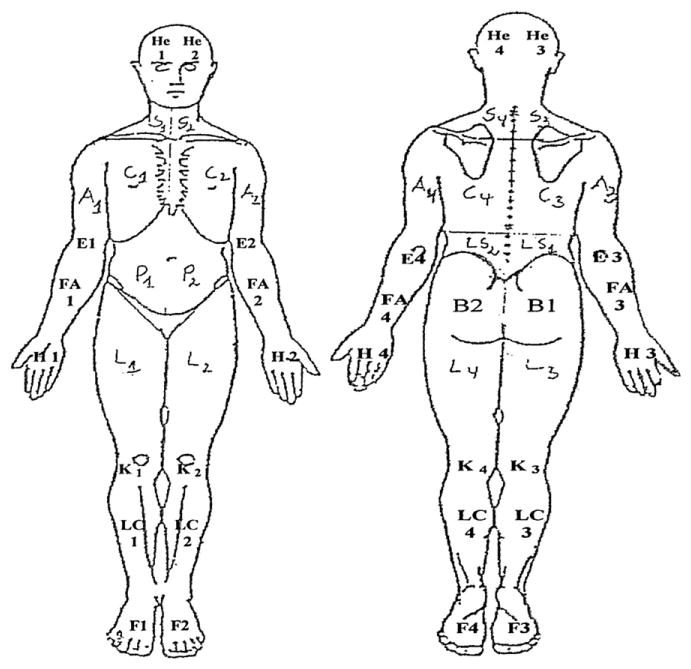
Body diagram used by participants to shade areas of current pain. Each shaded area was coded as 1. Each nonshaded area was coded as 0. A sum of shaded areas was calculated for each participant.
Windup and Aftersensation
Subjects underwent quantitative sensory testing using a computer-controlled Medoc Thermal Sensory Analyzer (Model TSA-II; Ramat Yishai, Israel) on day 7 of the observation period. A windup protocol was used as trains of 8 suprathreshold heat stimuli were delivered via a contact thermode (30 × 30 mm) placed on the thenar eminence of the palm. Each heat stimulus started at a baseline temperature of 39°C, peaked at 49°C, then returned to baseline with a rise and decline rate of 10°C/second. The duration of each stimulus was approximately 1 second with a 3-second interval separating the peak of each stimulus. Subjects were asked to attend to the delayed pain sensation (ie, second pain) felt after every pulse, and cued to verbally rate the intensity of that second pain after the 2nd, 4th, 6th, and 8th pulses using a numerical rating scale anchored with 0 (no pain sensation) and 100 (most intense pain sensation imaginable). Using the same numerical rating scale, ratings of painful aftersensations were obtained at 15 and 30 seconds following the 8th and final heat stimulus. Aftersensation ratings at 30 seconds have been shown to be a strong predictor of FM clinical pain in past research,45 and were used as a predictor of clinical pain intensity in the regression analyses. Each subject completed 2 trials of the windup protocol using their nondominant palm, and the aftersensation ratings from the 2 trials were averaged. Prior to the 2 trials, subjects completed 1 training trial on their dominant palm to familiarize them to the rating system and the range of heat.
Subjective Sleep
Participants completed a sleep diary24 each morning for 14 days, providing information on bed/wake/out-of-bed times, sleep onset latency, number of nighttime awakenings, wake after sleep onset, napping, and sleep quality. For purposes of the present study, only the following 2 derived, subjectively measured sleep variables were used: 1) total sleep time (TSTs) —computed by subtractingtotal wake time from timeinbed;and2)total wake time (TWTs) —time spent awake from bed time until out-of-bed time in the morning. TSTs and TWTs were averaged over the 14 days to obtain 1 mean for each participant. Across the sample, there was a 92.6% compliance rate with the sleep diaries (959 out of 1,036 possible observations). On average, participants completed 13.0 of 14 diaries. When calculating TST and TWT averages for each participant, missing diary data was handled by using the actual number of completed diaries as the denominator. For example, if a participant completed only 12 of 14 daily sleep diaries, TST for each of the 12 days was summed, then divided by 12 to obtain the average.
Objective Sleep
Participants wore an actigraph, the Actiwatch 2 (Phillips Respironics, Bend, OR), on their nondominant wrist 24 hours a day for the 14 days coincident to completing the sleep diaries. Actigraphy and polysomnography, the gold standard objective measurement of sleep, are highly correlated for total sleep time (.91–.97) in healthy adults2 and (.70) in individuals with insomnia.25 Correlations for nighttime wake variables tend to be more moderate (eg, .30 for sleep onset latency and .48 for wake after sleep onset in individuals with insomnia).25 The Actiwatch 2 records data on gross motor activity using a solid-state, piezo-electric accelerometer. The accelerometer continually measures the intensity and frequency of wrist movement at a sampling rate of 32 cycles per second. The sum of all wrist movements in a 30-second interval is recorded as an activity count. The activity counts are downloaded onto a PC and analyzed using Actiware Sleep Analysis Software v.5.3.2 which classifies each 30-second epoch as a sleep or wake state using validated al-gorithms.34 The high sensitivity setting in the Actiware software was used because it provides good correlation with PSG for total sleep time (.70) in individuals with insomnia25 (see McCrae et al27 for additional details regarding this algorithm). The bedtime and morning arise times reported on the sleep diaries were inserted into the corresponding actigraph day, and represented the time-in-bed period. Actiware determined the start of sleep by searching this time-in-bed period for the first 10-minute interval during which no more than 1 epoch was scored as awake. Similarly, sleep end was signified by the last 10-minute interval containing no more than 1 wake-state epoch. Nightly TWT, as measured by actigraphy, is the sum of all the wake epochs within the time-in-bed period. TST is the sum of all sleep epochs within the time-in-bed period. TST and TWT from the 14 days of actigraph were averaged to obtain 1 mean for each participant, and will be referred to as objective TST (TSTo) and objective TWT (TWTo).
Negative Mood
Negative mood was assessed with the Beck Depression Inventory II (BDI-II)5 and the State Trait Anxiety Inventory, State Version-Form Y1 (STAI-Y1).42 On day 14 of the observation period, participants were asked to think about their mood over the previous 14 days (corresponding to the time period assessed by the sleep and pain diaries) when responding to the questions on the BDI-II and STAI-Y1. The BDI-II is a 21-item self-report inventory that measures the severity of current depressive symptomotology,including cognitive, affective, and vegetative symptoms. Each item consists of a group of 4 descriptive statements centering around 1 symptom, and participants choose the statement that most accurately characterizes them. Each item is scored on a 0 to 3 scale, and total scores range from 0 to 63. The STAI-Y1 is a 20-item self-report questionnaire that measures current levels of anxiety. Each item consists of a self-descriptive statement (I feel ___, nervous, calm, etc), and participants rate their agreement with the statement on a 4-point Likert Scale (1 = “not at all” to 4 = “extremely”). A total score is obtained, ranging from 20 to 80. The BDI-II and STAI have been used extensively in a variety of populations, including those with chronic pain and other medical conditions. The instruments are well validated, show good reliability (alpha coefficients >.8), and can accurately distinguish between clinical and nonclinical populations.
Statistical Analysis
The Statistical Package for the Social Sciences (SPSS, v.17.0) was used for all statistical analyses. Descriptive statistics were calculated for demographic and clinical variables across the sample. Pearson r correlation statistic was used to determine the zero-order correlations between variables in the regression analysis. Because of the potential multicollinearity between the BDI and STAI total scores (BDI and STAI total scores were strongly correlated at the zero-order, r = .80, P < .001), Principle Axis Factoring with oblique rotation was used to determine if the 2 mood measures loaded onto a single latent negative mood factor. The analysis yielded 1 latent factor with an eigenvalueof1.80 and accounting for 89.94% of the variance among the measures. The corresponding factor regression scores were used to create a negative mood factor, which was used in the subsequent hierarchical regression analyses. The use of a negative mood factor supports previous research in our laboratory.20,33 Hierarchical linear regression was used to determine the variance in clinical pain accounted for by aftersensation ratings, sum of local pain areas, negative mood factor, TWTs, TWTo, TSTs, and TSTo. Each independent variable was entered as a separate block in the hierarchical regression, and the associated R2 change value established their unique contribution to the dependent variable, clinical pain intensity. Four hierarchical regression analyses were run. In all analyses, the variables found to be predictive of clinical pain in past research (aftersensation ratings, sum of local pain areas, negative mood factor) were entered into the first 3 blocks. Then each of the 4 analyses entered a different sleep variable (TSTs, TWTs, TSTo, or TWTo) into the final block to determine whether measures of sleep increased the predictive ability of the model.
Results
Demographic Characteristics
Seventy-four adults with FM provided data for the analysis. Demographic characteristics of the sample are presented in Table 1. The average age of the sample was 52.6 years. Consistent with population estimates of FM, the majority (94.6%) of participants were female. The racial composition was as follows: 82.4% Caucasian, 14.9% African American, 1.4% Asian, and 1.4% other. The marital status of the sample consisted of the following: 45.9% married, 18.9% single, 24.3% divorced, 2.7% separated, and 8.1% widowed.
Table 1.
Demographic Characteristics of the Sample (N = 74)
| N | % | Years | |
|---|---|---|---|
| Mean age in years | 52.6 (SD = 9.8) | ||
| Female gender | 70 | 94.6 | |
| Race | |||
| Caucasian | 61 | 82.4 | |
| African American | 11 | 14.9 | |
| Asian | 1 | 1.4 | |
| Other | 1 | 1.4 | |
| Marital status | |||
| Married | 34 | 45.9 | |
| Divorced | 18 | 24.3 | |
| Separated | 2 | 2.7 | |
| Widowed | 6 | 8.1 | |
| Single | 14 | 18.9 | |
| Mean years of education | 14.3 (SD = 2.4) | ||
| Employment | |||
| Employed | 39 | 52.7 | |
| Unemployed | 35 | 47.3 |
Clinical Characteristics
Pain, mood, and sleep characteristics of the sample are presented in Table 2. The mean rating of clinical pain intensity across the sample was 53.0 (range: 8.7–95.4, SD = 20.3). Subjects endorsed an average of 16.1 painful tender points (SD = 2.2), and shaded 21.9 (SD = 11.5) areas of pain on the body diagram. Following experimental induction of second pain, 30-second aftersensation ratings ranged from 0 to 77.5, with a mean of 27.4, SD = 22.9. Participants reported moderate levels of depressive and anxiety symptoms (mean BDI-II total score: 15.8, SD = 11.5; mean STAI-Y1 total score: 43.1, SD = 14.6). Self-report data from the sleep diaries indicated that the TSTs across the sample was 390.1 minutes (range: 213.5– 569.3.9, SD = 66.8) and the TWTs was 120.9 minutes (range: 28.2–361.3, SD = 67.8). Actigraphy data indicated that the TSTo across the sample was 396.5 minutes (range: 221.6–548.4, SD = 76.0), and the TWTo was 101.0 minutes (range: 5.8–305.2, SD = 52.0).
Table 2.
Clinical Characteristics of the Sample
| Mean | SD | Min–Max | |
|---|---|---|---|
| Clinical pain intensity | 53.0 | 20.3 | 8.7–95.4 |
| Tenderpoint count | 16.1 | 2.2 | 11–18 |
| Aftersensation | 27.4 | 22.9 | 0–77.5 |
| Sum of local pain areas | 21.9 | 11.2 | 4.0–48.0 |
| BDI-II total score | 15.8 | 11.5 | 0–46 |
| STAI total score | 43.1 | 14.6 | 20–78 |
| TSTo | 396.5 | 76.0 | 221.6–548.4 |
| TWTo | 101.0 | 52.0 | 5.8–305.2 |
| TSTs | 390.1 | 66.8 | 213.5–569.3 |
| TWTs | 120.9 | 67.8 | 28.2–361.3 |
Hierarchical Regression Analyses
Table 3 presents the zero-order correlations between predictor variables and the dependent variable, clinical pain intensity. Notably, clinical pain demonstrated significant correlations with aftersensation (r = .24, P = .04), sum of local pain areas (r = .55, P < .001), negative mood (r = .35, P = .002), and TWTo (r = .23, P = .05). In addition to clinical pain, negative mood was significantly correlated with sum of local pain areas (r = .29, P = .01), TWTo (r = .23, P = .006), and TWTs (r = .25, P = .03). Table 4 presents the results of the hierarchical regression analyses. Higher aftersensation ratings (ΔR2 = .06,P= .04), more bodily pain areas (ΔR2 = .26, P < .001), and higher negative mood (ΔR2 = .04, P = .04) were each significant predictors of higher clinical pain and together accounted for 36.0% of its variance. None of the 4 measures of sleep were significant predictors clinical pain at the P=.05 level (TSTo ΔR2 = .03, P = .06; TWTo ΔR2 = .00, P = .62; TSTs ΔR2 = .01, P=.38; TWTs ΔR2 = .00,P=.56); however, TSTo reached the level of statistical trend (ΔR2 = .03, P = .06).
Table 3.
Zero-Order Correlation Matrix Between Variables in the Regression Analyses
| Clinical Pain | After-sensation | Sum of Local Pain Areas | Negative Mood | TSTo | TWTo | TSTs | TWTs | |
|---|---|---|---|---|---|---|---|---|
| Clinical pain | – | *.24 (.04) | .55 (<.001) | .35 (.002) | −.18 (.12) | .23 (.05) | −.05 (.69) | .10 (.39) |
| Aftersensation | .24 (.04) | – | .14 (.24) | .03 (.83) | .18 (.12) | −.09 (.47) | .11 (.38) | −.00 (.98) |
| Sum of local pain areas | .55 (<.001) | .14 (.23) | – | .29 (.01) | −.01 (.91) | .29 (.01) | .08 (.52) | .22 (.06) |
| Negative mood | .35 (.002) | .03 (.83) | .29 (.01) | – | −.06 (.60) | .32 (.006) | −.06 (.61) | .25 (.03) |
| TSTo | −.18 (.12) | .18 (.12) | −.01 (.91) | −.06 (.60) | – | −.09 (.45) | .60 (<.001) | .17 (.16) |
| TWTo | .23 (.05) | −.09 (.47) | .29 (.01) | .32 (.006) | −.09 (.45) | – | .08 (.48) | .67 (<.001) |
| TSTs | −.05 (.69) | .11 (.38) | .08 (.52) | −.06 (.61) | .60 (<.001) | .08 (.48) | – | −.23 (.04) |
| TWTs | .10 (.39) | −.00 (.98) | .21 (.06) | .25 (.03) | .17 (.16) | .67 (<.001) | −24 (.04) | – |
Pearson r value (P value).
Table 4.
Results of the Hierarchical Regression Analyses Predicting Clinical Pain
| Block | Variable | ΔR2 | F Change | P Value |
|---|---|---|---|---|
| 1 | Aftersensation | .06 | 4.23 | .04 |
| 2 | Sum of local pain areas | .26 | 26.40 | <.001 |
| 3 | Negative mood factor | .04 | 4.57 | .04 |
| 4a | TSTo | .03 | 3.58 | .06 |
| 4b | TWTo | .00 | .26 | .62 |
| 4c | TSTs | .01 | .80 | .38 |
| 4d | TWTs | .00 | .34 | .56 |
| *Full Model | Beta (Standardized) | T Value | P Value | |
|
| ||||
| 1 | Aftersensation | .20 | 2.02 | .05 |
| 2 | Sum of local pain areas | .48 | 4.81 | <.001 |
| 3 | Negative mood factor | .20 | 2.03 | .05 |
| 4a | TSTo | −.18 | −1.89 | .06 |
| 4b | TWTo | .05 | .51 | .62 |
| 4c | TSTs | −.09 | −.89 | .38 |
| 4d | TWTs | −.06 | −.58 | .56 |
Full model for blocks 1–4a = aftersensation, sum of local pain areas, negative mood, and TSTo.
Full model for blocks 1–4b = aftersensation, sum of local pain areas, negative mood, and TWTo.
Full model for blocks 1–4c = aftersensation, sum of local pain areas, negative mood, and TSTs.
Full model for blocks 1–4d = aftersensation, sum of local pain areas, negative mood, and TWTs.
The full model entered the independent variables simultaneously.
Discussion
The purpose of this study was to determine the predictors of clinical pain intensity in adults with FM, and it represented a replication and extension of our work.44,45 Results confirm that a predictive model of aftersensation, the sum of local pain areas, and negative mood accounts for a significant portion of variance (36.0%) in clinical pain. None of the 4 measures of sleep accounted for significant additional variance, though TSTo reached the level of statistical trend in accounting for 3%of the variance in clinical pain.
The examination of sleep represented an extension of our predictive model of clinical pain in FM. To date, this was the first study to examine TST and TWT as a predictor of clinical pain at the between-person level in an FM population. All 4 sleep measures failed to significantly predict clinical pain in our study. Though the study designs and populations differ, longitudinal within-person findings on the effect of sleep duration on pain are generally contrary to our results. Edwards et al13,14 demonstrated that longer TST predicted better pain inhibitory control the next morning in temporomandibular joint disorder patients, and shorter TST predicted higher next day pain in a general population. Wilson et al49 found that more pain during the day predicted shorter TST at night in a chronic musculoskeletal pain population. Regarding insomnia, disturbed sleep has been found to predict higher pain in an FM population49 and in hospitalized burn patients.39 Additionally, laboratory studies in healthy subjects have found that sleep deprivation results in decreased mechanical and heat pain thresholds the following day.22,35
Several potential explanations exist to account for the lack of significance in the measures of sleep. It is possible that a sleep-pain relationship was washed out at the between-person level using 14-day averages. Rather, it may be that an individual night of poor sleep is followed by a day of higher pain or vice versa. Indeed, evidence for this daily variation between sleep and pain exists,13,14,39,49 and 2 studies have found significant relationships at the within-person level but not at the group level.1,12 It also may be that the effects of poor sleep (eg, daytime fatigue, inactivity, or perceived sleep quality), rather than measures of sleep or insomnia duration, are more important in determining clinical pain. There is evidence that fatigue and pain are related in FM samples,32 and the perception of sleep quality has been shown to be a predictor of clinical pain in FM as well.40 It is also possible that a sleep-pain relationship is dependent on disruption of specific sleep stages. Muldofsky et al30 and others35 have demonstrated that selective disruption of Stage 3, slow wave sleep in healthy subjects, resulted in symptoms similar to FM pain (eg, areas of muscle tenderness). Additionally, FM patients have been shown to exhibit abnormal patterns of alpha activity during slow wave sleep compared with normal controls.41 Finally, the magnitude of a sleep-pain relationship may be related to the timing of clinical pain measurement. In this study, clinical pain was measured in the evening; it may be that morning or afternoon pain is more strongly related to sleep duration or nightly wake time.
Statistically, 3 of the 4 sleep measures (TSTs, TWTs, TSTo) demonstrated nonsignificant zero order correlations with the dependent variable, clinical pain intensity. Therefore, it is unlikely that their inability to predict clinical pain can be explained by multicollinearity with other predictors. Though weak in strength, TWTo demonstrated significant zero-order correlations with clinical pain (r = .23, P = .05) and with negative mood (r = .32, P = .006), but in the regression model, TWTo failed to predict clinical pain. Thus, it is possible that the covariation with negative mood may play a role in the relationship between insomnia and pain, consistent with our previous research.33 To address the possibility that pain, sleep, or mood medication use may have affected sleep duration or nightly wake time, we conducted exploratory analyses controlling for usage of these medications. Results did not change; none of the 4 measures of sleep duration were significant predictors of clinical pain.
Central sensitization has been hypothesized to be the primary pathophysiological mechanism for the maintenance of FM pain as well as other musculoskeletal pain conditions.52 In central sensitization, neurons in the dorsal horn of the spinal cord become hyperexcitable and subsequently hyperresponsive due to prolonged noxious stimuli. One of the ways these neuroplastic changes are manifested is in prolonged poststimulus pain due to a disruption in endogenous pain inhibitory systems.10,28 Painful aftersensation ratings taken during experimental heat pain induction are a measure of this inhibitory dysfunction, and therefore a psychophysical correlate to central sensitization. In this study, higher aftersensation ratings predicted more clinical pain and accounted for 6% of its variance. This result is a confirmation of previous research,45 and taken together with findings of abnormal windup in FM,43 it suggests that central sensitization is an important mechanism in FM clinical pain.
Widespread pain (ie, a lack of spatial localization) is one of the diagnostic hallmarks for FM, and is also an indication of central sensitization. The sum of local pain areas was used as the measure of spatial extent in the analysis and proved to be a powerful predictor of daily clinical pain by accounting for 26% of its variance. This confirms past research suggesting that spatial extent plays an important role in determining clinical pain intensity in FM,44 and complements findings of spatial summation in pain threshold and tolerance during experimental pain in-duction.8,11,37 Additionally, the result supports the clinical utility of shading areas on a body diagram as an indicator of the magnitude of daily pain in FM patients, and to potentially guide localized treatment targets.
Higher negative mood predicted more clinical pain and accounted for 4% of its variance. The result confirms Staud's44,45 findings of the importance of negative mood in FM clinical pain, and adds evidence to a large literature linking depression and anxiety with chronic pain.4,19 Clinically, the presence of depression portends worse pain outcomes and greater functional limitations in those with chronic pain.15,48 Given their frequent co-occurrence, it follows that pain and negative mood may share similar biological and behavioral mechanisms. Regions of the brain involved with emotion regulation (amygdala, hypothalamus, medial prefrontal cortex) are intricately connected to those involved with pain modulation (periaqueductal gray). Thus, negative expectations and emotions may amplify pain signals, increasing the intensity and duration of pain experienced. Behaviorally, depression is associated with a lack of motivation and physical inactivity. Physical inactivity can contribute to the muscle stiffness experienced by patients with FM, and conversely, exercise has been shown to have a beneficial effect on symptoms of FM.17,26
In clinical application, the results of the present study suggest that FM patients may benefit from a 3-pronged approach to pain management. The significance of the sum of local pain areas to clinical pain indicates that peripheral nociceptive sources are playing an important role in the generalized pain hypersensitivity of FM. To decrease the spatial extent of pain, patients may benefit from anesthetic injection to or manual manipulation of myofascial trigger points. To address the implication of central sensitization in the aftersensation finding, treatments should be aimed at normalizing the hyperexcitability of neurons in the central nervous system. Pregabalin and duloxetine are centrally acting medications that have demonstrated some success in treating FM pain.3,9 Finally, psychobehavioral therapies should be considered to treat those with mood dysfunction and maladaptive pain coping. There is evidence to suggest that exercise18,23 and cognitive behavioral therapy23,47 are successful in treating depression as well as improving pain-related variables in FM patients. While these predictors of clinical pain offer logical treatment targets, it is important to note that directionality of influence cannot be assumed from these results. It is possible that reducing clinical pain intensity may reduce spatial extent, negative mood, and central sensitization, rather than the converse.
In conclusion, the current study confirmed that a model of aftersensation, sum of local pain areas, and negative mood is a strong predictor of clinical pain intensity in FM. The data did not support the hypothesis that measures of sleep would increase the predictive ability of the model. With an eye toward treatment targets and mechanisms of action, future research should examine other clinical correlates of FM to strengthen the predictive ability of the model. These might include known correlates like physical activity level, cognitive dysfunction, and symptoms of irritable bowel syndrome, or other sleep-related variables like fatigue, perception of sleep quality, or stage-specific abnormalities during sleep. Future research should also longitudinally examine the intra-individual, daily variation between sleep and pain. This can help to clarify whether a sleep-pain relationship in FM exists on a daily level, and if so, which of the 2 variables are driving the relationship. Despite its inability to predict pain in this sample, sleep dysfunction remains an important outcome measure in FM due to its high prevalence and detrimental effect on daily functioning and quality of life.
Perspective.
This study suggests that measures of sleep duration and nightly wake time do not predict fibromyalgia pain at the group level. Fibromyalgia patients may benefit from a 3-pronged approach to pain management: reducing pain's spatial extent, normalization of central nervous system hypersensitivity, and psychobehavioral therapies for negative mood.
Acknowledgments
Support provided by a grant from the National Institutes of Health (R01 AR055160) to Dr. McCrae and Dr. Robinson.
References
- 1.Affleck G, Urrows S, Tennen H, Higgins P, Abeles M. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain. 1996;68:363–368. doi: 10.1016/s0304-3959(96)03226-5. [DOI] [PubMed] [Google Scholar]
- 2.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 3.Arnold LM, Rosen A, Pritchett YL, D'Souza DN, Goldstein DJ, Iyengar S, Wernicke JF. A randomized, double-blind, placebo-controlled trial of duloxetine in the treatment of women with fibromyalgia with or without major depressive disorder. Pain. 2005;119:5–15. doi: 10.1016/j.pain.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 4.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: A literature review. Arch Intern Med. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 5.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 6.Bigatti SM, Hernandez AM, Cronan TA, Rand KL. Sleep disturbances in fibromyalgia syndrome: Relationship to pain and depression. Arthritis Rheum. 2008;59:961–967. doi: 10.1002/art.23828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Branco J, Atalaia A, Paiva T. Sleep cycles and alpha-delta sleep in fibromyalgia syndrome. J Rheumatol. 1994;21:1113–1117. [PubMed] [Google Scholar]
- 8.Coghill RC, Mayer DJ, Price DD. The roles of spatial recruitment and discharge frequency in spinal cord coding of pain: A combined electrophysiological and imaging investigation. Pain. 1993;53:295–309. doi: 10.1016/0304-3959(93)90226-F. [DOI] [PubMed] [Google Scholar]
- 9.Crofford LJ, Rowbotham MC, Mease PJ, Russell IJ, Dworkin RH, Corbin AE, Young JP, Jr, LaMoreaux LK, Martin SA, Sharma U. Pregabalin for the treatment of fibromyalgia syndrome: Results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2005;52:1264–1273. doi: 10.1002/art.20983. [DOI] [PubMed] [Google Scholar]
- 10.Dickenson AH, Sullivan AF. NMDA receptors and central hyperalgesic states. Pain. 1991;46:344–346. doi: 10.1016/0304-3959(91)90118-H. [DOI] [PubMed] [Google Scholar]
- 11.Douglass DK, Carstens E, Watkins LR. Spatial summation in human thermal pain perception: Comparison within and between dermatomes. Pain. 1992;50:197–202. doi: 10.1016/0304-3959(92)90161-4. [DOI] [PubMed] [Google Scholar]
- 12.Dzierzewski JM, Williams JM, Roditi D, Marsiske M, McCoy K, McNamara J, Dautovich N, Robinson ME, McCrae CS. Daily variations in objective nighttime sleep and subjective morning pain in older adults with insomnia: Evidence of covariation over time. J Am Geriatr Soc. 2010;58:925–930. doi: 10.1111/j.1532-5415.2010.02803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards RR, Almeida DM, Klick B, Haythornthwaite JA, Smith MT. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008;137:202–207. doi: 10.1016/j.pain.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards RR, Grace E, Peterson S, Klick B, Haythornthwaite JA, Smith MT. Sleep continuity and architecture: Associations with pain-inhibitory processes in patients with temporomandibular joint disorder. Eur J Pain. 2009;13:1043–1047. doi: 10.1016/j.ejpain.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engel CC, von Korff M, Katon WJ. Back pain in primary care: Predictors of high health-care costs. Pain. 1996;65:197–204. doi: 10.1016/0304-3959(95)00164-6. [DOI] [PubMed] [Google Scholar]
- 16.Eriksen HR, Ursin H. Subjective health complaints, sensitization, and sustained cognitive activation (stress) J Psychosom Res. 2004;56:445–448. doi: 10.1016/S0022-3999(03)00629-9. [DOI] [PubMed] [Google Scholar]
- 17.Fontaine KR, Conn L, Clauw DJ. Effects of lifestyle physical activity on perceived symptoms and physical function in adults with fibromyalgia: Results of a randomized trial. Arthritis Res Ther. 2010;12:R55. doi: 10.1186/ar2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gowans SE, deHueck A, Voss S, Silaj A, Abbey SE, Reynolds WJ. Effect of a randomized, controlled trial of exercise on mood and physical function in individuals with fibromyalgia. Arthritis Rheum. 2001;45:519–529. doi: 10.1002/1529-0131(200112)45:6<519::aid-art377>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Gureje O. Comorbidity of pain and anxiety disorders. Curr Psychiatry Rep. 2008;10:318–322. doi: 10.1007/s11920-008-0051-0. [DOI] [PubMed] [Google Scholar]
- 20.Hirsh AT, Waxenberg LB, Atchison JW, Gremillion HA, Robinson ME. Evidence for sex differences in the relationships of pain, mood, and disability. J Pain. 2006;7:592–601. doi: 10.1016/j.jpain.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Horne JA, Shackell BS. Alpha-like EEG activity in non-REM sleep and the fibromyalgia (fibrositis) syndrome. Electroencephalogr Clin Neurophysiol. 1991;79:271–276. doi: 10.1016/0013-4694(91)90122-k. [DOI] [PubMed] [Google Scholar]
- 22.Kundermann B, Spernal J, Huber MT, Krieg JC, Lautenbacher S. Sleep deprivation affects thermal pain thresholds but not somatosensory thresholds in healthy volunteers. Psychosom Med. 2004;66:932–937. doi: 10.1097/01.psy.0000145912.24553.c0. [DOI] [PubMed] [Google Scholar]
- 23.Lemstra M, Olszynski WP. The effectiveness of multidisciplinary rehabilitation in the treatment of fibromyalgia: A randomized controlled trial. Clin J Pain. 2005;21:166–174. doi: 10.1097/00002508-200503000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Lichstein KL, Riedel BW, Means MK. Psychological treatment of late-life insomnia. In: Schultz R, Maddox G, Lawton MP, editors. Annual Review of Gerontology and Geriatrics: Vol 19 Focus on Interventions Research With Older Adults. New York, NY: Springer; 1999. pp. 74–110. [Google Scholar]
- 25.Lichstein KL, Stone KC, Donaldson J, Nau SD, Soeffing JP, Murray D, Lester KW, Aguillard RN. Actigraphy validation with insomnia. Sleep. 2006;29:232–239. [PubMed] [Google Scholar]
- 26.Mannerkorpi K, Nordeman L, Cider A, Jonsson G. Does moderate-to-high intensity Nordic walking improve functional capacity and pain in fibromyalgia? A prospective randomized controlled trial. Arthritis Res Ther. 2010;12:R189. doi: 10.1186/ar3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCrae CS, Rowe MA, Tierney CG, Dautovich ND, Definis AL, McNamara JP. Sleep complaints, subjective and objective sleep patterns, health, psychological adjustment, and daytime functioning in community-dwelling older adults. J Gerontol B Psychol Sci Soc Sci. 2005;60:P182–P189. doi: 10.1093/geronb/60.4.p182. [DOI] [PubMed] [Google Scholar]
- 28.Meeus M, Nijs J. Central sensitization: A biopsychosocial explanation for chronic widespread pain in patients with fibromyalgia and chronic fatigue syndrome. Clin Rheumatol. 2007;26:465–473. doi: 10.1007/s10067-006-0433-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moldofsky H. The significance, assessment, and management of nonrestorative sleep in fibromyalgia syndrome. CNS Spectr. 2008;13:22–26. doi: 10.1017/s1092852900026808. [DOI] [PubMed] [Google Scholar]
- 30.Moldofsky H, Scarisbrick P. Induction of neurasthenic musculoskeletal pain syndrome by selective sleep stage deprivation. Psychosom Med. 1976;38:35–44. doi: 10.1097/00006842-197601000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Moldofsky H, Scarisbrick P, England R, Smythe H. Musculosketal symptoms and non-REM sleep disturbance in patients with “fibrositis syndrome” and healthy subjects. Psychosom Med. 1975;37:341–351. doi: 10.1097/00006842-197507000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Nicassio PM, Moxham EG, Schuman CE, Gevirtz RN. The contribution of pain, reported sleep quality, and depressive symptoms to fatigue in fibromyalgia. Pain. 2002;100:271–279. doi: 10.1016/S0304-3959(02)00300-7. [DOI] [PubMed] [Google Scholar]
- 33.O'Brien EM, Waxenberg LB, Atchison JW, Gremillion HA, Staud RM, McCrae CS, Robinson ME. Negative mood mediates the effect of poor sleep on pain among chronic pain patients. Clin J Pain. 2010;26:310–319. doi: 10.1097/AJP.0b013e3181c328e9. [DOI] [PubMed] [Google Scholar]
- 34.Oakley NR. Validation with polysomnography of the Sleepwatch sleep/wake scoring algorithm used by the Actiwatch activity monitoring system. Bend, OR: Mini Mitter Co, Inc; 1997. [Google Scholar]
- 35.Onen SH, Alloui A, Gross A, Eschallier A, Dubray C. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res. 2001;10:35–42. doi: 10.1046/j.1365-2869.2001.00240.x. [DOI] [PubMed] [Google Scholar]
- 36.Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3:57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- 37.Price DD, McHaffie JG, Larson MA. Spatial summation of heat-induced pain: Influence of stimulus area and spatial separation of stimuli on perceived pain sensation intensity and unpleasantness. J Neurophysiol. 1989;62:1270–1279. doi: 10.1152/jn.1989.62.6.1270. [DOI] [PubMed] [Google Scholar]
- 38.Price DD, Staud R, Robinson ME, Mauderli AP, Cannon R, Vierck CJ. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain. 2002;99:49–59. doi: 10.1016/s0304-3959(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 39.Raymond I, Ancoli-Israel S, Choiniere M. Sleep disturbances, pain and analgesia in adults hospitalized for burn injuries. Sleep Med. 2004;5:551–559. doi: 10.1016/j.sleep.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Raymond I, Nielsen TA, Lavigne G, Manzini C, Choiniere M. Quality of sleep and its daily relationship to pain intensity in hospitalized adult burn patients. Pain. 2001;92:381–388. doi: 10.1016/S0304-3959(01)00282-2. [DOI] [PubMed] [Google Scholar]
- 41.Roizenblatt S, Moldofsky H, Benedito-Silva AA, Tufik S. Alpha sleep characteristics in fibromyalgia. Arthritis Rheum. 2001;44:222–230. doi: 10.1002/1529-0131(200101)44:1<222::AID-ANR29>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 42.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory (“Self-Evaluation Questionnaire”) Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 43.Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJ., Jr Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain. 2003;102:87–95. doi: 10.1016/s0304-3959(02)00344-5. [DOI] [PubMed] [Google Scholar]
- 44.Staud R, Price DD, Robinson ME, Vierck CJ., Jr Body pain area and pain-related negative affect predict clinical pain intensity in patients with fibromyalgia. J Pain. 2004;5:338–343. doi: 10.1016/j.jpain.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Staud R, Robinson ME, Vierck CJ, Jr, Cannon RC, Mauderli AP, Price DD. Ratings of experimental pain and pain-related negative affect predict clinical pain in patients with fibromyalgia syndrome. Pain. 2003;105:215–222. doi: 10.1016/s0304-3959(03)00208-2. [DOI] [PubMed] [Google Scholar]
- 46.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 47.Turk DC, Okifuji A, Sinclair JD, Starz TW. Interdisciplinary treatment for fibromyalgia syndrome: Clinical and statistical significance. Arthritis Care Res. 1998;11:186–195. doi: 10.1002/art.1790110306. [DOI] [PubMed] [Google Scholar]
- 48.Wells KB, Golding JM, Burnam MA. Affective, substance use, and anxiety disordersinpersons with arthritis, diabetes, heart disease, high blood pressure, or chronic lung conditions. Gen Hosp Psychiatry. 1989;11:320–327. doi: 10.1016/0163-8343(89)90119-9. [DOI] [PubMed] [Google Scholar]
- 49.Wilson KG, Watson ST, Currie SR. Daily diary and ambulatory activity monitoring of sleep in patients with insomnia associated with chronic musculoskeletal pain. Pain. 1998;75:75–84. doi: 10.1016/S0304-3959(97)00207-8. [DOI] [PubMed] [Google Scholar]
- 50.Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38:19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- 51.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Franklin CM, Gattner RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, McCain GA, Reynolds WJ, Romano TJ, Russell IJ, Sheon RP. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 52.Yunus MB. Role of central sensitization in symptoms beyond muscle pain, and the evaluation of a patient with widespread pain. Best Pract Res Clin Rheumatol. 2007;21:481–497. doi: 10.1016/j.berh.2007.03.006. [DOI] [PubMed] [Google Scholar]


