Abstract
Dibenzo[def,p]chrysene (DBC) is a potent environmental carcinogen in rodents, fish, and human cells examined in culture. There are numerous similarities between the patterns of P450 activation of DBC and its covalent binding to DNA and proteins with another polycyclic aromatic hydrocarbon (PAH), 7-12, dimethylbenz[a]anthracene (DMBA). Our lab has previously shown that DMBA produces immunosuppression in rodents and human cell systems. Therefore, the purpose of these studies was to examine the immunotoxicity of DBC in a rodent model that was found to be sensitive to the immunosuppressive effects of DMBA. Data showed that DBC had similar potency to DMBA in producing suppression of a T-dependent antibody response (TDAR) and altered spleen cell subsets in a similar manner as DMBA when DMBA was given by gavage for 5 days in corn oil to mice at doses of 1-100 mg/kg total cumulative doses. T cell-independent antigen (TNP-Ficoll) responses were quantitatively less sensitive to DBC suppression. It was also found that as with DMBA, DBC produced a persistent immunosuppression which lasted for at least 4 weeks following dosing with a novel pill method for self-administration of DBC. In conclusion, DBC appears to possess many of the same characteristics of DMBA in terms of its immunotoxicity.
Keywords: immunosuppression; polycyclic aromatic hydrocarbons; TDAR; dibenzo[def,p] chrysene (DBC)
Introduction
Several high molecular weight polycyclic aromatic hydrocarbons (PAH) are carcinogenic and immunosuppressive. In our experience, PAHs such as 7,12-dimethylbenz[a]anthracene (DMBA) that are activated to diol-epoxides by CYP1B1 and epoxide hydrolase are genotoxic and produce particularly potent immunotoxicity in murine models (Gao et al, 2005, 2007). The purpose of the present studies was to examine the immunotoxicity of an environmentally relevant high molecular weight PAH known as dibenzo[def,p]chrysene (DBC), also formerly known as dibenzo[a,l]pyrene (DB[a,l]P). DBC is one of the most potent carcinogens ever discovered (Cavalieri et al.,1991, 2005; Higginbotham et al., 1993), and is present in coal-fired power plant emissions and other sources (World Health Organization, 2010), As with DMBA, DBC is genotoxic and forms covalent DNA adducts following CYP1B1 and epoxide hydrolase activation (Devanesan et al., 1990, 1999; Crowell et al., 2011). Because DBC has many properties that are similar to DMBA in its metabolic activation, we examined the potential immunotoxicity of DBC using a T-dependent antibody response (TDAR) ex vivo model that we have previously shown to be very sensitive to treatment with DMBA (Burchiel et al., 1988; Gao et al., 2005, 2007, 2008). The second aim was to evaluate a new method for administering test compounds using a self-ingestion pill method developed by Walker et al. (2012), which has been found to be less stressful than oral gavage.
Material and Methods
Chemicals
Reagents and chemicals were purchased from Sigma Aldrich unless otherwise stated. Dibenzo[def,p]chrysene (DBC), also previously known as dibenzo[a,l]pyrene (DB[a,l]P), was purchased from AccuStandard (New Haven, CT) and was greater than 95% pure.
Animals
All animal procedures were performed in accordance with our protocol. Protocols and procedures were approved by the University of New Mexico's Institutional Animal Use and Care Committee and were carried out by trained individuals. Mice were housed in a temperature-controlled environment with 12 hr light/dark cycles and received standard mouse chow and water ad libitum. Male C57BL/6J mice used for these studies were obtained from Jackson Laboratories (Bar Harbor, ME) and were used at 11 to 16 weeks of age and weighing approximately 24 g.
Care in handling DBC
DBC is known to be mutagenic/genotoxic and carcinogenic and therefore needs to be handled with caution. Appropriate protective equipment was used when handling this chemical or animals treated with this chemical. Disposal of all contaminated bedding, carcasses, and excess chemical was performed following local and state guidelines.
Dosing via oral gavage
Prior to dosing, mice were trained to accept the gavage needle by administering corn oil via oral gavage for two consecutive days and then transferred to disposable cages (Ancare Corp. Bellmore, NY) to acclimate. Approximately 48 hrlater mice were dosed daily for 5 days with either corn oil (vehicle control), 0.02, 0.2, 2 or 20 mg/kg/day DBC (0.1mg, 1 mg, 10 mg or 100 mg/kg cumulative dose) prepared in corn oil.
Preparation and dosing of pill
Pills containing DBC were prepared as described by Walker et al. (2012). Due to the hazards associated with DBC extra precautions were taken to avoid contaminating work areas and equipment. The work area was prepared by covering it with plastic backed bench cover. A piece of parafilm cut slightly larger than the pill mold (100 mg, Gallipot, St Paul, MN) was placed on the bench paper as the work surface. The parafilm was changed with each dose to prevent any cross contamination. DBC was dissolved in either corn oil or DMSO (for experiments with larger doses). The DBC was then incorporated into a sterile, bacon flavored, transgenic dough (Bio-Serv, Frenchtown, NJ) along with 0.1% bromophenol blue. Dosing calculations were based on the average mouse weight of the mice on study and 136 mg dough per pill. The mixture was folded together using a metal spatula until the dye was evenly distributed throughout the dough. Control pills were made using the same volume of corn oil or DMSO and dye. Training pills contained only dough. The pill molds were then filled with the treated dough using a metal spatula. Care was taken to make the pills uniform. The pill mold was then placed onto the pill stand and pills were released from the mold and dried overnight in a biosafety cabinet. After drying, the pills were collected into glass vials according to the dose and stored at 4°C. Prior to dosing, mice were trained to consume the dough in the pill form. Mice were housed individually in order to ensure that each mouse consumed the pill. To administer the pill the bedding was pushed away from the front of the cage and the pill was placed in the clearing. Mice would consume the pill within 30 min on the training days and by the first day of dosing they would consume it within min. Control pills or those with DBC were given to each mouse daily for 5 days. Forty-eight hr after the last dose mice were euthanized by CO2 narcosis. Spleens were aseptically harvested and spleen cells were isolated. One-half of the spleen was placed in sterile Hanks’ Balanced Salt Solution (Lonza, Walkersville, MD) for the T cell- dependent antibody response (TDAR) assay. The other one-half of the spleen was frozen in liquid nitrogen for subsequent studies and then stored at -80°C.
Spleen cell isolation
Spleen cell suspensions were prepared as described previously by Mitchell et al. (2007). In brief, spleens were weighed and then placed in approximately 5 ml of complete media [RPMI 1640 media supplemented with 10% fetal bovine serum (FBS, Hyclone, Logan, UT), 2mM L-glutamine (Gibco by Life Technologies, Grand Island, NY) 100 μg/ml streptomycin and 100 units/ml penicillin (Gibco by Life Technologies, Grand Island, NY) and 50 μM 2-mercaptoethanol (Sigma; suitable for cell culture)]. The spleens cells were then isolated by using the sterilized frosted ends of two microscope slides to homogenize the spleen. Cells were centrifuged at 280 × g for 10 min at 4°C, the media was aspirated and cell pellets were resuspended in 5 ml complete RPMI and held on ice. Viable and non-viable cells were counted using the trypan blue exclusion method and a hemacytometer.
Cell Surface Markers
Custom rat anti-mouse monoclonal antibody cocktails for CD45 and specific cell surface markers were obtained from BD Biosciences (BD Pharmingen, San Diego, CA). The specific cell surface markers used were CD3 (pan T cells), CD4 (TH cells), CD8 (TC cells), CD19 (B cells), pan NK(natural killer cells), and CD11b (Mac-1, macrophage cells). The cocktails and staining procedure are described elsewhere (Gao et al., 2005, 2007). Briefly, freshly isolated mouse spleen cells were aliquoted into flow tubes at 1 ×106 cells/ tube, incubated with CD16/CD32 Fc blocking antibody and then stained with an antibody cocktail listed here, the fluorochrome label is in parenthesis; IgG1+IgG2a(FITC)/IgM(PE)/CD45(PerCP)/IgG2a(APC) or CD3(FITC)/CD8a(PE)/CD45(PerCP)/CD4(APC) or CD3+CD19(FITC)/PanNK(PE)/CD45(PerCP)/Mac-1(APC). Red blood cells were then lysed and cell pellets washed twice with a phosphate buffered saline solution (PBS) containing sodium azide and 1% FBS. After the last wash cell pellets were resuspended in the PBS solution, capped and transported to the Flow Cytometry Core Facility for analysis on a FACScalibur Flow Cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA). Data was collected using CellQuest software. Data were acquired by gating for viable cells using forward angle light scatter (FALS), selecting CD45+ cells (all leukocytes),and acquiring 10,000 gated events for each sample.
T-Dependent Antibody Response (TDAR)
The TDAR assay was conducted as described by Gao et al., (2007). Briefly, 0.5 ml of 4 × 106 or 6 × 106 cell/ml mouse spleen cells were immunized ex vivo for 4 days by incubating them in a humidified, 37°C, 5% CO2 incubator with an equal volume (0.5 ml) of 1% sheep red blood cells (SRBC; Colorado Serum, Denver CO) using a modified Mishell-Dutton culture system. Plaques were developed using a glass slide modification of Jerne and Nordin Plaque-Forming Cell (PFC) assay as described by Gao et al., (2005). Briefly, immunized spleen cells were washed and resuspended in 100 μl of culture media and then mixed with 50 μl of a 67% SRBC suspension and 400 μl of a 43°C, 0.8% SeaPlaque® Agarose (Lonza, Rockland, ME) made in a 2X RPMI (Gibco) solution containing no supplements, pH 7.2. The mixture was then poured onto 0.15% SeaPlaque® Agarose pre-coated microscope slide and incubated face down on a custom slide tray in a humidified 37°C incubator (with no CO2) for 1 h. Following incubation slides were flooded with guinea pig complement (Colorado Serum, Denver, CO) diluted 1:20 (v:v) with Dulbecco's phosphate- buffered saline (DPBS+) containing calcium and magnesium. Slides were incubated and additional 2 hr under the same conditions. Slides were removed and held in a cold 0.85% sodium chloride solution. Anti-SRBC PFC were then identified using a Bausch and Lomb dissecting microscope. Results are expressed as the number PFC per total number of cultured cells.
TNP- Ficoll Immunization
The TNP-Ficoll activation takes advantage of the trinitro groups coupled to ficoll, derivatized with aminoethyl carboxymethyl (AECM) groups to elicit a T-cell independent antibody response which can be measured by using a plaque-forming cell (PFC) assay described above. The TNP-Ficoll assay was run in 48-well plates with 0.5 ml of 4 × 106 cells/ml in each well. To conduct the TNP-Ficoll assay as well as prepare all reagents we followed the procedure outlined in Current Protocols in Immunology (Bonada and Robertson, 2003). Briefly, TNP-Ficoll (Biosearch Technologies, Novato, CA) was solubilized with DPBS (without calcium or magnesium) to yield a 1 mg/ml solution which was sterile filtered then diluted with sterile media to yield a 20 ng/ml working solution which was then added to the well at a 1:1 ratio with the media and cells in the well. Plates were then incubated for 4 days in a humidified incubator at 37°C and 5% CO2. Following incubation plaques were developed using a glass slide modification described above after first coupling trinitrophenyl (TNP) hapten to SRBC. Briefly, 4.5 ml of SRBC were washed twice with 10 ml HBSS. The supernatant was removed and the pellet was washed once more with 14 ml 0.28M cacodylic acid buffer (pH 6.9) the SRBC were resuspended by pipetting up and down several times. 2,4,6-Trinitrobenzenesulfonic acid (picryl sulfonic acid) sodium salt solution (TNBS) was then added drop-wise and mixed with the SRBC solution by pipetting up and down. The tube was then covered with aluminum foil and gently rocked for 10 min at 20°C. After rocking 7.5 ml HBSS was added to the tube followed by centrifugation at 800 × g at 20°C. The supernatant was aspirated and the pellet was washed with 12 ml glycylglycine (Lonza, Walkerville, MD). The pellet was washed twice more with HBSS, following the final wash the pellet was resuspended to yield a 13% (v/v) TNP-SRBC suspension. To visualize plaques, immunized spleen cells were washed then resuspended in 100 μl culture media and then mixed with 50 μl of the TNP-SRBC suspension and 400 μl of a 43°C, 0.8% SeaPlaque® Agarose (Lonza, Rockland, ME) made in a 2X RPMI (Gibco) solution containing no supplements, pH 7.2. The mixture was then poured onto 0.15% SeaPlaque® Agarose pre-coated microscope slide and the PFC assay was completed as described above in the TDAR method.
Statistical analysis
All assays were run with at least 4 mice per exposure group and at least 4 replicate samples from each mouse. Differences between treatment groups were tested by one-way ANOVA or t-test followed by a Dunnett's t-test for multiple comparisons to control group using SigmaStat software version 3.5 (Systat Software, Inc., Richmond, CA). Error bars indicate the standard error (SE) of the sample set. Biostatistical significance was set at p ≤ 0.05.
Results
DBC given via oral gavage in corn oil suppresses the TDAR response to SRBC
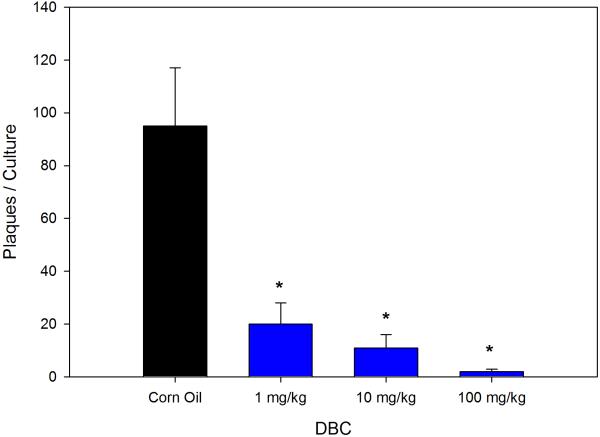
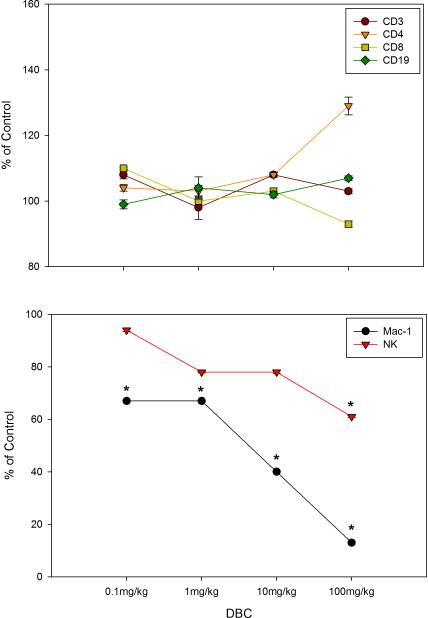
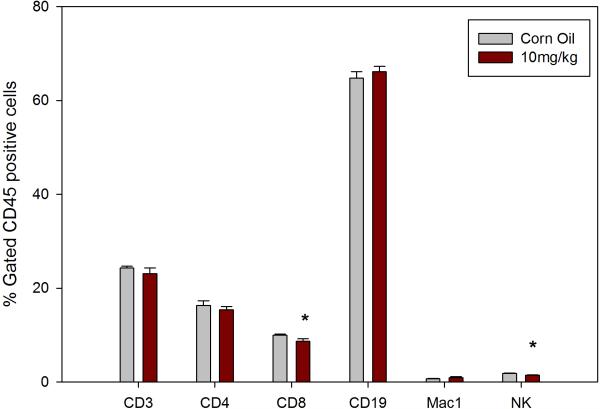
As shown in Figure 1 when administered by gavage DBC is immunosuppressive at 1, 10 and 100 mg/kg (total cumulative dose) as demonstrated by the PFC assays. Total cumulative exposures of mice to 1 mg/kg of DBC produced nearly 80% suppression of the TDAR. The 100 mg/kg DBC dose was generally toxic to mice and their spleen cells, as shown by a fall in body weights and spleen cell recoveries (Table 1), with a decrease in the spleen to body weight ratio seen only in the high dose mice (Data not shown). The spleen cell subsets recovered are shown in Figure 2, indicating that macrophage (Mac-1 positive cells) recoveries were decreased at all dose levels of DBC examined. Natural Killer (NK) cells were depleted at the 100 mg/kg dose, and there was a general increase in CD4+ cells relative to CD8+ cells. These results are quite similar to those previously reported for DMBA (Gao et al., 2007).
Figure 1.
Dose-dependent suppression of the T-dependent antibody response (TDAR) to SRBC by DBC given via gavage in corn oil for 5 consecutive days to mice and spleen cells were examined for in vitro antibody production two days later. Results are Mean ± SEM, (*) statistically significant compared to control at p ≤.05.
TABLE 1.
Body Weight and Cell Recovery
| DBC Treatment | Gavage | Pill |
|---|---|---|
| Body Weight | Mean Body Weight (g) ± SD | Mean Body Weight (g) ± SD |
| After 5 days of Dosing | ||
| Control | 27.8 ±1.3 | 25.4 ±1.1 |
| 0.1mg/kg | 28.9 ±1.4 | |
| lmg/kg | 29.8 ±1.2 | 25.9 ±1.8 |
| 10mg/kg | 26.7 ±1.7 | 24.7 ±1.4 |
| 100mg/kg | 22.8* ±2.8 | |
| Persistence (+ 4 week hold) | ||
| Control | 30.4±1.4 | 27.0 ±2.1 |
| lmg/kg | 29.9 ±2.3 | |
| 10mg/kg | 28.1±1.8 | 29.6 ±3.2 |
| Cell Recovery | Cells × 106 /mg spleen ± SD | Cells × 106 /mg spleen ± SD |
| After 5 days of Dosing | ||
| Control | 1.3 ±0.2 | 1.9 ±0.4 |
| 0.1mg/kg | 1.5 ±0.4 | |
| 1mg/kg | 1.3 ±0.3 | 1.9 ±0.4 |
| 10mg/kg | 1.3 ±0.4 | 1.7 ±0.5 |
| 100mg/kg | 1.1 ±0.7 | |
| Weights after 4 week hold | ||
| Control | 1.6 ±0.2 | 1.4 ±0.2 |
| 1mg/kg | 1.5 ±0.4 | |
| 10mg/kg | 1.6 ±0.4 | 1.6 ±0.3 |
Significantly different from Control p ≤ .05 ANOVA followed by Dunnett's t-test.
Figure 2.
Cell surface marker expression for viable (as defined by FALS) CD45+ spleen cells recovered on day 7 for mice examined in Figure 1. The percentage of cells obtained for each marker in controls were as follows: CD3+=25.2±3.6, CD4+=14.3±2.3, CD8+=12.2±1.5, CD19+=64.1±2.2, Mac-1+=1.5±0.3, and NK=3.6±0.6. Results shown are Mean ± SD for 4 replicate animals. * p ≤ .05
DBC given via gavage produces persistent immunosuppression 4 weeks after last exposure
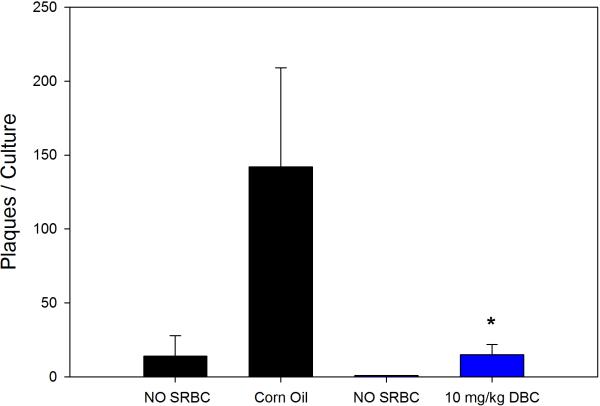
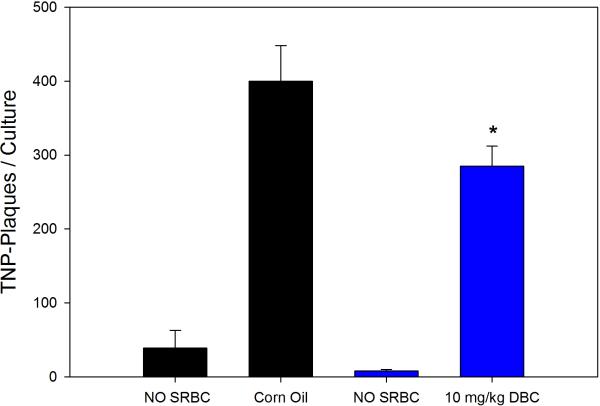
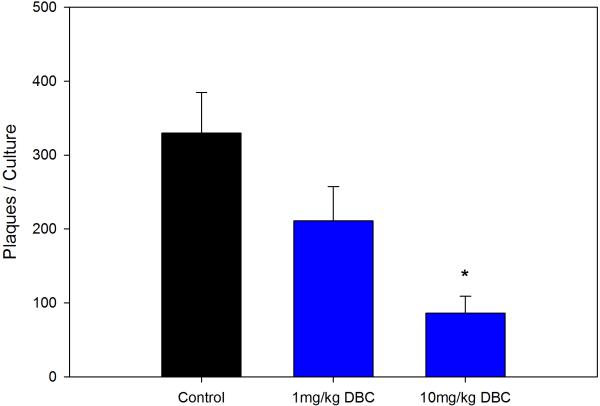
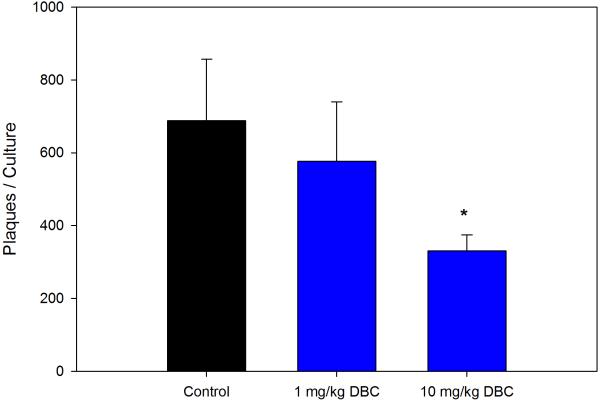
Previous studies with DMBA demonstrated that this chemical produces persistent immunosuppression at 4 and 8 weeks after the last exposure (Burchiel et al., 1988), which is long after the PAH has cleared the body (Archuleta et al., 1992; Crowell et al., 2011). Data showed that mice examined for their TDAR response after a 5 day exposure demonstrated a dose-dependent suppression of the TDAR response. The 5 day exposure was found to produce a persistent immunosuppression at a concentration of 10 mg/kg when mice were examined 4 weeks after last exposure for PFC response to SRBC (Figure 3A) and TNP-Ficoll (Figure 3B). The SRBC response was suppressed to a greater degree than the TNP-Ficoll response 4 weeks after the last exposure to 10 mg/kg DBC indicating that the TDAR is a more sensitive indicator of immune suppression. The No SRBC values are in vitro immunization controls indicating that SRBC specifically activated the PFC response. The cell subsets recovered from the animals held for 4 weeks are shown in Figure 4. In this experiment, it was determined that all cell subsets had essentially returned to normal levels 4 weeks after the last exposure to DBC, with the exception of CD4+ and NK cells that were still somewhat depressed in numbers.
Figure 3A.
Suppression of the TDAR to SRBC by 10 mg/kg total cumulative dose of DBC given via gavage. Murine spleen cells were analyzed for persistence of immunosuppression 4 weeks after the last dose of DBC. Results are Mean ± SEM and statistical significance is determined at p ≤.05 compared to a corn oil pill (*).
Figure 3B.
Results (Mean ± SD) are as described for the same group of murine spleens cells examine in Fig 3A, with the exception that TNP-Ficoll was used as the test antigen. *p ≤.05.
Figure 4.
Cell surface marker data for murine spleen cells examined 4 weeks after last exposure to DBC (Figures 3A and 3B) given via gavage at a total cumulative dose of 10 mg/kg . Values are expressed as Mean ± SD for 4 replicate animals. p ≤ .05.
DBC given via a pill suppresses the TDAR response to SRBC and TNP-Ficoll
A new method was examined for the administration of PAHs to determine whether a pill self-administration method would also produce the same immunosuppression observed with the more stressful corn oil gavage method (Walker et al., 2012). Results showed that a 5 day daily administration of DBC produced approximately the same level of immunosuppression as seen with the oral gavage methods. Total cumulative exposures of 10 mg/kg DBC given via the pill produced approximately a 70% decrease in PFC response to SRBC (Figure 5A), which is perhaps somewhat less than that seen via oral gavage of DBC at this dose (Figure 1). A second group of mice held for 4 weeks following dosing with pills were also immunosuppressed (Figure 5B). The amount of immunosuppression seen in the 4 week “recovery period” was only numerically less than that seen in the group of mice examined earlier. The significance of this finding is discussed below.
Figure 5A.
Dose-dependent suppression of the TDAR to SRBC when mice were examined two days after the last of five daily doses of DBC given as a pill containing test agent and self-administered (eaten) by mice. Results are the Means ± SEM for 5 mice per group determined to be statistically significant at the level of p ≤.05 (*)
Figure 5B.
Persistent immunosuppression of TDAR by DBC. As in Fig. 5A, except mice were held for four weeks after their last dose of DBC. Results are the Means ± SEM for 5 mice per group determined to be statistically significant at the level of p ≤.05 (*)
Discussion
Previous studies showed that polycyclic aromatic hydrocarbons (PAH) produce immunosuppression in mice and humans (Burchiel and Luster, 2001). There are significant differences between the immunosuppression produced by different PAHs. Our lab showed that there is a reasonably good correlation between the genotoxicity of PAH and their immunosuppressive properties, with a few exceptions. While benoz(a)pyrene (BaP) and its metabolites are immunosuppressive in vitro (White and Holsapple,1984, Kawabata and White, 1987), BaP produces low amounts of immunosuppression in mice in vivo due to the upregulation of CYP1A1 which mostly detoxifies BaP (Uno et al., 2006, Shi et al., 2010).
The present studies focused on an examination of the immunotoxicity of DBC, an environmentally-relevant high molecular weight PAH that is the most potent carcinogenic PAH tested to date (Cavalieri et al., 1991, 2005; Higginbotham et al., 1993). The immunotoxicity of DBC has not been previously characterized and there is little data on the on human exposures to DBC via ingestion or inhalation. The purpose of this paper was not to establish the environmental risk of exposure to DBC per se, but rather to determine whether DBC is similar to DMBA in its effects on the TDAR, a sensitive test recommended by EPA for immunotoxicity assessment. DBC is similar to DMBA in many respects, requiring metabolic activation by CYP1B1 and epoxide hydrolase resulting in the formation of diol-epoxides that covalently bind to DNA (Devanesan et al., 1990, 1999; Mahadevan et al., 2005, 2006, 2007; Crowell et al., 2011). By analogy, it is known that DMBA requires CYP1B1 and epoxide hydrolase metabolism to be immunotoxic (Gao et al., 2005, 2007).
In addition to demonstrating a new method to administer PAH and other xenobiotics through a less stressful pill self-administration system, our results demonstrate three important points. Firstly, DBC is similar to DMBA in the degree of immunosuppression produced as detected with the TDAR PFC assay. The concentration of DBC and the degree of suppression is similar to our published studies with DMBA (Gao et al., 2005, 2007). Secondly, DBC suppresses both the TDAR and a T-independent antibody response to TNP-Ficoll, which is similar to the result of our studies with DMBA (Burchiel et al., 1988). The amount of suppression of the T-independent response was found to be less than the TDAR suggesting that T cells may play an important role in immune suppression. Thirdly, DBC produces a persistent immunosuppression that is similar to that seen with DMBA (Burchiel et al., 1988). This is an intriguing finding that is currently being examined in terms of immunologic and perhaps epigenetic mechanisms. Taken collectively, our results demonstrate that DBC is as potent as DMBA in producing immunosuppression in mice. It was also found that DBC produces immunosuppression of murine spleen during in vitro exposures suggesting the spleen cells have the necessary biochemical mechanisms needed to form immunotoxic metabolites of DBC (Li et al., 2010). Further studies are needed to resolve these points as well as to determine whether p53 is required for immunosuppression, as previously demonstrated for DMBA (Gao et al., 2008)
Acknowledgments
The authors would like to acknowledge the financial support of a UNM Health Science Center Research Allocations (RAC) grant, funds from the DeSantis Endowed Chair of Pharmacogenomics held by Dr. Burchiel, and NIH RO1ES 019968-01A1. The authors are also grateful to Ms. Mary Walsh for her technical assistance.
Abbreviations
- DMBA
7,12-dimethylbenz[a]anthracene
- DBC
dibenzo[def,p]chrysene
- PAHs
polycyclic aromatic hydrocarbons
- TDAR
T-dependent antibody response
- SRBC
sheep red blood cells
- PFC
plaque forming cell
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
References
- Archuleta MM, Born JL, Montano RM, Burchiel SW. Covalent binding of 7,12-dimethylbenz[a]anthracene to lymphoid and nonlymphoid tissues following oral administration to B6C3F1 mice. Toxicol Appl Pharmacol. 1992;113:133–137. doi: 10.1016/0041-008x(92)90017-m. [DOI] [PubMed] [Google Scholar]
- Bondada S, Robertson DA. Current Protocols in Immunology. John Wiley & Sons, Inc; New York: 2003. pp. 3.8.1–3.8.24. [DOI] [PubMed] [Google Scholar]
- Burchiel SW, Hadley WM, Barton SL, Fincher RH, Lauer LD, Dean JH. Persistent suppression of humoral immunity produced by 7,12-dimethylbenz(a)anthracene (DMBA) in B6C3F1 mice: Correlation with changes in spleen cell surface markers detected by flow cytometry. Int J Immunopharmacol. 1988;10:369–376. doi: 10.1016/0192-0561(88)90123-3. [DOI] [PubMed] [Google Scholar]
- Burchiel SW, Luster MI. Signaling by environmental polycyclic aromatic hydrocarbons in human lymphocytes. Clin. Immunol. 2001;98:2–10. doi: 10.1006/clim.2000.4934. [DOI] [PubMed] [Google Scholar]
- Cavalieri EL, Higginbotham S, RamaKrishna NV, Devanesan PD, Todorovic R, Rogan EG, Salmasi S. Comparative dose-response tumorigenicity studies of dibenzo[alpha,l]pyrene versus 7,12-dimethylbenz[alpha]anthracene, benzo[alpha]pyrene and two dibenzo[alpha,l]-pyrene dihydrodiols in mouse skin and rat mammary gland. Carcinogenesis. 1991;12:1939–1944. doi: 10.1093/carcin/12.10.1939. [DOI] [PubMed] [Google Scholar]
- Cavalieri EL, Rogan EG, Li KM, Todorovic R, Ariese F, Jankowiak R, Grubor N, Small GJ. Identification and quantification of the depurinating DNA adducts formed in mouse skin treated with dibenzo[a,l]pyrene (DB[a,l]P) or its metabolites and in rat mammary gland treated with DB[a,l]P. Chem. Res. Toxicol. 2005;18:976–83. doi: 10.1021/tx049682k. [DOI] [PubMed] [Google Scholar]
- Crowell SR, Amin SG, Anderson KA, Krishnegowda G, Sharma AK, Soelberg JJ, Williams DE, Corley RA. Preliminary physiologically based pharmacokinetic models for benzo[a]pyrene and dibenzo[def,p]chrysene in rodents. Toxicol Appl Pharmacol. 2011;257:365–376. doi: 10.1016/j.taap.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanesan P, Ariese F, Jankowiak R, Small GJ, Rogan EG, Cavalieri EL. A novel method for the isolation and identification of stable DNA adducts formed by dibenzo[a,l]pyrene and dibenzo[a,l]pyrene 11, 12-dihydrodiol 13,14-epoxides in vitro. Chem Res Toxicol. 1999;12:796–801. doi: 10.1021/tx980203p. [DOI] [PubMed] [Google Scholar]
- Devanesan PD, Cremonesi P, Nunnally JE, Rogan EG, Cavalieri EL. Metabolism and mutagenicity of dibenzo[a,e]pyrene and the very potent environmental carcinogen dibenzo[a,l]pyrene. Chem Res Toxicol. 1990;3:580–586. doi: 10.1021/tx00018a014. [DOI] [PubMed] [Google Scholar]
- Gao J, Lauer FT, Dunaway S, Burchiel SW. Cytochrome P450 1B1 is required for 7,12-dimethylbenz(a)-anthracene (DMBA) induced spleen cell immunotoxicity. Toxicol. Sci. 2005;86:68–74. doi: 10.1093/toxsci/kfi176. [DOI] [PubMed] [Google Scholar]
- Gao J, Lauer FT, Mitchell LA, Burchiel SW. Microsomal expoxide hydrolase is required for 7,12-dimethylbenz[a]anthracene (DMBA)-induced immunotoxicity in mice. Toxicol Sci. 2007;98:137–44. doi: 10.1093/toxsci/kfm089. [DOI] [PubMed] [Google Scholar]
- Gao J, Mitchell LA, Lauer FT, Burchiel SW. p53 and ATM/ATR regulate 7,12-dimethylbenz[a]anthracene-induced immunosuppression. Mol. Pharmacol. 2008;73:137–146. doi: 10.1124/mol.107.039230. [DOI] [PubMed] [Google Scholar]
- Higginbotham S, RamaKrishna NV, Johansson SL, Rogan EG, Cavalieri EL. Tumor-initiating activity and carcinogenicity of dibenzo[a,l]pyrene versus 7,12-dimethylbenz[a]anthracene and benzo[a]pyrene at low doses in mouse skin. Carcinogenesis. 1993;14:875–878. doi: 10.1093/carcin/14.5.875. [DOI] [PubMed] [Google Scholar]
- Kawabata TT, White KL., Jr Suppression of the vitro humoral immune response of mouse splenocytes by benzo(a)pyrene metabolites and inhibition of benzo(a)pyrene-induced immunosuppression by alpha-naphthoflavone. Cancer Res. 1987;47:2317–2322. [PubMed] [Google Scholar]
- Li Q, Lauer FT, Liu KJ, Hudson LG, Burchiel SW. Low-dose synergistic immunosuppression of T-dependent antibody responses by polycyclic aromatic hydrocarbons and arsenic in C57BL/6J murine spleen cells. Toxicol Appl Pharmacol. 2010;245:344–351. doi: 10.1016/j.taap.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan B, Luch A, Atkin J, Haynes M, Nguyen T, Baird WM. Inhibition of human cytochrome p450 1b1 further clarifies its role in the activation of dibenzo[a,l]pyrene in cells in culture. J Biochem Mol Toxicol. 2007;21:101–109. doi: 10.1002/jbt.20168. [DOI] [PubMed] [Google Scholar]
- Mahadevan B, Luch A, Bravo CF, Atkin J, Steppan LB, Pereira C, Kerkvliet NI, Baird WM. Dibenzo[a,l]pyrene induced DNA adduct formation in lung tissue in vivo. Cancer Lett. 2005;227:25–32. doi: 10.1016/j.canlet.2004.11.056. [DOI] [PubMed] [Google Scholar]
- Mahadevan B, Luch A, Atkin J, Nguyen T, Sharma AK, Amin S, Baird WM. Investigation of the genotoxicity of dibenzo[c,p]chrysene in human carcinoma MCF-7 cells in culture. Chem Biol Interact. 2006;164:181–191. doi: 10.1016/j.cbi.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell LA, Gao J, Wal RV, Gigliotti A, Burchiel SW, McDonald JD. Pulmonary and systemic immune response to inhaled multiwalled carbon nanotubes. Toxicol. Sci. 2007;100:203–214. doi: 10.1093/toxsci/kfm196. [DOI] [PubMed] [Google Scholar]
- Shi Z, Dragin N, Gálvez-Peralta M, Jorge-Nebert LF, Miller ML, Wang B, Nebert DW. Organ-specific roles of CYP1A1 during detoxication of dietary benzo[a]pyrene. Mol. Pharmacol. 2010;78:46–57. doi: 10.1124/mol.110.063438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno S, Dalton TP, Dragin N, Curran CP, Derkenne S, Miller ML, Shertzer HG, Gonzalez FJ, Nebert DW. Oral benzo[a]pyrene in Cyp1 knockout mouse lines: CYP1A1 important in detoxication, CYP1B1 metabolism required for immune damage independent of total-body burden and clearance rate. Mol. Pharmacol. 2006;69:1103–1114. doi: 10.1124/mol.105.021501. [DOI] [PubMed] [Google Scholar]
- Walker MK, Boberg JR, Walsh MT, Wolf V, Trujillo A, Duke MS, Palme R, Felton LA. A less stressful alternative to oral gavage for pharmacological and toxicological studies in mice. Toxicol Appl Pharmacol. 2012;260:65–69. doi: 10.1016/j.taap.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White KL, Jr, Holsapple MP. Direct suppression of in vitro antibody production by mouse spleen cells by the carcinogen benzo(a)pyrene but not by the noncarcinogenic congener benzo(e)pyrene. Cancer Res. 1984;44:3388–3393. [PubMed] [Google Scholar]
- World Health Organization. International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans . Some Non-Heterocyclic Polcyclic Aromatic Hydrocarbons and Some Related Exposures. Vol. 92. Lyon, France: 2010. [PMC free article] [PubMed] [Google Scholar]