Abstract
The colonic epithelium is composed of a polarized monolayer sheathed by a layer of pericryptal myofibroblasts (PCMFs). We mimicked these cellular compartments in vitro to assess the effects of paracrine-acting PCMF-derived factors on tight junction (TJ) integrity, as measured by transepithelial electrical resistance (TER). Co-culture with 18Co PCMFs, or basolateral administration of 18Co conditioned medium (CM), significantly reduced TER of polarized Caco-2 cells. Amongst candidate paracrine factors, only keratinocyte growth factor (KGF) reduced Caco-2 TER; basolateral KGF treatment led to time- and concentration-dependent increases in claudin-2 levels. We also demonstrate amphiregulin (AREG), produced largely by Caco-2 cells, increased claudin-2 levels, leading to epidermal growth factor receptor-mediated TER reduction. We propose that colonic epithelial TJ integrity can be modulated by paracrine KGF and autocrine AREG through increased claudin-2 levels. KGF-regulated claudin-2 induction may have implications for inflammatory bowel disease, where both KGF and claudin-2 are upregulated.
Keywords: Tight junction, pericryptal myofibroblasts, keratinocyte growth factor, amphiregulin, claudin-2
Introduction
A critical function of the polarized intestinal epithelium is to act as a semi-permeable barrier that separates the contents of the intestinal lumen from the internal milieu. Movement across epithelial barriers proceeds by two mechanisms: transcellular transport occurs by bidirectional transit from apical to basolateral membranes, whereas paracellular transport occurs between adjacent epithelial cells and is regulated by tight junctions (TJs) (3, 36, 41, 67). TJs comprise the most apical contact point between adjacent epithelial cells; they are composed of membrane and membrane-associated proteins that form a paracellular diffusion barrier and interact with intracellular cytoskeletal and signaling molecules (8, 18, 22, 60). Major TJ proteins include occludin, claudins, tricellulin, and junctional adhesion molecules (JAMs) (4, 8, 18, 36, 41).
The claudin family of tetraspan membrane proteins is composed of 27 members that form a network of intramembrane strands via homo- and limited heterophilic interactions both within the cytoplasm of epithelial cells and between adjacent epithelial cells to form an ion-selective paracellular diffusion pore (3, 4, 35, 41, 47, 49, 70). Individual claudins are expressed differentially across mammalian tissues and exhibit variable functional properties. Unique combinations of claudin expression govern the size and charge selectivity, as well as the resulting transepithelial electrical resistance (TER) of paracellular routes across different epithelial tissues (18, 19, 36, 41, 67, 68). Of the 27 claudins, claudin-2 has been identified as a cation-selective pore-forming claudin that is expressed in leaky epithelial tissues, such as intestinal crypts and proximal renal tubules (2,4,19,26,40). In contrast to the other claudins, claudin-2 expression increases TJ permeability to small cations and causes a subsequent decrease in TER (2, 34, 69).
Barrier integrity in the continuously self-renewing colonic epithelium is critical for maintenance of homeostasis. The colonic epithelium is organized into individual invaginations (crypts), which contain a stem cell compartment at the crypt base that gives rise to differentiated cells that transit to the crypt surface and are sloughed into the intestinal lumen. Contained in the lamina propria, surrounding the crypt epithelium, are pericryptal myofibroblasts (PCMFs) that participate in extensive interactions with the epithelium. PCMFs secrete growth factors, prostaglandins, hormones, cytokines, inflammatory mediators, matrix-modifying molecules, and basement membrane/extracellular matrix proteins that promote epithelial growth, maintenance, repair, and remodeling (52, 53, 62). The reciprocal crosstalk between PCMFs and the overlying polarized epithelium is recognized as having an important role in regulating normal gastrointestinal physiology, as well as influencing the pathogenesis of diseases such as cancer and inflammatory bowel disease (IBD) (9, 20, 52, 53, 54, 55, 77).
Due to the important signaling crosstalk that occurs between these two cell types, we modeled PCMF-epithelial interactions in vitro to observe their effects on intestinal epithelial barrier function, using 18Co and Caco-2 cells. We found that paracrine keratinocyte growth factor (KGF), produced by 18Co cells, decreases TER of polarized Caco-2 cells by increasing claudin-2 levels. Additionally, we found that the epidermal growth factor receptor (EGFR) ligand, amphiregulin (AREG), largely produced by Caco-2 cells, has a similar effect on TER through upregulation of claudin-2 expression. Thus, paracrine KGF and autocrine AREG act to modulate TJ integrity through upregulation of claudin-2 in Caco-2 cells.
Materials and Methods
Cell culture conditions and preparation of 18Co conditioned medium (CM)
Human Caco-2 colon cancer cells and 18Co PCMF cells were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, nonessential amino acids, 100 units/ml penicillin, and 100 μg/ml streptomycin (HyClone, Logan, UT). 18Co cells were a generous gift from Randy C. Mifflin (University of Texas Medical Branch, Galveston, TX). The 18Co myofibroblast phenotype was verified by immunohistochemical staining for α-smooth muscle actin and vimentin. For polarization studies, Caco-2 cells were seeded at a density of 1 × 105 (5 × 105) on 12 mm (24 mm) polycarbonate culture inserts (pore size, 0.4 μm) of Transwell® filters (Corning, Acton, MA), which were placed in 12-well (6-well) plates and incubated with 0.5 (1.5) ml of apical medium and 1.5 (2.5) ml of basolateral medium. Monolayer polarization was monitored by TER measurements (see below) (Millicell-ERS; Millipore, Bedford, MA). For co-culture experiments in Figure 1, 18Co cells were plated in the outer compartment of wells containing Transwell® filters (1×105 cells/well) and grown overnight in 5% FBS-containing culture medium. Caco-2 cells were then plated on Transwell® filter membranes (1×105 cells/well) and the co-cultured cells were maintained in 5% FBS-containing culture medium.
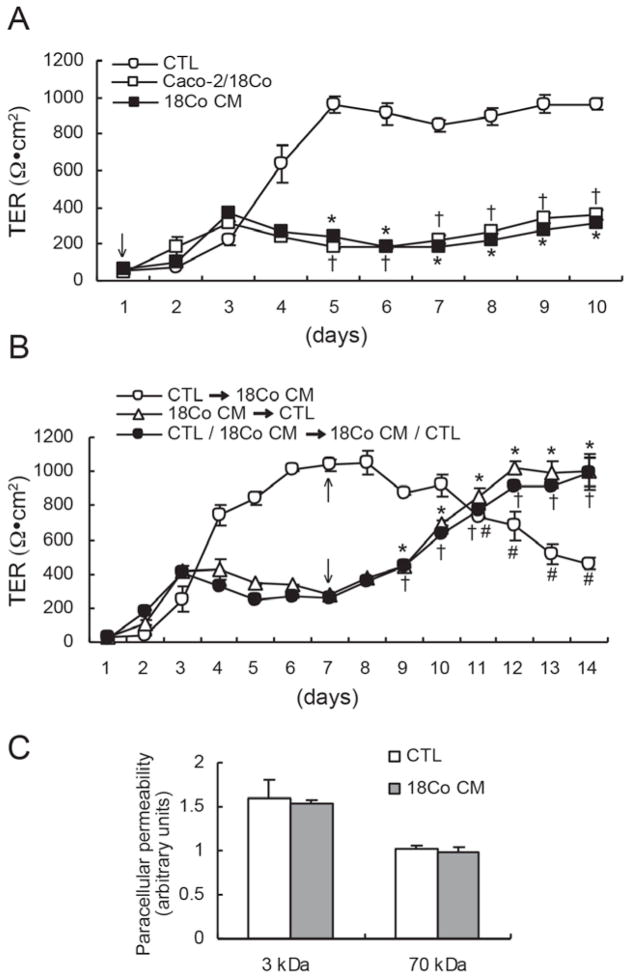
Figure 1.
Reduction of TER in polarized Caco-2 cells co-cultured with 18Co cells or cultured in 18Co CM. (A) In CTL (5% FBS-supplemented) medium, Caco-2 TER increased steadily for five days and maintained a relatively constant level thereafter. Co-culture with 18Co cells or growth in 18Co CM decreased Caco-2 TER compared to CTL medium. Data shown are mean ± S.E. of three independent experiments. *†p < 0.001, compared to TER in CTL medium. (B) Caco-2 cells were grown for seven days under three experimental conditions: CTL medium, 18Co CM, or CTL/18Co CM (CTL medium in the upper apical chamber and 18Co CM in the lower basolateral chamber). Seven days after plating (arrows), culture conditions were changed as indicated. For the first seven days, TER remained low when 18Co CM was in both apical and basolateral compartments, or in the basolateral compartment with CTL medium in the apical compartment (CTL/18Co CM). When media was changed on day seven, effects on TER were reversed so that when 18Co CM replaced CTL medium, TER decreased, whereas, when CTL medium replaced 18Co CM, TER increased; reversal of CTL/18Co CM to 18Co CM/CTL also increased Caco-2 TER. Data shown are mean ± S.E. of three independent experiments. *†#p < 0.001, compared to TER at seven days. (C) Paracellular permeability to FITC-dextran (3 kDa) and Rhodamine B-dextran (70 kDa) was measured in polarized Caco-2 cells on Transwell® filters treated with CTL medium or 18Co CM. 18Co CM did not modify transepithelial flux of either tracer. Values are presented as mean ± S.E. of three independent experiments.
To prepare 18Co CM, 18Co cells were gown to confluency in 75 cm2 flasks in serum-supplemented culture medium. Cells were washed with phosphate-buffered saline (PBS) and starved overnight in serum-free medium. The following day, CM was prepared by incubating cells in fresh serum-free culture medium for one day. This medium was harvested, clarified by centrifugation (2000 r.p.m. for 10 minutes), and stored at −20°C.
18Co CM (in Figure 1) was prepared by mixing 10% FBS-supplemented culture medium and serum-free CM from 18Co cells in equal amounts, yielding 5% FBS-containing 50% 18Co CM. In Figures 4 and 5, 18Co CM was prepared by combining equal amounts of 5% FBS-containing culture medium and 5% FBS-containing 50% 18Co CM (as above). This 5% FBS-containing 50% 18Co CM was then mixed in equal amounts with 5% FBS-containing culture medium to yield a final 5% FBS-containing 25% 18Co CM. In the experiments described, control (CTL) medium was DMEM supplemented with 5% FBS. Culture medium was changed every two days.
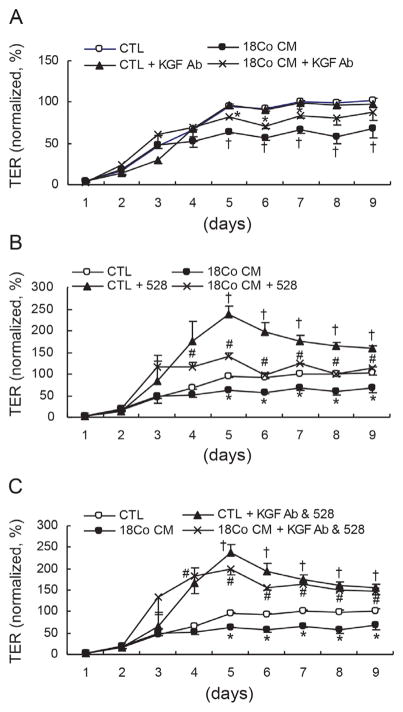
Figure 4.
18Co CM-induced TER-reducing effects are specific to KGF and EGFR ligands. Polarized Caco-2 cells were treated with CTL medium or 18Co CM with or without an anti-KGF neutralizing antibody (A), an anti-EGFR blocking antibody (528 mAb) (B), or both (C). (A) Addition of an anti-KGF antibody to CTL medium had no effect on Caco-2 TER, while addition of an anti-KGF antibody to 18Co CM attenuated the TER-reducing effect of 18Co CM alone. Data shown are mean ± S.E. of three independent experiments. *p < 0.05, compared to TER in 18Co CM. †p < 0.05, compared to TER in CTL medium. (B) Addition of 528 mAb to CTL medium and 18Co CM enhanced Caco-2 TER, although 18Co CM containing 528 mAb did not increase TER to the level of CTL medium with 528 mAb. Data shown are mean ± S.E. of three independent experiments. #p < 0.05, compared to TER in 18Co CM. *p < 0.05, compared to TER in CTL medium. †p < 0.05, compared to TER in CTL medium. (C) Addition of both an anti-KGF antibody and 528 mAb to CTL medium had similar effects on Caco-2 TER as that of CTL medium with 528 mAb alone. Addition of both an anti-KGF antibody and 528 mAb to 18Co CM increased Caco-2 TER to levels similar to those observed with CTL medium containing both antibodies. Data shown are mean ± S.E. of three independent experiments. #p < 0.05, compared to TER in 18Co CM. *p < 0.05, compared to TER in CTL. †p < 0.05, compared to TER in CTL medium. Values are normalized to the average of plateaued TER values (from day five to day nine) after polarization of Caco-2 cells in CTL medium.
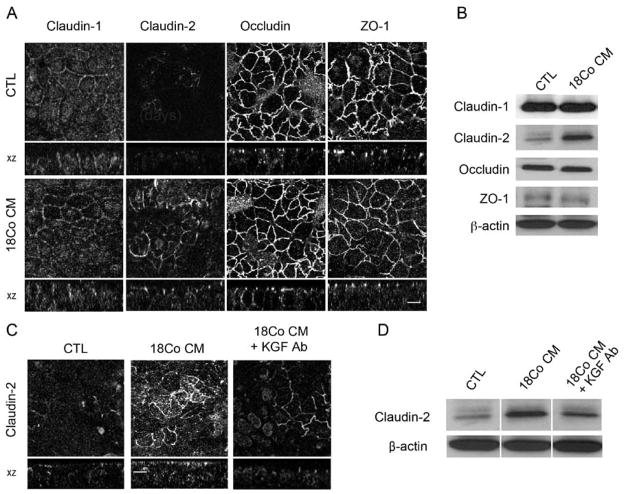
Figure 5.
18Co CM increases claudin-2 expression in a KGF-dependent manner. (A) Polarized Caco-2 cells were cultured in CTL medium or 18Co CM for 24 hours, and immunofluorescence of candidate TJ proteins (claudin-1, claudin-2, occludin, and ZO-1) was examined. Fluorescent intensity of claudin-2 staining was increased by addition of 18Co CM, compared to CTL medium, without significant changes in claudin-1, occludin, or ZO-1. (B) Western blotting was performed using cell lysates from cells grown under the same conditions as in (A). 18Co CM induced an increase in claudin-2 expression without significant changes in claudin-1, occludin, and ZO-1. (C, D) Using the same culture conditions as in (A), an anti-KGF neutralizing antibody (2.5 μg/ml) was added to 18Co CM, and immunofluorescence (C) and western blot analysis (D) for claudin-2 were performed. Addition of anti-KGF neutralizing antibody to 18Co CM attenuated the 18Co CM-dependent increase in claudin-2 expression. Scale bars, 10 μm.
Reagents and antibodies
Recombinant human epidermal growth factor (EGF), transforming growth factor-α (TGFα), AREG, heparin-binding EGF-like growth factor (HB-EGF), as well as anti-human KGF, anti-human AREG, and anti-human HB-EGF antibodies used for neutralization were obtained from R&D Systems (Minneapolis, MN). Recombinant human KGF, hepatocyte growth factor (HGF), transforming growth factor beta 1 (TGF- β1), and insulin-like growth factor 2 (IGF-2) were obtained from PeproTech (Rocky Hill, NJ).
Dr. Hideo Masui (Memorial Sloan-Kettering Cancer Center) generously provided the EGFR-blocking mouse monoclonal antibody, 528 mAb (44,45). The anti-AREG neutralizing antibody, 6R1C2.4 (48,65), was obtained from Bristol Myers Squib (Seattle, WA). EKI-785 (45) was generously provided by Jay Gibbons from Wyeth-Ayerst (Pearl River, NY). Rabbit polyclonal antibodies against claudin-2 and ZO-1, mouse monoclonal antibodies against claudin-1 and occludin, and Alexa Fluor 488- and Alexa Fluor 594-conjugated secondary antibodies were purchased from Invitrogen (Zymed; Carlsbad, CA). The mouse monoclonal anti-β-actin antibody was obtained from Sigma (St. Louis, MO) and the mouse monoclonal antibody against α-tubulin was purchased from Millipore (Billerica, MA).
TER measurements
TER across Transwell® filters was measured using a Millicell Electrical Resistance System (Millipore). For TER measurements, Caco-2 cells were cultured on 12 mm filter inserts. One Transwell® chamber was left blank to determine the intrinsic resistance of the membrane. Final TER values were obtained by subtracting the blank value, and the results expressed as Ω·cm2 in Figures 1–3. In Figure 4, TER was normalized to the average of plateau values (from days five to nine) after polarization of Caco-2 cells in CTL medium.
Figure 3.
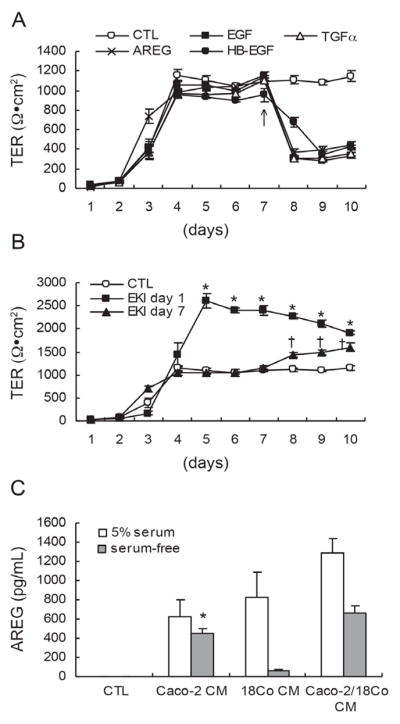
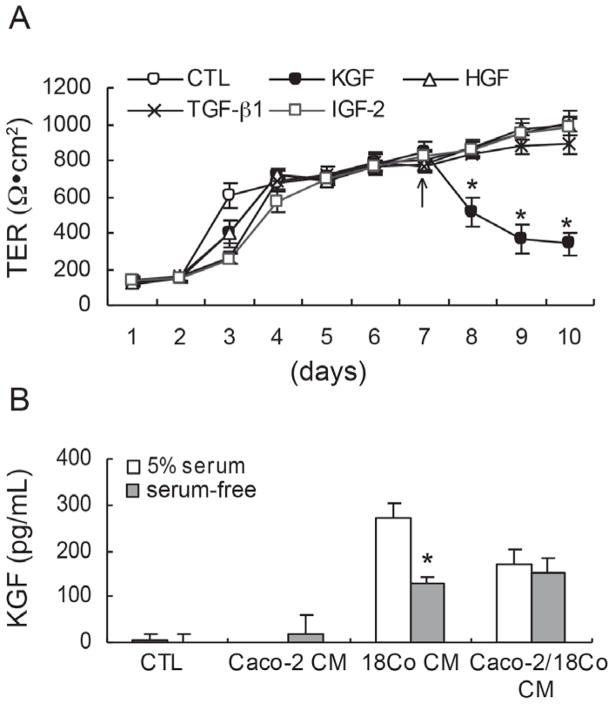
EGFR ligands reduce TER of polarized Caco-2 cells and AREG is detected in CM from Caco-2 and 18Co cells. (A) Polarized Caco-2 cells were treated with 10 ng/ml of EGF, TGFα, AREG, or HB-EGF on day seven after plating on Transwell® filters (arrow). All of the EGFR ligands tested significantly decreased Caco-2 TER (p < 0.001). Data shown are mean ± S.E. of three independent experiments. (B) EKI-785 (1μM), an irreversible EGFR kinase inhibitor, was added to polarized Caco-2 cells one (squares) or seven (triangles) days after plating on Transwell® filters. Addition of EKI-785 increased Caco-2 TER compared to CTL medium under both experimental conditions. Data shown are mean ± S.E. of three independent experiments. *†p < 0.001, compared to TER in CTL medium. (C) CM from Caco-2 cells, 18Co cells, and Caco-2/18Co co-cultured cells was collected, and AREG levels were determined by ELISA. When Caco-2 cells were grown in serum-free medium, AREG levels were seven-fold greater than that in CM from 18Co cells grown in serum-free medium. AREG levels detected in 18Co CM were increased by 13-fold from cells cultured in 5% serum-containing medium, compared to serum-free medium. Data shown are mean ± S.E. of three independent experiments. *p < 0.001, compared to that in serum-free 18Co CM.
Measurement of paracellular permeability
Cells were grown on Transwell® filters for three days in CTL medium as described above and treated with fresh CTL medium or 18Co CM for 36 hours. Following the 36 hour incubation, media was replaced with fresh CTL medium or 18Co CM. The cultures were allowed to equilibrate at 37°C for 30 minutes. At this time, 100 μg/ml FITC-dextran (3 kDa) or 1 mg/ml Rhodamine B-dextran (70 kDa) was individually added with CTL medium to the inner chamber of Transwell® filters. After a three-hour incubation, the basal medium was collected and the fluorescence of the diffused tracers in the outer chamber was measured with a fluorescence spectrometer, Fluorstar Optima (BMG Labtech), at λ484 nm (excitation) and λ520 nm (emission) for FITC-dextran, and λ544 nm (excitation) and λ590 nm (emission) for Rhodamine B-dextran.
ELISA of EGFR ligands and KGF
Individually cultured Caco-2 cells grown on 24 mm Transwell® filters (Caco-2 CM), 18Co cells grown in the outer compartment of 24 mm Transwell® filters (18Co CM), or co-cultured Caco-2 and 18Co cells with Caco-2 cells grown on the filter and 18Co cells grown in the outer compartment (Caco-2/18Co CM), were serum deprived overnight and fresh serum-free medium or 5% serum-containing culture medium was added the following day. After 24 hours, media from the above three conditions were collected and analyzed by ELISA for levels of the EGFR ligands AREG, EGF, HB-EGF, and TGFα by Luminex ELISA. Antibody ELISA pairs and recombinant proteins for AREG (Human AREG DuoSet ELISA kit), EGF (Human EGF DuoSet ELISA kit), HB-EGF (Human HB- EGF DuoSet ELISA kit), and TGFα (Human TGFα DuoSet ELISA kit) were purchased from R&D Systems. The capture antibodies from each kit were coupled to four spectrally distinct Bio-Plex carboxy-coated beads (COOH beads) (Bio-Rad, Hercules, CA) using the Bio-Plex Amine Coupling Kit following the manufacturer’s recommendations. Antigen binding was detected on a Bio-Plex 200 Workstation (Bio-Rad).
For measurement of KGF in CM, the biotinylated anti-human KGF antibody and monoclonal anti-human KGF antibody were purchased from R&D Systems, and used as a detection and capture antibody, respectively. The procedure was performed following the manufacturer’s recommendations.
Immunofluorescence and western blotting
For immunofluorescence, Caco-2 cells were cultured on Transwell® filters, treated as indicated, washed with cold PBS, fixed with 4% paraformaldehyde for 20 minutes, permeabilized with 0.5% Triton X-100 for 10 minutes, and blocked for one hour in PBS containing 2% bovine serum albumin (PBS blocking buffer). Samples were incubated with primary antibodies diluted in PBS blocking buffer overnight at 4°C, washed three times with PBS, followed by incubation for one hour with the respective fluorophore-conjugated secondary antibodies diluted in PBS blocking buffer at room temperature. Samples were washed three times in PBS, mounted, and examined using an Olympus FV-1000 confocal microscope at the Vanderbilt Cell Imaging Core Resource.
Cell lysates were prepared for western blotting (Figure 5), by scraping Caco-2 cell monolayers grown on Transwell® filters into 4°C lysis buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% Triton X-100, 1% SDS, 2 mM 4-[2-aminoethyl] benzenesulfonyl fluoride, 1 mM EDTA, 130 μM bestatin, 14 μM E-64, 1 μM leupeptin, and 0.3 μM aprotinin, 10 mM sodium fluoride, 2 mM sodium orthovanadate). Collected cell lysates were sonicated with three 10 second pulses, rotated at 4°C for 30 minutes, followed by centrifugation at 10 000 × g for 20 minutes. For cell lysis in preparation for western blotting in Figure 6, the filter membranes containing Caco-2 monolayers were removed from the plastic insert and rotated in lysis buffer for one hour at 4°C. Filter membranes were removed and lysates were centrifuged as above. Protein concentrations of cell lysates were determined using a microBCA protein assay kit (Pierce). Equal amounts of protein were subjected to SDS/PAGE and transferred to PVDF membranes for immunoblotting. Membranes were blocked with TBST (10 mM Tris-HCl, 0.09% NaCl, 0.1% Tween 20) containing 5% non-fat milk for one hour at room temperature. Primary antibodies were incubated at 4°C overnight in TBST containing 0.3% BSA (claudin-2) or 5% milk (all others). Membranes were washed three times in TBST, followed by incubation with HRP-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, Inc) at room temperature for one hour, and developed using chemiluminescent detection reagents (Pierce, Rockford, IL).
Figure 6.
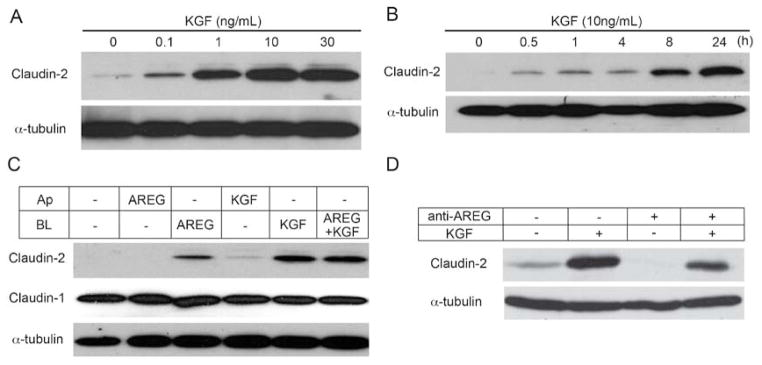
KGF and AREG induce claudin-2 expression predominantly through basolateral signaling. (A) KGF was added to the basolateral compartment of polarized Caco-2 cells in increasing concentrations for 24 hours. Western blot analysis demonstrated that increases in claudin-2 were observed with as low as 0.1 ng/ml KGF. (B) Ten ng/ml KGF was added to the basolateral side of polarized Caco-2 cells and claudin-2 expression was determined over 24 hours. KGF-dependent claudin-2 expression increased with time. (C) AREG (10 ng/ml) and/or KGF (10 ng/ml) were added to either the apical (Ap) or basolateral (BL) medium of polarized Caco-2 cells on Transwell® filters. Claudin-2 levels increased in response to basolateral AREG and/or KGF. Apical KGF had a minor effect on claudin-2 expression and combined AREG and KGF did not enhance claudin-2 expression compared to either growth factor alone. (D) Polarized Caco-2 cells were treated with basolateral KGF, an anti-AREG neutralizing antibody, or both for 24 hours. Neutralization of AREG eliminated basal levels of claudin-2 by western blot analysis. Additionally, AREG neutralization attenuated claudin-2 induction by basolateral KGF.
Statistical analysis
Data are presented as mean ± standard error (S.E.). Statistical analysis was performed using a two-tailed Student’s t-test. Significance was accepted at a p value of less than 0.05.
Results
18Co CM reduces TER of polarizing Caco-2 cells
To determine how PCMFs affect TJ integrity, we utilized a co-culture system to model intestinal epithelial-mesenchymal interactions in vitro. Our model utilized Caco-2 epithelial cells and 18Co (CCD-18) PMCF cells. Caco-2 cells are derived from a human colorectal adenocarcinoma and retain the capacity to form a uniform polarizing monolayer when cultured on Transwell® filters (59). Human colorectal cell lines with the ability to polarize in vitro are uncommon (15); we chose the Caco-2 line because it is well characterized and often used in studies of intestinal barrier function, as well as in co-culture experiments that mimic the juxtaposition of intestinal epithelium and mesenchyme. 18Co cells are human colon-derived myofibroblasts that express vimentin and α-smooth muscle actin, two characteristic markers of PCMFs (data not shown) (66). In this study, TER measurements of Caco-2 monolayers allowed us to assess the effects of PCMFs on Caco-2 TJ integrity; TER measurements were used to quantify the paracellular permeability of epithelial monolayers, which is largely regulated by TJs (51). In initial experiments, we determined that 18Co cells exhibited identical effects on Caco-2 TER when they were cultured on the undersurface of Transwell® filter membranes or on the bottom of Transwell®-containing wells (data not shown). In control (CTL) medium (5% FBS-containing DMEM), TER of the Caco-2 monolayer peaked around 1000 Ω·cm2 five days after plating, and remained relatively constant thereafter (Figure 1A). However, when Caco-2 cells were grown in conditioned medium from 18Co cells (18Co CM), or co-cultured with 18Co cells (Caco-2/18Co), TER remained significantly lower compared to what was observed with CTL medium (Figure 1A). These experiments suggest that 18Co cells secrete factors that alter Caco-2 TER. Given this result and the comparable effects on Caco-2 TER observed between exposure to 18Co CM and co-culture with 18Co cells, 18Co CM was used in all subsequent experiments.
To examine the effects of 18Co CM on fully polarized Caco-2 cells (TER > 300 Ω), we grew cells on Transwell® filters for seven days in CTL medium in both compartments, 18Co CM in both compartments, or CTL medium in the apical compartment and 18Co CM in the basolateral compartment. Seven days after plating, media was changed such that 18Co CM replaced CTL medium, and CTL medium replaced 18Co CM. These results show that when CTL medium was replaced with 18Co CM, Caco-2 TER decreased; in contrast, Caco-2 TER increased when 18Co CM was replaced with CTL medium (Figure 1B), suggesting paracrine factors in 18Co CM reduce TJ integrity of Caco-2 cells. To determine whether these paracrine factors acted via the basolateral and/or apical compartment, seven days after plating Caco-2 cells in apical CTL medium and basolateral 18Co CM, media was reversed so that 18Co CM was apical and CTL medium was basolateral. After reversal, Caco-2 TER increased (Figure 1B). Combined, these results suggest that paracrine factors released from 18Co cells act basolaterally to reduce TER of polarized Caco-2 monolayers.
TJs also regulate the movement of non-ionic molecules through the paracellular pathway. Therefore, to determine whether 18Co CM modulates non-ionic paracellular permeability in polarized Caco-2 cells, we measured the apical-to-basolateral flux of 3 kDa FITC- and 70 kDa Rhodamine B-dextrans. We found 18Co CM did not significantly modify the flux of either tracer (Figure 1C), indicating that the 18Co CM-induced decrease in Caco-2 TER does not affect paracellular transport of inert molecules.
KGF reduces TER of polarized Caco-2 cells and is detected in 18Co CM
To identify stromal factors that contribute to the 18Co CM-induced reduction in Caco-2 TER, we administered exogenous candidate stromal factors, including KGF, HGF, TGF- β1, and IGF-2 to both apical and basolateral compartments of polarized Caco-2 cells seven days after plating and monitored TER daily. Only KGF induced a significant decrease in TER compared to CTL medium (Figure 2A). Among paracrine factors released by PCMFs, KGF (FGF-7) is an epithelial-specific mitogen that acts on overlying intestinal epithelial cells expressing its cognate receptor tyrosine kinase, FGFR2b (KGFR) (30, 32, 33, 46, 58). To determine whether CM from Caco-2 and/or 18Co cells contained KGF, KGF levels were measured by ELISA in CM from Caco-2 cells alone, 18Co cells alone, and co-cultured cells grown under serum-free and 5% serum-containing culture conditions. As expected, KGF was detected in 18Co CM, but not in Caco-2 CM (Figure 2B), confirming KGF is a candidate paracrine factor produced by 18Co cells that affects Caco-2 TER.
Figure 2.
KGF reduces TER of polarized Caco-2 cells and is detected in 18Co CM. (A) KGF (10 ng/ml), HGF (40 ng/ml), TGF-β1 (2 ng/ml), and IGF-2 (20 ng/ml) were added to polarized Caco-2 cells seven days after plating. Only KGF selectively reduced TER. Data shown are mean ± S.E. of three independent experiments. *p < 0.05, compared to TER in CTL medium. (B) CM from polarized Caco-2 cells, 18Co cells, or co-cultured Caco-2/18Co cells grown in 5% serum or serum-free culture conditions was collected and analyzed by ELISA for KGF. KGF was detected in 18Co CM and Caco-2/18Co CM, but not in Caco-2 CM. Data shown are mean ± S.E. of three independent experiments. *p < 0.001, compared to that in serum-free Caco-2 CM.
Autocrine amphiregulin decreases TER of polarized Caco-2 cells
As EGFR activation has been shown to affect levels of TJ proteins (23), we also considered that EGFR ligands might modulate TER of polarized Caco-2 cells. We tested four recombinant EGFR ligands: EGF, TGFα, AREG, and HB-EGF. Ligands were added at a concentration of 10 ng/ml to both apical and basolateral sides of Caco-2 cells seven days after plating. We observed significantly decreased TER in response to all four EGFR ligands compared to CTL medium (Figure 3A). As Caco-2 cells are known to express multiple EGFR ligands (21), we investigated the effect of autocrine EGFR activation on Caco-2 TER. An irreversible EGFR kinase inhibitor, EKI-785 (1 μM), was added one or seven days after plating Caco-2 cells on Transwell® filters and TER was monitored daily. We observed a sustained two-fold increase in TER after cells were exposed to EKI-785 for four or more days (up to 10 days) (Figure 3B). Addition of EKI-785 to fully polarized Caco-2 cells on day seven induced a further increase in TER (Figure 3B). These findings suggest that EGFR activation in Caco-2 cells decreases TER.
We next examined which EGFR ligands were present in CM from Caco-2 and/or 18Co cells by ELISA. EGF and TGFα were not detected in any CM (data not shown). AREG and HB-EGF were detected in CM from Caco-2, 18Co, and co-cultured cells (Figure 3C and data not shown). AREG levels were much greater than HB-EGF levels in both cell lines (data not shown). In 5% serum-containing medium, similar levels of AREG were detected in CM from Caco-2 and 18Co cells, while 18-fold more AREG was detected in serum-containing 18Co CM compared to serum-free 18Co CM (Figure 3C). Serum, however, is a non-physiological fluid. Under more physiological serum-free conditions, seven-fold more AREG was detected in CM from Caco-2 cells than from 18Co cells (Figure 3C). We detected additive levels of AREG in CM from co-cultured Caco-2 and 18Co cells (Figure 3C). We did not measure levels of epigen, epiregulin, or betacellulin, so we cannot exclude these EGFR ligands are produced by these cells. These results suggest that under physiological conditions, Caco-2 cells are likely to be largely responsible for AREG levels detected in CM of these co-cultured cells as 18Co cells produce far less AREG. Therefore, we conclude that the effects of AREG on Caco-2 TER are largely autocrine.
18Co CM-induced TER reduction is due to paracrine KGF and autocrine EGFR ligands
The results above implicate KGF and AREG in the regulation of Caco-2 TJ integrity. To test if paracrine KGF and autocrine AREG directly affect Caco-2 TER, polarized Caco-2 cells were treated with 18Co CM containing an anti-KGF neutralizing antibody (2.5 μg/ml) and/or an anti-EGFR blocking antibody (528 mAb, 5.6 μg/ml). The 18Co CM-induced decrease in Caco-2 TER was attenuated by addition of the anti-KGF neutralizing antibody to 18Co CM, whereas its addition to CTL medium had no effect (Figure 4A). Thus, functional KGF is secreted by stromal 18Co cells and acts on Caco-2 cells in a paracrine manner to reduce TER.
When Caco-2 cells were grown in CTL medium that contained an EGFR-blocking antibody (528 mAb), Caco-2 TER increased, supporting our earlier observation that endogenous EGFR ligands act in an autocrine fashion to activate EGFR and reduce TER of polarizing Caco-2 cells (Figure 4B). Addition of 528 mAb to 18Co CM also resulted in increased Caco-2 TER; however, the increase was not as great as observed with CTL medium containing 528 mAb, presumably due to the presence of KGF in 18Co CM (Figure 4B).
Finally, addition of both the anti-KGF neutralizing antibody and 528 mAb to 18Co CM increased TER to levels comparable to CTL medium supplemented with both antibodies (Figure 4C). Therefore, these data indicate that paracrine KGF and autocrine EGFR ligands predominantly account for the decrease in Caco-2 TER observed under our experimental conditions.
Furthermore, addition of CTL medium or 18Co CM containing an anti-AREG neutralizing antibody or combined anti-AREG and anti-KGF neutralizing antibodies mimicked results seen in Figure 4A–C (data not shown). However, an anti-HB-EGF neutralizing antibody had no significant effect on Caco-2 TER (data not shown), suggesting that although HB-EGF is secreted by Caco-2 cells, it does not account for changes in TER. Taken together, these results further support that paracrine KGF and autocrine AREG are the major growth factors that decrease TER of polarized Caco-2 cells cultured in 18Co CM.
18Co CM induces claudin-2 levels in Caco-2 cells in a KGF-dependent manner
Paracellular permeability of epithelial monolayers is regulated by TJs. To elucidate the mechanism by which 18Co CM lowered Caco-2 TER, we assessed the effects of 18Co CM on steady state levels of TJ components, including claudin-1, -2, occludin, and zonula occludens (ZO)-1. Immunofluorescent staining and western blot analysis demonstrated that exposure of Caco-2 cells to 18Co CM for 24 hours increased claudin-2 levels, whereas levels of claudin-1, occludin, and ZO-1 were unchanged (Figure 5A&B); addition of an anti-KGF neutralizing antibody attenuated this effect (Figure 5C&D), indicating that increased claudin-2 expression in response to 18Co CM exposure was largely KGF-dependent.
KGF induces claudin-2 expression in polarized Caco-2 cells
Thus far, we have shown that KGF is produced by 18Co cells and acts in a paracrine manner to reduce TER in overlying polarized Caco-2 cells. To directly show that the mechanism by which KGF affects Caco-2 TER is through induction of claudin-2, recombinant human KGF was added over a concentration range of 0.1–30 ng/ml to the basolateral side of polarized Caco-2 cells for 24 hours. Western blot analysis demonstrated that KGF produced a concentration-dependent increase in claudin-2 levels (Figure 6A). In a time-course experiment, claudin-2 levels increased after a 30-minute exposure to 10 ng/ml KGF, and levels progressively increased over 24 hours (Figure 6B). Basolateral administration of KGF to a second polarizing human colorectal cancer cell line, HCT-8, also resulted in induction of claudin-2 at 24 hours (data not shown), suggesting a more generalized effect of KGF on claudin-2 expression in polarized human colon cell lines.
We then compared individual and combined compartment-selective effects of KGF and AREG on claudin-2 expression in polarized Caco-2 cells. Exogenous AREG and/or KGF were applied to the basolateral or apical compartment of polarized Caco-2 cells for 24 hours. Basolateral, but not apical, AREG increased claudin-2 levels. Although apical KGF had a minor effect on claudin-2 expression, basolateral KGF led to a more robust increase (Figure 6C). Combined basolateral AREG and KGF treatment did not further enhance claudin-2 induction, indicating they may act through the same or parallel downstream pathways to modulate claudin-2 levels. Therefore, KGF and AREG act in a basolateral-dominant manner to increase claudin-2 levels.
To further demonstrate that paracrine KGF and autocrine AREG are the major factors that act to decrease Caco-2 TER through claudin-2 induction, we added an anti-AREG neutralizing antibody to polarized Caco-2 cells and observed claudin-2 levels upon exposure to exogenous KGF. As described previously, addition of an anti-AREG neutralizing antibody to CTL medium or 18Co CM increased Caco-2 TER, compared to untreated medium (data not shown). Consistent with that result, addition of the anti-AREG neutralizing antibody reduced basal claudin-2 protein to almost undetectable levels (Figure 6D). Upon combined AREG neutralization and KGF treatment, induction of claudin-2 was slightly less than with KGF alone. Collectively, these experiments further support that paracrine KGF and autocrine AREG are the major growth factors in our experimental system that act to decrease Caco-2 TER through induction of claudin- 2 protein levels.
Discussion
Communication between PCMFs and the overlying colonic epithelium is critical for maintenance of tissue homeostasis. We sought to simulate these conditions in vitro by co-culturing polarized colonic epithelial cells (Caco-2) and PCMF cells (18Co) on and underneath Transwell® filters, respectively. We monitored Caco-2 TER as a measure of epithelial TJ integrity. TER decreased when cells were co-cultured and this effect was reproduced when 18Co CM was added to the basolateral, but not apical, compartment. The observation that basolateral 18Co CM decreased Caco-2 TER led us to consider candidate stromal-derived growth factors that influenced TER in this system and the mechanism by which this occurred. Detection of KGF in 18Co CM and AREG largely in Caco-2 CM, their ability to decrease Caco-2 TER when given exogenously, as well as increased TER observed with neutralization of each factor, led us to conclude these are the major growth factors affecting Caco-2 TJ integrity.
We show here that 18Co CM decreases TER of polarized Caco-2 cells through modulation of TJs, as shown by changes in claudin-2 levels. Decreased Caco-2 TER was coincident with increased claudin-2 expression, without effects on claudin-1, occludin, or ZO-1 expression, implicating claudin-2 as a major TJ component that is altered by the paracrine effect of 18Co CM. Exogenous KGF treatment resulted in a time- and concentration-dependent increase in claudin-2 protein. We also demonstrate an autocrine mechanism of reducing TER through EGFR and its ligands, specifically AREG. Exogenous AREG treatment, like KGF, results in increased claudin-2 expression, but combined AREG and KGF treatment does not enhance the effect compared to KGF alone. Neutralization of AREG upon KGF treatment demonstrated that claudin-2 induction is probably due to activation of FGFR2b/EGFR parallel pathways. Thus, under these experimental conditions, we conclude that KGF and AREG are the predominant growth factors that influence the paracellular permeability of Caco-2 epithelial cell monolayers, at least in part, through claudin-2 induction.
A recent study by Dhawan et al. (2011) demonstrated EGFR-dependent induction of claudin-2 in Caco-2 cells upon treatment with a high concentration of exogenous EGF (100 ng/ml) and, based on EGFR kinase inhibition, suggested that baseline claudin-2 levels can be attributed to autocrine EGFR activation (23). Based on our findings, we implicate AREG as the candidate EGFR ligand in this autocrine activation, since an anti- AREG neutralizing antibody eliminates baseline levels of claudin-2 (Figure 6D).
Interestingly, there is evidence for crosstalk between the KGF/FGFR2b and EGFR pathways. In human and mouse keratinocytes, as well as in human fetal small intestine explants, KGF indirectly stimulated EGFR-induced cell growth via upregulation of TGFα (24, 7). We did not detect TGFα in CM from Caco-2 cells stimulated by co-culture with 18Co cells, although we cannot rule out that crosstalk may exist between these two pathways under different conditions.
The importance of stromal-derived KGF in epithelial barrier protection, as well as in epithelial cell proliferation and differentiation, is well established in intestinal physiology. KGF exerts a cytoprotective effect on the intestinal epithelium in multiple models of intestinal injury in mouse and rat, including those caused by dextran sodium sulfate (DSS) (17,25), 2,4,6-trinitrobenzenesulfonic acid (TNB)-induced colitis (75), 5-fluorouracil, carboplatin, combined methotrexate and whole body irradiation, and total body irradiation alone (28). Systemic delivery of KGF to mice results in increased cell proliferation at the crypt base (28, 50, 75) and an increased number of mucin-producing goblet cells (25, 38, 75). Additionally, Visco and colleagues observed that similar co-culture conditions (polarized Caco-2 epithelial cells and 18Co PCMFs) as used in our study, increased proliferation and differentiation of Caco-2 cells, as determined by increased expression of carcinoembryonic antigen (CEA); these results were reproduced by treating Caco-2 cells with KGF (71). Of relevance, KGF is upregulated in cases of human IBD; this overexpression is thought to be cytoprotective and to play a significant role in damage repair, consistent with the effects of KGF on cell proliferation and differentiation (5, 12, 31, 72).
The mechanism of increased KGF expression during intestinal epithelial damage is thought to be a result of cytokine release by immune cells. In mice, KGF expression is observed in activated γδ-TCR+ lymphocytes (10, 17, 74) and production by this cell population plays an important role in promoting intestinal repair and proliferation in models of DSS-induced colitis, mucosal atrophy, and villus hypertrophy (17,74). Numerous studies have demonstrated that KGF mRNA and/or protein expression are induced in multiple cell types from various tissues, by interleukin (IL)-1α(16, 29), tumor necrosis factor-α (TNF-α) (6, 7, 11), IL-1β, and IL-6 (11). These studies provide a connection between immune cell activity in the mesenchyme and the proliferative epithelial response to damage or inflammation observed in vivo (5, 42).
The in vivo physiological significance of KGF-mediated upregulation of claudin-2 expression is not clear. Interestingly, increased expression of claudin-2 is observed in IBD and in vitro studies indicate immune cytokines such as TNFα, IL-4, IL-13, IL-17, and IL-6 can regulate its expression in cultured human intestinal cell lines (1, 37, 39, 43, 56, 63, 73, 76). Increased claudin-2 expression results in decreased TER and increased paracellular cation permeability, and, therefore, may contribute to ion flux and epithelial permeability associated with TJ alterations observed in IBD (2, 4, 14, 19, 26, 34, 40, 56, 61). Increased paracellular permeability may lead to damage by allowing inappropriate diffusion of molecules through the TJ, which may activate the immune system, or, in the case of growth factors, may bind and activate their basolateral receptors, initiating aberrant signaling cascades (14, 23). Increased TJ permeability can have a normal physiological role; one study implicated TJs expressing claudin-2 in Na+-coupled water transport in MDCK cells, demonstrating that such TJs can act as water channels (57). This provides a mechanism for a leaky epithelium to wash out molecules that may inappropriately diffuse from the intestinal lumen through a leaky TJ (14). Additionally, KGF has also been implicated in increased epithelial cell proliferation, which may, in part, be mediated by claudin-2.
There is evidence of a role for claudin-2 in promoting intestinal epithelial cell proliferation and migration. Claudin-2 is detected only at the colonic crypt base in the proliferative progenitor zone; normal human colon expresses a low level of claudin-2, which is upregulated in IBD (27, 56). In HT-29 clone 16E cells, Buchert and colleagues demonstrated claudin-2 acts in relocalization of the ZONAB transcription factor from TJs to the nucleus, where it can induce transcription of cyclin D1, resulting in a proliferative response (13). In another study, SW480 and HCT116 cells overexpressing claudin-2 were more proliferative than their control counterparts, and claudin-2 knockdown prevented EGF-induced proliferation, suggesting claudin-2 may play a role in growth factor-induced cell proliferation (23). Finally, Takehara and colleagues demonstrated that claudin-2 overexpression in Caco-2 cells increases cell migratory activity (64). Collectively, these data suggest that increased claudin-2 expression plays a role in promoting cell proliferation and migration, which may augment epithelial repair processes in IBD where KGF has been shown to be upregulated.
Conclusions
Our in vitro results implicate PCMF-derived paracrine KGF and largely autocrine AREG in the upregulation of claudin-2 in Caco-2 epithelial monolayers and consequent disruption of TJ integrity, as observed by decreased TER. The role of these events in vivo is yet to be determined; it is likely that additional cell types will release factors that affect TJ integrity. These results may have implications in human intestinal diseases, such as IBD.
Acknowledgments
This work was supported by the National Cancer Institute CA151566 and CA46413, Gastrointestinal Specialized Program of Research Excellence P50CA095103 to R.J.C., and T32GM008554 to E.J.P. Cell imaging was performed in part through the VUMC Cell Imaging Shared Resource (supported by NIH grant CA68485, DK20593, DK58404, HD15052, DK59637, and EY08126).
Footnotes
Declaration of Interest
The authors report no declarations of interest.
References
- 1.Amasheh M, Fromm A, Krug SM, Amasheh S, Andres S, Zeitz M, Fromm M, Schulzke JD. TNFalpha-induced and berberine-antagonized tight junction barrier impairment via tyrosine kinase, Akt and NFkappaB signaling. J Cell Sci. 2010;123:4145–4155. doi: 10.1242/jcs.070896. [DOI] [PubMed] [Google Scholar]
- 2.Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969–4976. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JM. Molecular structure of tight junctions and their role in epithelial transport. News Physiol Sci. 2001;16:126–130. doi: 10.1152/physiologyonline.2001.16.3.126. [DOI] [PubMed] [Google Scholar]
- 4.Angelow S, Ahlstrom R, Yu AS. Biology of claudins. Am J Physiol Renal Physiol. 2008;295:F867–876. doi: 10.1152/ajprenal.90264.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bajaj-Elliott M, Breese E, Poulsom R, Fairclough PD, MacDonald TT. Keratinocyte growth factor in inflammatory bowel disease. Increased mRNA transcripts in ulcerative colitis compared with Crohn’s disease in biopsies and isolated mucosal myofibroblasts. Am J Pathol. 1997;151:1469–1476. [PMC free article] [PubMed] [Google Scholar]
- 6.Bajaj-Elliott M, Pender SL, Poulsom R, Macdonald TT. Upregulation of keratinocyte growth factor during T-cell immunity in the gut mucosa. Ann N Y Acad Sci. 1998;859:184–187. doi: 10.1111/j.1749-6632.1998.tb11123.x. [DOI] [PubMed] [Google Scholar]
- 7.Bajaj-Elliott M, Poulsom R, Pender SL, Wathen NC, MacDonald TT. Interactions between stromal cell--derived keratinocyte growth factor and epithelial transforming growth factor in immune-mediated crypt cell hyperplasia. J Clin Invest. 1998;102:1473–1480. doi: 10.1172/JCI2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balda MS, Matter K. Tight junctions and the regulation of gene expression. Biochim Biophys Acta. 2009;1788:761–767. doi: 10.1016/j.bbamem.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 9.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boismenu R, Havran WL. Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science. 1994;266:1253–1255. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- 11.Brauchle M, Angermeyer K, Hubner G, Werner S. Large induction of keratinocyte growth factor expression by serum growth factors and pro-inflammatory cytokines in cultured fibroblasts. Oncogene. 1994;9:3199–3204. [PubMed] [Google Scholar]
- 12.Brauchle M, Madlener M, Wagner AD, Angermeyer K, Lauer U, Hofschneider PH, Gregor M, Werner S. Keratinocyte growth factor is highly overexpressed in inflammatory bowel disease. Am J Pathol. 1996;149:521–529. [PMC free article] [PubMed] [Google Scholar]
- 13.Buchert M, Papin M, Bonnans C, Darido C, Raye WS, Garambois V, Pelegrin A, Bourgaux JF, Pannequin J, Joubert D, Hollande F. Symplekin promotes tumorigenicity by up-regulating claudin-2 expression. Proc Natl Acad Sci U S A. 2010;107:2628–2633. doi: 10.1073/pnas.0903747107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bücker RSM, Amasheh S, Schulzke J-D. Claudins in Intestinal Function. In: YA, editor. Claudins. Vol. 65. Elsevier Inc; Burlington, MA: 2010. pp. 195–227. [Google Scholar]
- 15.Chantret I, Barbat A, Dussaulx E, Brattain MG, Zweibaum A. Epithelial polarity, villin expression, and enterocytic differentiation of cultured human colon carcinoma cells: a survey of twenty cell lines. Cancer research. 1988;48:1936–1942. [PubMed] [Google Scholar]
- 16.Chedid M, Rubin JS, Csaky KG, Aaronson SA. Regulation of keratinocyte growth factor gene expression by interleukin 1. The Journal of biological chemistry. 1994;269:10753–10757. [PubMed] [Google Scholar]
- 17.Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc Natl Acad Sci U S A. 2002;99:14338–14343. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiba H, Osanai M, Murata M, Kojima T, Sawada N. Transmembrane proteins of tight junctions. Biochim Biophys Acta. 2008;1778:588–600. doi: 10.1016/j.bbamem.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 19.Colegio OR, Van Itallie C, Rahner C, Anderson JM. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol Cell Physiol. 2003;284:C1346–1354. doi: 10.1152/ajpcell.00547.2002. [DOI] [PubMed] [Google Scholar]
- 20.Cutler NS, Graves-Deal R, LaFleur BJ, Gao Z, Boman BM, Whitehead RH, Terry E, Morrow JD, Coffey RJ. Stromal production of prostacyclin confers an antiapoptotic effect to colonic epithelial cells. Cancer research. 2003;63:1748–1751. [PubMed] [Google Scholar]
- 21.Damstrup L, Kuwada SK, Dempsey PJ, Brown CL, Hawkey CJ, Poulsen HS, Wiley HS, Coffey RJ., Jr Amphiregulin acts as an autocrine growth factor in two human polarizing colon cancer lines that exhibit domain selective EGF receptor mitogenesis. Br J Cancer. 1999;80:1012–1019. doi: 10.1038/sj.bjc.6690456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denker BM, Nigam SK. Molecular structure and assembly of the tight junction. The American journal of physiology. 1998;274:F1–9. doi: 10.1152/ajprenal.1998.274.1.F1. [DOI] [PubMed] [Google Scholar]
- 23.Dhawan P, Ahmad R, Chaturvedi R, Smith JJ, Midha R, Mittal MK, Krishnan M, Chen X, Eschrich S, Yeatman TJ, Harris RC, Washington MK, Wilson KT, Beauchamp RD, Singh AB. Claudin-2 expression increases tumorigenicity of colon cancer cells: role of epidermal growth factor receptor activation. Oncogene. 2011;30:3234–3247. doi: 10.1038/onc.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dlugosz AA, Cheng C, Denning MF, Dempsey PJ, Coffey RJ, Jr, Yuspa SH. Keratinocyte growth factor receptor ligands induce transforming growth factor alpha expression and activate the epidermal growth factor receptor signaling pathway in cultured epidermal keratinocytes. Cell Growth Differ. 1994;5:1283–1292. [PubMed] [Google Scholar]
- 25.Egger B, Procaccino F, Sarosi I, Tolmos J, Buchler MW, Eysselein VE. Keratinocyte growth factor ameliorates dextran sodium sulfate colitis in mice. Dig Dis Sci. 1999;44:836–844. doi: 10.1023/a:1026642715764. [DOI] [PubMed] [Google Scholar]
- 26.Enck AH, Berger UV, Yu AS. Claudin-2 is selectively expressed in proximal nephron in mouse kidney. Am J Physiol Renal Physiol. 2001;281:F966–974. doi: 10.1152/ajprenal.2001.281.5.F966. [DOI] [PubMed] [Google Scholar]
- 27.Escaffit F, Boudreau F, Beaulieu JF. Differential expression of claudin-2 along the human intestine: Implication of GATA-4 in the maintenance of claudin-2 in differentiating cells. J Cell Physiol. 2005;203:15–26. doi: 10.1002/jcp.20189. [DOI] [PubMed] [Google Scholar]
- 28.Farrell CL, Bready JV, Rex KL, Chen JN, DiPalma CR, Whitcomb KL, Yin S, Hill DC, Wiemann B, Starnes CO, Havill AM, Lu ZN, Aukerman SL, Pierce GF, Thomason A, Potten CS, Ulich TR, Lacey DL. Keratinocyte growth factor protects mice from chemotherapy and radiation-induced gastrointestinal injury and mortality. Cancer research. 1998;58:933–939. [PubMed] [Google Scholar]
- 29.Finch PW, Cheng AL. Analysis of the cellular basis of keratinocyte growth factor overexpression in inflammatory bowel disease. Gut. 1999;45:848–855. doi: 10.1136/gut.45.6.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finch PW, Cunha GR, Rubin JS, Wong J, Ron D. Pattern of keratinocyte growth factor and keratinocyte growth factor receptor expression during mouse fetal development suggests a role in mediating morphogenetic mesenchymal-epithelial interactions. Dev Dyn. 1995;203:223–240. doi: 10.1002/aja.1002030210. [DOI] [PubMed] [Google Scholar]
- 31.Finch PW, Pricolo V, Wu A, Finkelstein SD. Increased expression of keratinocyte growth factor messenger RNA associated with inflammatory bowel disease. Gastroenterology. 1996;110:441–451. doi: 10.1053/gast.1996.v110.pm8566591. [DOI] [PubMed] [Google Scholar]
- 32.Finch PW, Rubin JS. Keratinocyte growth factor/fibroblast growth factor 7, a homeostatic factor with therapeutic potential for epithelial protection and repair. Adv Cancer Res. 2004;91:69–136. doi: 10.1016/S0065-230X(04)91003-2. [DOI] [PubMed] [Google Scholar]
- 33.Finch PW, Rubin JS, Miki T, Ron D, Aaronson SA. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science. 1989;245:752–755. doi: 10.1126/science.2475908. [DOI] [PubMed] [Google Scholar]
- 34.Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol. 2001;153:263–272. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol. 1999;147:891–903. doi: 10.1083/jcb.147.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta. 2008;1778:729–756. doi: 10.1016/j.bbamem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 37.Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Burgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke JD. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Housley RM, Morris CF, Boyle W, Ring B, Biltz R, Tarpley JE, Aukerman SL, Devine PL, Whitehead RH, Pierce GF. Keratinocyte growth factor induces proliferation of hepatocytes and epithelial cells throughout the rat gastrointestinal tract. J Clin Invest. 1994;94:1764–1777. doi: 10.1172/JCI117524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kinugasa T, Sakaguchi T, Gu X, Reinecker HC. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118:1001–1011. doi: 10.1016/s0016-5085(00)70351-9. [DOI] [PubMed] [Google Scholar]
- 40.Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol. 2002;13:875–886. doi: 10.1681/ASN.V134875. [DOI] [PubMed] [Google Scholar]
- 41.Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochim Biophys Acta. 2008;1778:631–645. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 42.MacDonald TT, Monteleone G, Pender SL. Recent developments in the immunology of inflammatory bowel disease. Scand J Immunol. 2000;51:2–9. doi: 10.1046/j.1365-3083.2000.00658.x. [DOI] [PubMed] [Google Scholar]
- 43.Mankertz J, Amasheh M, Krug SM, Fromm A, Amasheh S, Hillenbrand B, Tavalali S, Fromm M, Schulzke JD. TNFalpha up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol-3-kinase signaling. Cell Tissue Res. 2009;336:67–77. doi: 10.1007/s00441-009-0751-8. [DOI] [PubMed] [Google Scholar]
- 44.Merchant NB, Rogers CM, Trivedi B, Morrow J, Coffey RJ. Ligand-dependent activation of the epidermal growth factor receptor by secondary bile acids in polarizing colon cancer cells. Surgery. 2005;138:415–421. doi: 10.1016/j.surg.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 45.Merchant NB, Voskresensky I, Rogers CM, Lafleur B, Dempsey PJ, Graves-Deal R, Revetta F, Foutch AC, Rothenberg ML, Washington MK, Coffey RJ. TACE/ADAM-17: a component of the epidermal growth factor receptor axis and a promising therapeutic target in colorectal cancer. Clin Cancer Res. 2008;14:1182–1191. doi: 10.1158/1078-0432.CCR-07-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miki T, Bottaro DP, Fleming TP, Smith CL, Burgess WH, Chan AM, Aaronson SA. Determination of ligand-binding specificity by alternative splicing: two distinct growth factor receptors encoded by a single gene. Proc Natl Acad Sci U S A. 1992;89:246–250. doi: 10.1073/pnas.89.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mineta K, Yamamoto Y, Yamazaki Y, Tanaka H, Tada Y, Saito K, Tamura A, Igarashi M, Endo T, Takeuchi K, Tsukita S. Predicted expansion of the claudin multigene family. FEBS letters. 2011;585:606–612. doi: 10.1016/j.febslet.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 48.Piepkorn M, Underwood RA, Henneman C, Smith LT. Expression of amphiregulin is regulated in cultured human keratinocytes and in developing fetal skin. J Invest Dermatol. 1995;105:802–809. doi: 10.1111/1523-1747.ep12326567. [DOI] [PubMed] [Google Scholar]
- 49.Piontek J, Winkler L, Wolburg H, Muller SL, Zuleger N, Piehl C, Wiesner B, Krause G, Blasig IE. Formation of tight junction: determinants of homophilic interaction between classic claudins. FASEB J. 2008;22:146–158. doi: 10.1096/fj.07-8319com. [DOI] [PubMed] [Google Scholar]
- 50.Potten CS, O’Shea JA, Farrell CL, Rex K, Booth C. The effects of repeated doses of keratinocyte growth factor on cell proliferation in the cellular hierarchy of the crypts of the murine small intestine. Cell Growth Differ. 2001;12:265–275. [PubMed] [Google Scholar]
- 51.Powell DW. Barrier function of epithelia. The American journal of physiology. 1981;241:G275–288. doi: 10.1152/ajpgi.1981.241.4.G275. [DOI] [PubMed] [Google Scholar]
- 52.Powell DW. Myofibroblasts: paracrine cells important in health and disease. Trans Am Clin Climatol Assoc. 2000;111:271–292. discussion 292–273. [PMC free article] [PubMed] [Google Scholar]
- 53.Powell DW, Adegboyega PA, Di Mari JF, Mifflin RC. Epithelial cells and their neighbors I. Role of intestinal myofibroblasts in development, repair, and cancer. Am J Physiol Gastrointest Liver Physiol. 2005;289:G2–7. doi: 10.1152/ajpgi.00075.2005. [DOI] [PubMed] [Google Scholar]
- 54.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. The American journal of physiology. 1999;277:C1–9. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- 55.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. The American journal of physiology. 1999;277:C183–201. doi: 10.1152/ajpcell.1999.277.2.C183. [DOI] [PubMed] [Google Scholar]
- 56.Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, Collins JE. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest. 2005;85:1139–1162. doi: 10.1038/labinvest.3700316. [DOI] [PubMed] [Google Scholar]
- 57.Rosenthal R, Milatz S, Krug SM, Oelrich B, Schulzke JD, Amasheh S, Gunzel D, Fromm M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci. 2010;123:1913–1921. doi: 10.1242/jcs.060665. [DOI] [PubMed] [Google Scholar]
- 58.Rubin JS, Osada H, Finch PW, Taylor WG, Rudikoff S, Aaronson SA. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci U S A. 1989;86:802–806. doi: 10.1073/pnas.86.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sambuy Y, De Angelis I, Ranaldi G, Scarino ML, Stammati A, Zucco F. The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol Toxicol. 2005;21:1–26. doi: 10.1007/s10565-005-0085-6. [DOI] [PubMed] [Google Scholar]
- 60.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 61.Schulzke JD, Ploeger S, Amasheh M, Fromm A, Zeissig S, Troeger H, Richter J, Bojarski C, Schumann M, Fromm M. Epithelial tight junctions in intestinal inflammation. Ann N Y Acad Sci. 2009;1165:294–300. doi: 10.1111/j.1749-6632.2009.04062.x. [DOI] [PubMed] [Google Scholar]
- 62.Shao J, Sheng GG, Mifflin RC, Powell DW, Sheng H. Roles of myofibroblasts in prostaglandin E2-stimulated intestinal epithelial proliferation and angiogenesis. Cancer research. 2006;66:846–855. doi: 10.1158/0008-5472.CAN-05-2606. [DOI] [PubMed] [Google Scholar]
- 63.Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. The Journal of biological chemistry. 2011;286:31263–31271. doi: 10.1074/jbc.M111.238147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takehara M, Nishimura T, Mima S, Hoshino T, Mizushima T. Effect of claudin expression on paracellular permeability, migration and invasion of colonic cancer cells. Biol Pharm Bull. 2009;32:825–831. doi: 10.1248/bpb.32.825. [DOI] [PubMed] [Google Scholar]
- 65.Thorne BA, Plowman GD. The heparin-binding domain of amphiregulin necessitates the precursor pro-region for growth factor secretion. Mol Cell Biol. 1994;14:1635–1646. doi: 10.1128/mcb.14.3.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valentich JD, Popov V, Saada JI, Powell DW. Phenotypic characterization of an intestinal subepithelial myofibroblast cell line. The American journal of physiology. 1997;272:C1513–1524. doi: 10.1152/ajpcell.1997.272.5.C1513. [DOI] [PubMed] [Google Scholar]
- 67.Van Itallie CM, Anderson JM. The molecular physiology of tight junction pores. Physiology (Bethesda) 2004;19:331–338. doi: 10.1152/physiol.00027.2004. [DOI] [PubMed] [Google Scholar]
- 68.Van Itallie CM, Anderson JM. The role of claudins in determining paracellular charge selectivity. Proc Am Thorac Soc. 2004;1:38–41. doi: 10.1513/pats.2306013. [DOI] [PubMed] [Google Scholar]
- 69.Van Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol. 2003;285:F1078–1084. doi: 10.1152/ajprenal.00116.2003. [DOI] [PubMed] [Google Scholar]
- 70.Van Itallie CM, Mitic LL, Anderson JM. Claudin-2 forms homodimers and is a component of a high molecular weight protein complex. The Journal of biological chemistry. 2011;286:3442–3450. doi: 10.1074/jbc.M110.195578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Visco V, Bava FA, d’Alessandro F, Cavallini M, Ziparo V, Torrisi MR. Human colon fibroblasts induce differentiation and proliferation of intestinal epithelial cells through the direct paracrine action of keratinocyte growth factor. J Cell Physiol. 2009;220:204–213. doi: 10.1002/jcp.21752. [DOI] [PubMed] [Google Scholar]
- 72.Weber CR, Nalle SC, Tretiakova M, Rubin DT, Turner JR. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab Invest. 2008;88:1110–1120. doi: 10.1038/labinvest.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wisner DM, Harris LR, 3rd, Green CL, Poritz LS. Opposing regulation of the tight junction protein claudin-2 by interferon-gamma and interleukin-4. J Surg Res. 2008;144:1–7. doi: 10.1016/j.jss.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 74.Yang H, Antony PA, Wildhaber BE, Teitelbaum DH. Intestinal intraepithelial lymphocyte gamma delta-T cell-derived keratinocyte growth factor modulates epithelial growth in the mouse. J Immunol. 2004;172:4151–4158. doi: 10.4049/jimmunol.172.7.4151. [DOI] [PubMed] [Google Scholar]
- 75.Zeeh JM, Procaccino F, Hoffmann P, Aukerman SL, McRoberts JA, Soltani S, Pierce GF, Lakshmanan J, Lacey D, Eysselein VE. Keratinocyte growth factor ameliorates mucosal injury in an experimental model of colitis in rats. Gastroenterology. 1996;110:1077–1083. doi: 10.1053/gast.1996.v110.pm8612996. [DOI] [PubMed] [Google Scholar]
- 76.Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zigrino P, Loffek S, Mauch C. Tumor-stroma interactions: their role in the control of tumor cell invasion. Biochimie. 2005;87:321–328. doi: 10.1016/j.biochi.2004.10.025. [DOI] [PubMed] [Google Scholar]