Abstract
The teleost Astyanax mexicanus is a single species consisting of two radically different forms: a sighted pigmented surface-dwelling form (surface fish) and a blind depigmented cave dwelling form (cavefish). The two forms of Astyanax have favorable attributes, including descent from a common ancestor, ease of laboratory culture, and the ability to perform genetic analysis, permitting their use as a model system to explore questions in evolution and development. Here we review current research on the molecular, cellular and developmental mechanisms underlying the loss of eyes and pigmentation in Astyanax cavefish. Although functional eyes are lacking in adults, cavefish embryos begin to develop eye primordia, which subsequently degenerate. The major cause of eye degeneration appears to be apoptotic cell death of the lens, which prevents the growth of other optic tissues, including the retina. Ultimately, the loss of the eye is the cause of craniofacial differences between cavefish and surface fish. Lens apoptosis is induced by enhanced activity of the Hedgehog signaling system along the cavefish embryonic midline. The absence of melanin pigmentation in cavefish is due to a block in the ability of undifferentiated melanoblasts to accumulate L-tyrosine, the precursor of L-DOPA and melanin, in melanosomes. Genetic analysis has shown that this defect is caused by a hypomorphic mutation in the p/oca 2 gene encoding an integral melanosomal membrane protein. We discuss how current studies eye and pigment regression have revealed some of the mechanisms in which cavefish development has been changed during evolution.
Introduction
Many studies in evolutionary developmental biology have been centered exclusively on the generation of novel traits. Although molecular and developmental analysis of trait loss is often more tractable than analysis of gain, considerably less attention has been focused on the reduction and loss of traits. It can be argued that trait modification or loss is just as important as gain in providing a complete understanding of evolution as a developmental process, and may be one of the first steps in the cascade of events leading to evolutionary innovations. For example, during the evolution of flippers in marine mammals, significant changes, including reductions and losses, must have occurred in the limbs of their terrestrial ancestors prior to their conversion to perform a swimming function. Therefore, it is important to study the evolution of novelties, referred to here as constructive traits, within the context of reduced or lost traits, referred to here as regressive traits. One of the most important animal models for studying regressive and constructive traits in the same context is the teleost Astyanax mexicanus (Jeffery, 2001; 2008).
Astyanax mexicanus consists of two conspecific forms: a surface dwelling form (surface fish) and a cave dwelling form (cavefish). Surface fish adults have large eyes and three different types of pigment cells, whereas cavefish have reduced or lost both these traits (Fig. 1), a phenotype shared with a diverse community of cave animals (Culver, 1982). Cavefish have also gained constructive features, larger jaws, more taste buds, larger cranial neuromasts, fat reserves, and possibly a more sensitive olfactory system than their surface fish counterparts. At least 30 different populations of Astyanax cavefish are present in limestone caverns in Mexico, having been isolated from their surface fish conspecifics for the past few million years (Porter et al., 2007). Each cavefish population is named after their cave of origin (Mitchell et al., 1977). For example, Pachón, Chica, Los Sabinos, and Subterráneo, and Molino cavefish are found in La Cueva de El Pachón, La Cueva Chica, La Cueva de los Sabinos, La Cueva de la Subterráneo, and Sótano de El Molino, respectively. In this article, unless another cavefish population is named specifically, reference to cavefish will imply the Pachón population, which has been the subject of the most extensive developmental analysis. Genetic (Wilkens, 1971; Borowsky, 2008) and phylogenetic (Dowling et al., 2002; Strecker et al., 2003; 2004) evidence suggests that some of these cavefish populations have evolved regressive and constructive phenotypes independently. Thus, Astyanax cavefish are an excellent model system to study convergent evolution of developmental mechanisms.
Figure 1.
Gradual loss of eyes and absence of body pigment development in cavefish. Surface fish (above) and cavefish (below) are shown in each frame. A. One day post-fertilization (dpf). B. Three dpf. C. One week post-fertilization (wpf). D. Two wpf. E. One month post-fertilization (mpf). F. Adults. Note developmental arrest and progressive loss of eyes in cavefish, development and rapid growth of eyes in surface fish, body pigment cell development in surface fish, and absence of eye and body pigmentation in cavefish. Scale bars are 62.5 mm (A), 125 mm (B), 250 mm (C–D), and 500 mm (F). F. From Yamamoto and Jeffery (2000).
The Astyanax system has many advantages for studies in evolutionary developmental biology. First and perhaps foremost is its value as a laboratory model. Surface fish and cavefish are easy to maintain in the laboratory, spawn frequently, and produce fairly large and robust embryos. Second, the polarity of evolutionary changes in this system is known with certainty: cavefish lacking eyes and pigment have evolved from surface fish ancestors that exhibited both of these traits. Evolutionary polarity is must be inferred by phylogenetic analysis and rarely understood with such confidence in other cases. Third, the similarity of present day surface fish to the historical source of cavefish provides an excellent comparative system in which an evolutionary product can be compared to a prototype of its ancestral form. Finally, cavefish and surface fish are completely interfertile, allowing the power of genetic analysis to be applied to the evolution of constructive and regressive traits.
It has been said that evolution is the effect of ecology on development (Van Valen, 1973). Accordingly, not only do Astyanax cavefish provide an excellent model system to study the evolution of development, but they also provide a context in which evolutionary events can be understood with respect to the environmental conditions that forged them. In most instances, the ecological effects that led to the emergence of new phenotypes are difficult to discern because they occurred in the distant past and are no longer in existence. In contrast, perpetual darkness, the ecological cue leading to evolutionary changes in Astyanax cavefish and other cave animals has remained constant through time. Thus, it is likely that present conditions in the caves harboring cavefish are the same as they were when surface fish first entered and begin the process of evolutionary change leading to cavefish. This article reviews the molecular, cellular, and developmental mechanisms responsible for loss of eyes and pigmentation, which have occurred in a background of constructive changes in Astyanax cavefish.
Eye Development and Degeneration
Vertebrate eyes develop from three different parts of the ectoderm. The ocular lens is formed from a thickening in the surface ectoderm, known as the lens placode. The retina and retinal pigment epithelium (RPE) are formed from bilateral protrusions of forebrain neuroectoderm, which are called optic vesicles. The optic vesicles arise from bilaterally symmetric optic fields in the anterior neural plate. Each optic vesicle rotates about 90° and then buckles inward to form the optic cup, with the future RPE on the convex side and retina on the concave side. The connection of the optic cup to the forebrain will become the optic stalk, which encases the optic nerve fibers. As the optic vesicles are forming, the lens placode reorganizes into a vesicle, which detaches from the surface epithelium and enters the concave opening of the optic cup. The neural crest is the third part of the ectoderm responsible for eye development. Cranial neural crest cells migrate from the anterior neural tube region into spaces between the lens and surface epithelium to form the inner parts of the cornea, between the lens and the distal edges of the retina to contribute to the iris and ciliary body, and into the areas surrounding the RPE to form the choroid and sclera. Neural crest cells probably also contribute to the ocular dermal bones that develop much later around the orbit, forming a part of the adult craniofacial skeleton.
The eye primordium also consists of three major parts, the lens, retina, and RPE, which differentiate in concert. The lens vesicle produces fiber cells, which synthesize crystallin proteins, and becomes transparent, leaving behind a layer of undifferentiated stem cells. The retina differentiates into several layers. From distal to proximal, they consist of (1) the ganglion cell layer, which transmits neural signals to the brain via axons extending through the optic stalk into the optic tectum, (2) the intermediate layers, which consist of inter-neurons and glial cells, and (3) the photoreceptor layer, where rod and cone cells translate photons into neural signals. The RPE forms tight connections with the photoreceptor layer and produces melanin pigment. Pigment cells also become organized around the RPE, but outside the eye proper. These body pigment cells have a different origin from those of the RPE, and will be discussed later in this article.
The sequence of events during surface fish and cavefish eye development are compared in Figure 2A. The cavefish eye primordium is slightly smaller than its surface fish counterpart (Fig. 2B, C). This difference in size is due to a smaller lens and optic cup, which appears to be missing its ventral sector. In contrast to the surface fish eye, cavefish optic tissues either fail to be induced (cornea, iris, ciliary body) or begin to differentiate and then degenerate (lens, retina, and probably the RPE). However, the most important flaw in the cavefish eye primordium is the absence of net optic growth after the conclusion of the embryonic stages (Fig. 2A). Eventually, the arrested cavefish eye primordium, which has not markedly increased in size during the larval stages, is overgrown by head epidermis and connective tissue, and disappears into the orbit, making adult cavefish appear eyeless.
Figure 2.
Eye development and degeneration in Astyanax. A. Development of the eye primordium from up to about 20 hrs post fertilization (hpf) in cavefish and surface fish (left). The surface fish eye differentiates and rapidly increases in size (top) from 1 dpf to 1 mpf, whereas the cavefish eye arrests in growth, degenerates, and gradually sinks into the orbit. B, C. Size differences in the 24 hpf surface fish (B) and cavefish (C) eye primordia. D–G. Sections of 2 (D, E) and 3 (F, G) dpf surface fish (D. F) and cave fish (E, G) eye primordia showing apoptosis (dark stained spots) in various eye tissues. In cavefish, apoptosis begins in the lens (arrowheads) and spreads to the retina (arrows). There is no apoptosis in these tissues in surface fish. H. The roles of cell proliferation and apoptosis during retina/RPE growth in surface fish cavefish. Clear retinal areas: embryonic retina and central part of growing retina derived from embryonic retina. Shaded area: part of retina derived from cell proliferation at the CMZ after the embryonic stages. X: Apoptotic areas. The surface fish retina grows continuously due to cell proliferation at the CMZ, whereas the cavefish retina is arrested in growth because the products of cell proliferation at the CMZ die before they contribute to the differentiated retina. B, C. From Yamamoto and Jeffery (2000). D–G: From Strickler et al. (2007a).
Because cavefish eye development involves growth arrest, it is important to consider the possible effects on the origin of new cells. Stem cells in the epithelial layer are the source of new lens fiber cells. The source of most new retinal and all new RPE cells is a stem cell niche at the edge of the optic cup, a region known as the ciliary marginal zone (CMZ). As the eye enlarges during larval development, it is surrounded by orbital bones, which form a part of the craniofacial skeleton. The orbital bones presumably differentiate from mesenchyme of neural crest origin and their number, size, and organization is distinct between surface fish and cavefish, and even among different cavefish populations (Alvarez, 1947; Yamamoto et al., 2003). As described below, the presence or absence of a functional eye is critical in the morphogenesis of the orbital bones and organization of the craniofacial skeleton.
Cellular Mechanisms of Eye Degeneration
A block in cell proliferation, an increase in programmed cell death, or a combination of these processes could be the caused of arrested eye development in cavefish. Current evidence suggests that cell death has a major role in this process (Jeffery and Martasian, 1998; Yamamoto and Jeffery, 2000). If cell death is restricted to a single eye tissue, or starts in one tissue and later spreads to others, then the tissue that dies first is a candidate to initiate the entire degeneration process. The early cavefish eye primordium is largely free of cell death, except for one tissue: the lens (Fig. 2E). Apoptosis is not detected in the surface fish lens (Fig. 2D). Although cell proliferation does not cease in the cavefish lens, the rate of apoptosis is very high, eventually obliterating the lens, or reducing it to a tiny vestige in the adult (Soares et al., 2004). A few days after the initiation of lens apoptosis, the cavefish retina also begins to undergo apoptosis (Fig. 2G) (Alunni et al., 2007; Strickler et al., 2007a). Retinal cell death is restricted to the intermediate layers and regions adjacent to the CMZ. Later in development, the cavefish RPE also shows dying cells (Strickler et al., 2007a). As for the lens, cell death is not observed in the surface fish retina (Fig. 2F) or RPE. Clearly, the lens is the first tissue to undergo cell death in the cavefish eye primordium, suggesting that its absence may be the trigger for eye degeneration.
In contrast to cell death, there is no evidence that cell proliferation stops in the degenerating cavefish eye. In the surface fish retina the primary zone of cell proliferation is the CMZ, in which proliferating cells can be detected by labeling replicating DNA or the presence of DNA replication enzymes, such as proliferating cell nuclear antigen (PCNA). Continuous cell proliferation in the CMZ displaces newly born cells into the adjacent retinal layers and RPE, where they differentiate and increase the general mass of the retina. The profile of cell proliferation in the cavefish retina is not changed compared to surface fish. The cell proliferation markers BrdU and PCNA are expressed normally in the cavefish CMZ during the period in which the retina does not markedly increase in size (Strickler et al., 2002; 2007a; Alunni et al., 2007). The reason that the cavefish retina does not show net growth is that the new cells are quickly removed by apoptosis, which persists during cavefish larval development and into adult life (Strickler et al., 2007a). Thus, the cavefish eye is arrested in growth because newly born cells die before they are able to differentiate and join the retinal layers. The relationship between growth, cell proliferation, and apoptosis in the surface fish and cavefish retina is illustrated in Figure 2H.
Does the absence of a functional lens play a role in the survival of newly born cavefish retinal cells? This possibility has been tested by transplantation of embryonic lenses between surface fish and cavefish (Yamamoto and Jeffery, 2000). The lens transplantation method is illustrated in Figure 3A. The embryonic lens is removed from a donor embryo shortly after its formation and transplanted into the optic cup of a host embryo. Lens transplantation is done unilaterally, with the unoperated eye of the host serving as a control, and reciprocally: a surface fish lens is transplanted into a cavefish optic cup and vice versa. When a cavefish lens was transplanted into a surface fish optic cup it died on schedule, just as if it had not been removed from the donor embryo. In contrast, when a surface fish lens was transplanted into a cavefish optic cup it continued to grow and differentiate, just as it would have done in the surface fish host. These results indicate that the cavefish lens is autonomously fated for apoptosis.
Figure 3.
Lens transplantation. A. Diagram showing the transplantation method in which a donor lens is removed from the optic cup of one form of Astyanax embryo and transplanted unilaterally into the optic cup of another form after the host lens is removed. This operation is carried out at about 1 dpf. B–J. Changes in eye development after lens transplantation during embryogenesis. B, C, F, G. Surface fish lens was transplanted into a Pachón (B, C) or Los Sabinos (F, G), cavefish host. D, E, I, H. Changes in eye development after a Pachón (D, E) or Los Sabinos (H, I) cavefish lens was transplanted into a surface fish host. J, K. Changes in orbital bone structure after a surface fish lens was transplanted into a cavefish optic cup unilaterally. B, D, F, H, J: Control (unoperated) side. C, E, G, I, K. Transplant side. B–E. From Yamamoto and Jeffery (2000). F–I. From Jeffery et al. (2003). J, K. From Yamamoto et al. 2003.
Cavefish with a transplanted surface fish lens show a dramatic restoration of eye development. The eye primordium of Pachón or Los Sabinos cavefish containing a surface fish lens begins to grow (Fig. 3C, H) (Yamamoto and Jeffery, 2000; Jeffery et al., 2003). Eventually, the cornea and iris appear, and the enlarged retina is more highly organized. Further growth results in the presence of a highly developed eye containing a cornea, iris, and photoreceptor cells. In contrast to the eye with a transplanted lens, the unoperated eye of the cavefish host degenerates and disappears into the orbit (Fig. 3B, G). Likewise, after obtaining a cavefish lens, development of the surface fish eye is retarded, the cornea and iris does not differentiate, and the size and organization of the retina is reduced. The degenerate surface fish eye eventually disappears into the orbit, mimicking the cavefish eye (Fig. 3E, I), whereas the unoperated eye develops normally, producing a one eyed surface fish (Fig. 3D, H).
Several important conclusions can be made from the lens transplantation experiments. First, the lens is required for normal development of the retina, cornea, and iris. Second, as a result of apoptosis the cavefish lens has lost the ability to organize eye development. Third, the cavefish optic cup (RPE/retina) has retained the ability to respond to signals generated by a normal surface fish lens. Fourth, the lens has a role in promoting the survival of retinal cells: a transplanted surface fish lens can protect the cavefish retina from apoptosis (Strickler et al., 2007a). Finally, the lens has an indirect role in determining craniofacial morphology. When a surface fish lens is transplanted into a cavefish optic cup, an orbital bone phenotype is obtained resembling surface fish rather than cavefish (Fig. 3J, K) (Yamamoto et al., 2003). The cavefish host develops with a hybrid craniofacial morphology, one side (the lens transplant side) resembling surface fish and the other (the control side) cavefish.
Clearly, the lens has a major role in regulating cavefish eye degeneration. Whether the death of the lens is the only cause of eye degeneration, or other optic alterations, such as independent changes in the retina or RPE, are also involved (Strickler et al., 2007a), remains to be determined.
Molecular Mechanisms of Eye Degeneration
Understanding the molecular mechanisms of eye degeneration requires identification of the genes involved in this process and how they function during development. Many eye development genes are known in vertebrates, allowing a candidate gene approach to be used for gene identification (Jeffery, 2005). In addition, many genes that are differentially expressed in cavefish have been identified by a microarray-based approach (Strickler and Jeffery, 2009). A list of some of the differentially expressed genes is provided in Table 1. These genes encode transcription factors that function near the top of eye gene hierarchies, as well as structural genes encoding proteins that function at the bottom of these cascades. In many cases, in situ hybridization or staining with specific antibodies was used to determine their expression patterns.
Table 1.
Differentially expressed genes in cavefish embryos relative to surface fish embryos
| Gene | Status | Expression | Identification* | Reference |
|---|---|---|---|---|
| hsp90α | upregulated | lens | candidate analysis | Hooven et al. (2004) |
| shhA | upregulated | midline, brain | candidate analysis | Yamamoto et al. (2004) |
| shhB | upregulated | midline | candidate analysis | Yamamoto et al. (2004) |
| patched 1, 2 | upregulated | midline | candidate analysis | Yamamoto et al. (2004) |
| pax2.1a | upregulated | optic vesicles | candidate analysis | Yamamoto et al. (2004) |
| nkx 2.1a, b | upregulated | midline, brain | candidate anaylsis | Yamamoto et al. (2004); Menuet et al. (2007) |
| vax1 | upregulated | optic vesicles | candidate analysis | Yamamoto et al. (2004) |
| downregulatedretina | candidate analysis | Alunni et al. (2007) | ||
| lhx6, 7 | upregulated | brain | candidate analysis | Menuet et al. (2007) |
| ubiquitin-specific protease 53 | upregulated | unknown | microarray analysis | Strickler and Jeffery (2009) |
| pax6 | downregulatedoptic vesicles | candidate analysis | Strickler et al. (2001) | |
| gamma M-crystallin | downregulatedlens | microarray analysis | Strickler and Jeffery (2009) | |
| gamma B crystallin | dowregulated | unknown | microarray analysis | Strickler and Jeffery (2009) |
| αA- crystallin | downregulatedlens | candidate analysis | Behrens et al. (1998); Strickler et al. (2007b) | |
| rhodopsin | downregulated retina | microarray analysis | Strickler and Jeffery (2009) | |
| neurofilament protein M | downregulatedunknown | microarray analysis | Strickler and Jeffery (2009) | |
| guanosine nucleotide binding proteins 1, 2 | downregulatedunknown | microarray analysis | Strickler and Jeffery (2009) | |
In microarray analysis only genes with at least a 10-fold difference are included.
Most of the genes surveyed by candidate gene analysis do not show expression changes in surface fish and cavefish embryos. For example, the Prox1 transcription factor is expressed normally in the developing lens and retina of cavefish until after the eye begins to degenerate (Jeffery et al., 2000). Likewise, prior to lens degeneration, genes encoding the membrane proteins MIP and MP19 are expressed normally (Strickler et al., 2007b). Many genes also show the same or similar expression patterns in the developing surface and cave fish retinas (Jeffery et al., 2000; Strickler et al., 2002; Menuet et al., 2007). However, some genes are downregulated or upregulated in cavefish (Table 1). For example, gamma-M crystallin and rhodospin genes are reduced in the cavefish lens and retina respectively (Strickler and Jeffery, 2009; see also Langecker et al., 1993 for rhodopsin). These downregulated genes are consistent with respective lack of lens fiber cell differentiation and degeneration of the retinal photoreceptor layer in cavefish.
Among the upregulated genes is one related to human ubiquitin specific protease 53 and several other genes (not shown in Table 1) encoding factors related to apoptotic cell death. Two genes related to apoptosis are especially interesting. First, the hsp90α gene is specifically activated in the cavefish lens vesicle just prior to apoptosis (Hooven et al., 2004). Outside of the lens, hsp90α expresion remains unchanged, and expression of its close relative hsp90β, remains unchanged between cavefish and surface fish (Hooven et al., 2004). Pharmacological inhibition of Hsp90α suppresses lens apoptosis and rescues lens differentiation. Second, the αA-crystallin gene, which encodes a potent anti-apoptotic factor, is strongly downregulated in the lens vesicles of Piedras (Behrens et al., 1998) and Pachón cavefish (Strickler et al., 2007b). αA-crystallin may normally protect the lens from apoptosis and is a required chaperone for the normal function of other crystallins in the lens. It is possible that αA-crystallin and Hsp90α interact in a cascade leading to lens apoptosis.
Some of the changes in expression detected by in situ hybridization are more subtle than those described above. The pax6 gene encodes a transcription factor that is expressed in the lens, retina, RPE, and their precursors early in teleost eye development (Krauss et al., 1991; Püschel et al., 1998). Later, pax6 expression becomes restricted to the lens epithelial cells, some of the retinal layers, and the corneal epithelium. In surface fish embryos, the pax6 expression domains in the bilateral optic fields connect across the midline at their anterior margins (Fig. 4A). In cavefish embryos, however, the corresponding pax6 domains are diminished in size and show a large gap across the midline (Strickler et al., 2001) (Fig. 4B). The division of the optic vesicle into the optic cup and stalk is controlled by reciprocal antagonistic interactions between the Pax6, Pax2, and Vax1 transcription factors (Schwarz et al., 2000). Pax6 directs optic cup development, whereas Pax2 and Vax1 control optic stalk development. Accordingly, a reduction of pax6 levels (or an increase in pax2 and vax1 levels) increases the optic stalk at the expense of the optic cup. The reduction of pax6 expression coupled with the overexpression of pax2a (Fig. 4C–F) and vax1 (Fig. 4G–L) genes accounts for the ventrally reduced optic cup in cavefish embryos (Yamamoto et al., 2004). The vax1 gene is also expressed on the ventral side of the developing retina in surface fish and other teleosts. However, vax1 expression is missing in the ventral portion of the cavefish retina (Alunni et al., 2007), showing that this gene is either upregulated or downregulated in cavefish depending on developmental stage.
Figure 4.
Optic vesicle (A–F) and optic cup (G–L) development in surface fish and cavefish. A, B. Neural plate stage embryo showing differences in pax6 expression in the surface fish and cavefish optic fields (OF). Arrowhead shows the midline pax6 expression gap, which is wider in cavefish. C, D. Optic vesicles (OV) showing size and pax2a expression (arrowheads) differences in surface fish and cavefish. In A–D. embryos are viewed dorsally with anterior at the top. E, F. Diagram showing size differences in the surface fish and cavefish optic vesicles. Territories fated to form optic stalk are lightly shaded and those fated to form retina/RPE are darkly shaded. G, H. Surface fish and cavefish optic cups (OC) showing ventral size reduction in the latter. L: lens. I, J. The vax1 gene is overexpressed ventrally in the cavefish optic cup relative to surface fish. In G–J, embryos are viewed laterally with dorsal at the top. K, L. Diagram showing size and relative optic cup territorial differences between cavefish and surface fish. The optic stalk (OS) is lightly shaded and the optic cup is darkly shaded. A–D, G–J. From Yamamoto et al. (2004). E, F, K, L. From Strickler et al. (2003)
The wider gap between pax6-expressing optic fields in the cavefish neural plate provides further insight into how eye degeneration is controlled. During vertebrate development, the presumptive optic cup is initially determined as a single medial optic field, which is subsequently split into two bilateral eye domains by Hedgehog (Hh) signals emanating from the underlying prechordal plate (Macdonald et al. 1995; Ekker et al. 1995). Hh signaling inhibits pax6 expression along the midline to divide the original eye domain into bilateral eyes. Teleosts have at least two-hh midline signaling genes, sonic hedgehogA (shhA) and shhB, which show overlapping expression patterns (Ekker et al. 1995). Yamamoto et al. (2004) compared shhA and shhB expression patterns during surface fish and cavefish development and demonstrated that the midline expression domains of both genes are expanded in cavefish relative to surface fish (see Fig. 5A, B for shhA). Later in cavefish development, shhA expression is also expanded anteriorly, curling around the rostrum in the presumptive oral area (Fig. 5C, D). The expression patterns of genes acting downstream of shhA and shhB in the Hh midline signaling pathway, such as patched1 and patched2, encoding Shh receptors, and nkx2.1a and nkx2.1b, encoding Shh dependent transcription factors, are also expanded (Yamamoto et al., 2004), suggesting that a general increase in midline Hh signaling has evolved in cavefish.
Figure 5.
Role of Hh midline signaling in cavefish eye degeneration. A–D. The cavefish embryonic midline shows a wider shh expression domain than its surface fish counterpart. The expression of dlx3 and pax2a marker genes does not change. A, B. Tailbud stage. C, D. Ten somite stage. E–J. Effects of shh overexpression in surface fish. E, F. Increased shh expression (compare F with C) and reduced pax6 expression (E) on one side of the midline of an embryo injected with shh mRNA. G, H. As a result of shh overexpression, the optic cup (retina/RPE) is missing its ventral sector and the adult eye has degenerated (G) and adult (H). Arrowhead in G: missing ventral sector of the retina. Arrowhead in H: missing eye. I, J. Lens apoptosis (J) after injection of an embryo with shh mRNA. Arrowheads: lens. H. Diagram showing antagonistic relationship between Pax6, Pax2, and Vax1 transcription factors, ventralization of the optic cup, and lens apoptosis in cavefish. Arrows: activations. Blocked lines: inhibitions. A–J. From Yamamoto et al. (2004).
The shh genes are expressed in many places in vertebrate embryos. Does Shh expansion also occur in these places in cavefish? Although further studies are needed to completely investigate this important question, the answer appears to be yes and no. Although shhA is overexpressed early in the notochord as well as the anterior midline, at later stages of development the notochord expression domain appears normal (Yamamoto and Jeffery, unpublished), suggesting that compensatory mechanisms are active during later cavefish development. Likewise, there appear to be no differences in the size or intensity of shhA expression domains in cavefish and surface fish fin buds (Yamamoto and Jeffery, unpublished). In contrast, early shhA expansion is continued in various regions of the cavefish embryonic forebrain, where working through downstream transcription factors such as Nkx2.1a and Lhx 6/Lhx7, it seems to be instrumental in increasing the size of the cavefish hypothalamus and ventral forebrain (Menuet et al., 2007). The conclusion is that persistent expansion of Shh signaling is restricted to the anterior midline, a region known as the prechordal plate, as well as the developing forebrain immediately dorsal to this region.
Role of Hedgehog Signaling in Eye Degeneration
The epicenter of expanded shh expression along the cavefish anterior midline places is a critical position with respect to eye development. The role of enhanced Hh signaling in cavefish eye development was investigated by increasing shh expression in surface fish embryos (Yamamoto et al., 2004). When shhA mRNA was injected into one side of a cleaving embryo, shhA expression was expanded along that side of the prechordal plate (anterior embryonic midline), and pax6 expression was down regulated unilaterally in the corresponding optic field (Fig. 5E, F). Surface fish larvae that developed from embryos overexpressing Shh were missing an eye missing on one side of the head (Fig. 5G, H). Thus, blind cavefish were phenocopied by increasing the levels of shh gene expression in surface fish, demonstrating a key role for Shh signals in eye degeneration. Importantly, lens apoptosis is also induced by shh overexpression in surface fish embryos (Yamamoto et al., 2004) (Fig. 5I, J). A diagram of the proposed gene network leading to eye degeneration via hyperactive Shh signaling, reduction of the optic vesicle, and lens cell death is shown in Figure 5K.
In summary, a sequence of regulatory events beginning with expanded midline signaling, proceeding through reduction in size of the eye primordia, lens apoptosis, and retinal apoptosis, and resulting in arrested eye growth and alteration of craniofacial morphology, is responsible for cavefish optic degeneration (Fig. 7). Alterations in the activity of many different genes and their upstream regulators are likely to control these changes. Early genetic studies showed that eye degeneration is a multigenic trait (Wilkens, 1988). More recently, it has been determined that at least 12 quantitative trait loci (QTL) are involved in the loss of eyes in Pachón cavefish (Protas et al., 2007). None of these QTL are in near the locations of shhA or shhB on the Astyanax genetic map, showing that shh genes themselves are not mutated to cause eye degeneration. One possibility for further consideration is that some or all of these QTL may act upstream in the pathway leading to shh overexpression in cavefish
Figure 7.
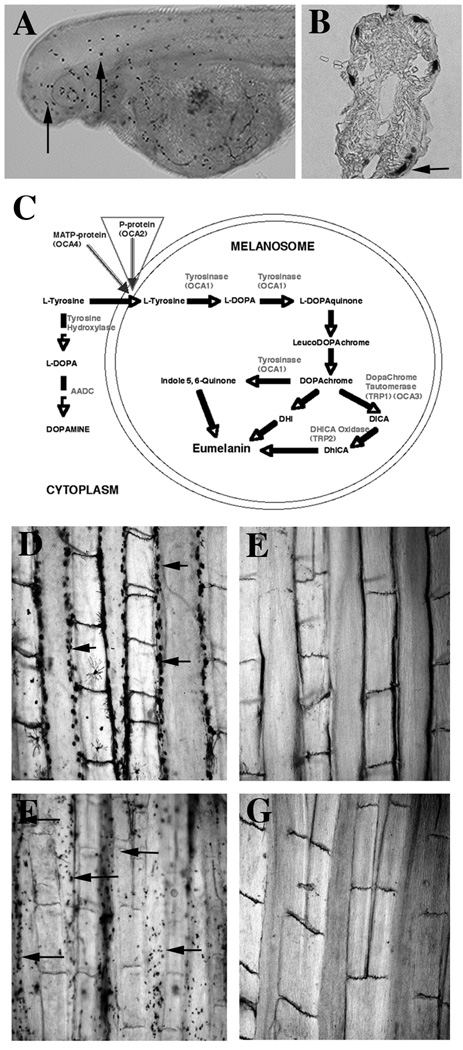
Neural crest development in cavefish. A–C. Detection of migratory neural crest cells in cavefish embryos by DiI injection and subsequent tracing of labeled cells. A. A 1.5 dpf cavefish embryo showing the site of Dil injection (arrowhead). B. Fluorescence image of the embryo in A showing migration of Dil injected cells. DiI: original injection site. C, D. Higher magnification images of insets in B showing morphology of injected cells (arrows). E. Diagram of pigment cell development from the neural crest derived precursor cells showing the location of the pigmentation block in cavefish. A–D. From McCauley et al. (2004).
Pigment Cell Regression
Astyanax surface fish have three types of body pigment cells: light reflecting iridophores, yellow-orange xanthophores, and black melanophores. Pigmentation normally functions in protection from the damaging effects of sunlight, in camouflage, and in species and sex recognition. Selective pressure for retaining these functions is relaxed in the absence of light. What are the consequences in Astyanax cavefish?
The early studies of Rasquin (1947) showed that melanophores are decreased in numbers although xanthophores seem to be present at the same levels in Chica cavefish. Very little is known about changes in iridophores. Of the three types of pigment cells, most is known about melanophores (Wilkens, 1988; McCauley et al., 2004). Subterráneo cavefish show a modest reduction in melanophore pigmentation, Chica, Curva, Los Sabinos, and Tinaja cavefish show substantial decreases in melanophore pigmentation, and most Molino and Pachón cavefish show little if any melanophores. In addition to changes in the number of melanophores, cavefish also show defects in the ability to produce melanin, the pigment found in melanophores. In Pachón cavefish, melanin pigment seems to be entirely absent, both in body pigment cells (including those surrounding the eye) and in the pigment-containing layer of the RPE. Loss of pigmentation is a typical feature of a diverse assemblage of cave animals and may indeed represent one of the most broad examples of evolutionary convergence in nature. What are the mechanisms of pigment cell regression?
All types of body pigment cells are derived from the neural crest, a unique class of migratory cells derived from the border of the neural tube and surface ectoderm (Erickson, 1993; LeDouarin and Kalcheim, 1999). Vertebrate neural crest cells produce a myriad of different cell types, including sensory ganglia, the peripheral nervous system, cranial cartilage and bone, endocrine and fat cells, as well as body pigment cells. Considering the diversity of their derivatives, it is unlikely that neural crest cells could be modified without inducing lethality. However, a subset of neural crest cells involved in pigment cell development could be missing in cavefish. To test this possibility, cell tracing, immunological, and tissue culture methods have been used to follow neural crest development in Astyanax (McCauley et al., 2004; Jeffery, 2006). In DiI labeling experiments neural crest cells migrate into the epidermis (Fig. 7A–D), suggesting that there is no defect in neural crest cells during cavefish development.
Another possibility to explain the regression of pigment cells would be cell death. We have already seen how lens cell death mediated by Shh overexpression along the embryonic midline has major effects on cavefish eye regression. Neural crest cells that do not migrate properly or receive normal differentiation signals often die by apoptosis (Morales et al., 2005). Therefore, apoptosis could remove neural crest derived precursors in cavefish embryos before they differentiate into pigment cells. When this possibility was tested only a few dying neural crest cells were observed in cavefish embryos, and their number was about the same as in surface fish embryos (Fig. 7A–D; Jeffery, 2006). Therefore, melanophores or their progenitor cells do not undergo massive apoptosis during cavefish embryogenesis. Cavefish pigmentation defects must arise downstream of the generation, migration, and divergence of pigment cell types. This conclusion is supported by the fact that iridophores and xanthophores, which are also products of the migratory neural crest, are apparently present in cavefish that are completely lacking melanophores.
Defective Melanogenesis and Undifferentiated Melanoblasts in Cavefish
The early events of pigment cell formation and diversification are not completely understood in vertebrates. However, the fates of iridophores, xanthophores, and melanophores, which are derived from the same neural crest cell lineage, may be somewhat interchangeable (Fig. 7E). The presence of appreciable numbers of other pigment cell types in cavefish lacking melanophores (Rasquin, 1947; McCauley et al., 2004) suggests that the lesion in melanophore development lies downstream of the split between the pigment cell progenitors.
Melanophore differentiation involves the initial formation of colorless melanoblasts, which subsequently synthesize black melanin pigment and become functional melanophores. The biochemical steps involved in melanin synthesis during the transition from melanoblast to melanophore are well known and conserved throughout the vertebrates (Fig. 8C). First, cytoplasmic L-tyrosine is transported into the melanosome, where it is converted to L-DOPA by the multifunctional enzyme tyrosinase. Next, L-DOPA is converted into melanin by a series of enzymatic reactions, the first of which is also catalyzed by tyrosinase. Most subsequent reactions in this biosynthetic pathway are spontaneous. If adequate L-tyrosine is available and the tyrosinase, tyrosinase-related protein-1 (TRP-1), and TRP-2 enzymes are active, then melanin will be produced. This series of reactions have been investigated to determine the lesion in cavefish melanin synthesis.
Figure 8.
Block in melanogenesis in cavefish. A, B. Cavefish embryos after L-DOPA assay showing tyrosinase positive melanoblasts (arrows). A. Whole mount viewed laterally. B. Section through the trunk. C. Eumelanin and dopamine synthesis from L-tyrosine in the melanosome and cytoplasm respectively. Substrates and products, enzymes, and melanosome membrane proteins involved in the reactions are indicated at their position(s) in the pathways. Red inverted triangle indicates the lesion in cavefish melanogenesis involving P/OCA2. D–G. Whole mounts of tail fins of adult surface fish (D) and cavefish (E–G). D. The surface fish fin has melanophores (arrows). E. The cavefish fin lacks melanophores. F. The cavefish fin has melanoblasts (arrows) that can convert exogenously supplied L-DOPA to melanin. G. Cavefish melanoblasts lack the ability to convert L-tyrosine to L-DOPA and melanin. A, B, D–G from McCauley et al. (2004).
Tyrosinase is the limiting enzyme in melanogenesis. Do cavefish pigment progenitor cells have functional tyrosinase? Tyrosinase activity was determined by the L-DOPA assay, in which melanin production is determined after exogenous L-DOPA is provided to fixed specimens. The L-DOPA assay showed that Pachón, Chica, Los Sabinos, Tinaja, and Curva cavefish all exhibit active tyrosinase in cells resembling melanoblasts in their morphology and location within the embryo (Fig. 8A, B). Tyrosinase positive melanoblasts were also observed in the scales and fins of adult cavefish in the positions in which differentiated melanophores are found in their surface fish counterparts (Fig. 8D, E). The results of this experiment show that the inability to synthesize melanin in cavefish is due to a block in the melanogenic pathway immediately upstream of the tyrosinase dependent steps.
The first step in melanin synthesis is the conversion of L-tyrosine to L-DOPA, which is also catalyzed by tyrosinase (Fig. 8C). Cavefish must have L-tyrosine itself because it is required for protein synthesis. However, because cavefish seem to lack endogenous melanosomal L-DOPA there may be a deficiency in the ability of L-tyrosine to be converted to L-DOPA. This possibility was investigated by a modified L-DOPA assay in which exogenous L-tyrosine was provided to fixed specimens instead of L-DOPA (McCauley et al., 2004). If cavefish can convert L-tyrosine to L-DOPA then black pigment would be deposited in the same cells that have active tyrosinase. However, melanin deposition was not detected in cavefish exposed to excess L-tyrosine (Fig. 8E, G). The results indicate that cavefish melanoblasts are unable to convert L-tyrosine to melanin, implying that melanogenesis is blocked because cytoplasmic L-tyrosine cannot be transported into cavefish melanosomes.
Genetic Basis of Cavefish Albinism
A single gene controls cavefish albinism (Sadoglu, 1957; Borowsky and Wilkens, 2002). Accordingly, all F1 progeny of surface fish × Pachón cavefish cross are pigmented and their F2 progeny show a 3:1 ratio of pigmented to unpigmented fishes. Further, crosses between Pachón or Molino cavefish and albino Curva, Los Sabinas, Piedras, Japones, Tinaja, and Yerbanez cavefish (Wilkens, 1988; Wilkens and Strecker, 2003; Jeffery, unpublished), generate albino F1 offspring, suggesting that mutations in the same gene underlie albinism in many different Astyanax cavefish populations.
Using crosses between surface fish and Pachón or Molino cavefish, Protas et al. (2006) determined the location of the albinism gene on a microsatellite map of the Astyanax genome. The albinism gene was mapped to the same position in linkage group 16 in both cavefish populations. This result could be explained either by the same mutation in the same gene, different mutations in the same gene, or mutations in different but very closely linked genes. To address this issue, a complementation test was performed in which Pachón and Molino cavefish were crossed and pigmentation was examined in the offspring. If the progeny are pigmented this would suggest that different genes are responsible for albinism, whereas if they are colorless the same gene locus would be implicated. Colorless progeny were obtained showing that the same gene is responsible for albinism in Pachón and Molino cavefish.
Human tyrosinase-positive albinisms have been classified as OCA1, OCA2, OCA3, and OCA4, which are defined by mutations in different genes (Oeting and King, 1999). OCA1 albinism is caused by mutations in the mulifunctional enzyme tyrosinase, which acts at three different points in the melanin biosynthetic pathway (Fig. 8C). As described above, cavefish can convert L-DOPA to melanin. This means that functional tyrosine must be present in melanoblasts and that cavefish are not OCA1 albinos (McCauley et al., 2004). OCA3 albinism is due to mutations in the gene encoding tyrosinase-related protein 1 (DOPAChrome tautomerase), which functions downstream of the initial tyrosinase-catalyzed steps. By the same reasoning as applied immediately above, this enzyme is also likely to be functional in cavefish, which are therefore not OCA3 albinos. OCA2 and OCA4 albinisms are caused by mutations in the pink-eyed dilution/oca2 (p/oca2) (Rinchik et al., 1993) and matp (Baxter and Pavan, 2002) genes respectively, which encode melanosome membrane proteins. Mutations in p/oca2 also cause albinism in mice, in which the mutant gene was originally named pink-eyed dilution (p), and in a teleost, the Medaka (Fukamachi et al., 2004). The matp gene is responsible for hypopigmentation in the mouse underwhite mutant, where it encodes a putative membrane transporter (Newton et al., 2001). Protas et al. (2006) compared the positions of three candidate genes, tyrosinase (OCA1), p/oca2 (OCA2), and tyrosinase-related protein 1 (OCA3) to the albinism locus on the Astyanax genome map. These studies identified p/oca2 as the cavefish albinism gene. These results suggest that cavefish are OCA2 albinos, which is also the most common form of albinism in humans.
The mammalian p/oca2 gene contains 24 exons encoding a putative twelve-pass membrane protein (Rosenblatt et al., 1994; Brilliant et al., 1994) (Fig. 9A). Several functions have been proposed. One possibility is that P/OCA2 transports L-tyrosine into the melanosome (Toyofuku et al., 2002), thus explaining why cavefish melanosomes can use exogenous L-DOPA, but not L-tyrosine as a tyrosinase substrate. Another possibility is that P/OCA2 modulates the processing and transport of tyrosinase (Toyofuko et al., 2002). However, the conservation of tyrosinase activity in cavefish is inconsistent with this possibility. Finally, it has been proposed that P/OCA2 is a proton transporter responsible for regulating melanosomal pH, a key factor in melanogenesis (Brilliant, 2001). Further studies are needed to define the molecular function of P/OCA2 and the physiological lesion it mediates in cavefish melanosomes.
Figure 9.
Mutations in p/oca2 responsible for albinism in Pachón and Molino cavefish. A. The predicted structure of the human P/OCA2 protein showing 12 membrane spanning domains. N: N terminus. C: C terminus. Thick bar: Melanosome membrane. Thin line: P/OCA2 protein. After Brilliant et al. (1994). B. Diagram showing the positions of single amino acid changes (asterisks) and deletions (peaked thin lines) in the Pachón (P) and Molino (M) cavefish P/OCA2 proteins. S: The intact surface fish P/OCA2 protein consisting of 24 exons of the p/oca2 gene. P: The non-functional Pachón cavefish P/OCA2 protein in showing loss of a major part of exon 24. For clarity, additional translanted sequence in Pachón cavefish P/OCA2 protein corresponding to part of intron 23 (see text) is not indicated in the diagram. M: The non-functional P/OCA2 protein in Molino cavefish showing the loss of exon 21. Thick black lines: Exon sequence. Sequence lengths are not drawn to scale. After Protas et al. (2006).
The molecular basis of loss of function was determined by identifying cavefish p/oca2 mutations. Protas et al. (2006) isolated and compared surface fish, Pachón cavefish, and Molino cavefish p/oca2 cDNAs. Three differences from surface fish were discovered in Pachón cavefish p/oca2: two point mutations resulting in conserved amino acid substitutions and a large deletion extending from within intron 23 through most of exon 24. Because of this deletion, the Pachón P/OCA2 protein would contain a part of intron 23 as a translated sequence and would be missing most of exon 24 (Fig. 9B). In Molino cavefish, there was a single change, another large deletion encompassing exon 21 that would also shorten the P/OCA2 protein. Both deletions are in regions predicted to be parts of membrane spanning domains.
To determine which of these mutations cause p/oca2 loss of function, Protas et al. (2006) examined the ability of DNA constructs containing wild-type surface fish p/oca2 and the individual polymorphisms in Pachón and Molino cavefish p/oca2 to rescue the colorless phenotype in a melanocyte cell line derived from a P/OCA2 deficient albino mouse (Sviderskaya et al., 1997). The surface fish p/oca2 DNA construct and the two Pachón cavefish p/oca2 DNA constructs with different amino acid polymorphisms rescue melanogenesis in the cell line, indicating that the corresponding point mutations do not prevent melanogenesis. In contrast, p/oca2 DNA constructs containing the large deletions found in Pachón and Molino p/oca2 do not induce melanin synthesis, suggesting that they are responsible for loss of function. Although the p/oca2 gene appears to be responsible for loss of melanin pigment in many different cavefish populations, the mutations are distinct in Pachón and Molino cavefish, suggesting that cavefish albinism evolved by convergence.
Evolution of Development
The comparative studies of Astyanax provide important insights into the evolution of development in cavefish. In the final section of this article, we discuss evolutionary insights gleaned from the studies described above pertaining to cavefish eye and pigment regression.
Developmental Constraints
It is clear that cavefish regressive evolution is channeled to a large extent by developmental constraints, which restrict the amplitude of evolutionary changes, or make them unlikely or impossible, by limiting developmental flexibility. This lack of flexibility appears to have a very important role in cavefish eye and pigment evolution. Consider the following. If these traits are ultimately lost, why is it necessary to construct an eye or produce melanoblasts in the first place? The answer may be that early steps in eye and pigment development are required for other essential steps in development, and the elimination of these steps would be fatal.
Eyes are initially formed and then degraded during larval or adult development in all sightless cave dwelling vertebrates (Eigenmann, 1909; Durand, 1976; Berti et al., 2001). Indeed, we feel that cave vertebrates lacking embryonic eye primordia will not be discovered because of this strong developmental constraint. Because all vertebrates have bilateral eyes arising from a single medial optic field, the subsequent separation of optic fields is likely to be an ancient vertebrate trait that evolved in concert with other head features. Thus, if the Shh midline-signaling pathway is altered, as we have seen in cavefish, there may be automatic consequences on eye development, in this case leading to degeneration (Jeffery, 2005).
In cavefish, a block in the pigment cell-generating pathway occurs relatively late in the developmental pathway, during the conversion of melanoblasts into melanophores. Earlier steps in this pathway, such as the determination and migration of neural crest cells, the restriction to pigment cell fate, and the diversification of different pigment cell lineages is apparently not changed, even though the usefulness of any pigment cell type is questionable in cavefish. The reason neural crest cells are formed is clear: they have many critical derivatives and their loss would be lethal. Why are any pigment cell types are formed in cavefish? The constraint might be that progression toward making a general set of pigment cells precursors (including melanoblasts) may be required to produce other types of pigment cells (e. g. iridophores and xanthophores), whose function is in some unknown way essential in cave dwelling teleosts.
The process in which retinal development is arrested in cavefish may be another example of a developmental constraint. We have shown that the arrest of retinal development is not caused by inhibition of cell division at the CMZ, which would seem to be the simplest way to stop growth. Instead, retinal growth is curtailed by apoptosis of newly born cells (Strickler et al., 2007a). This must be a very costly process in terms of energy expenditure, so why has inhibition of cell proliferation, the most parsimonious and least expensive route to preventing retina development, not been taken? The probable answer lies in the fact that the retina is actually a part of the brain. In both retina and brain, stem cells replenish the laminated areas through the same course of action, which may be a fundamental property of nervous system development and difficult to modify. Accordingly, killing new cells after they proliferate in the retina may be more allowable than blocking stem cell division in the CMZ because of an ancient constraint on how different parts of the brain grow in concert during development.
Developmental Amplification
Cavefish show how large-scale changes in the phenotype can occur rapidly during evolution. The differences in craniofacial skeletons between cavefish and surface fish, in particular the ocular bones surrounding the eye, are so extreme that they were formerly used to support their designation as separate genera (Alvarez, 1947). However, the majority of these changes are related to whether or not a large eye punctuates the craniofacial skeleton. When the eye is absent from the surface of the head, as in cavefish, the craniofacial skeleton is patterned differently from when an eye is present. Major changes in the craniofacial skeleton can be elicited by transplanting a surface fish lens into a cavefish optic cup during early development (Yamamoto et al., 2003). The sequence of events is as follows: a normal lens induces anterior eye parts and promotes the growth of a normal retina, producing a large growing eye, which in turn dictates the morphology of surrounding bones in the adult (Fig. 8). Cavefish show that slight changes in early development can be amplified to have major impacts in the adult.
Pleiotropy and Tradeoffs
Pleiotropy, the control multiple, often seemingly unrelated phenotypes, by a single gene is a possible mechanism for the evolution of regressive traits in cave animals (Barr, 1968). Accordingly, if downregulation of genes controlling eye development simultaneously increases the development of a beneficial trait, such as olfaction or another sensory system, the latter might be adaptive and subject to natural selection. The potential for trait linkage is the reason that it is important to study regressive traits in the context of constructive traits. The discovery of enhanced midline signaling mediated by highly pleiotropic hh genes (Yamamoto et al., 2004) opens many possibilities that may be able to explain eye degeneration in cavefish. As we have seen, Hh overexpression has a negative effect on eye development, and it is known from studies on other vertebrates that Hh signaling has positive effects on many other developmental traits. Thus, selection for the positive traits would automatically affect the negative ones. In the future, it will be important to determine the identity of positive traits influenced by Hh signaling.
Evolutionary Forces
Why have eyes and pigment been lost in cavefish? No one really knows the answer but the regressive features of cave animals are usually explained by one of two hypotheses: (1) the accumulation of selectively neutral (loss of function) mutations and genetic drift (Wilkens, 1988) or (2) indirect selection based on energy conservation and/or antagonistic pleiotropy (Culver, 1982; Jeffery, 2005). Although neither hypothesis has been proved in the case of Astyanax cavefish, developmental and genetic studies generally support one or the other for loss of pigmentation and eyes respectively.
In the case of eye loss, the developmental information seems to support selection over neutral mutation. First, the genes involved in eye development that have been studied thus far do not appear to have mutated to a degree in which they have lost function. In addition, the restoration of eyes by lens transplantation suggests that all genes that act downstream of lens function are present and potentially active in cavefish. Also supportive of selection is that most genes with modified expression patterns, such as those in the Shh signaling pathway and hsp90α, increase rather than decrease their activity in cavefish. Genetic analysis is also consistent with selection (Protas et al., 2007). QTL have only been found that result in a decrease in eye formation; none have been reported that result in an increase, which would be expected if genetic drift were involved.
In contrast to eye regression, developmental studies on loss of pigmentation could support either selection or neutral mutation. On one hand, the accumulation of neutral mutations resulting in loss of melanophores might be possible, especially if the oca2 gene is not pleiotropic and its disruption does not affect other important developmental pathways. Genetic analysis, in which individual QTL governing the extent of melanophore development have been shown to either increase or decrease melanophore abundance, support the role of neutral mutation and genetic drift (Protas et al., 2007). On the other hand, melanogenesis could be disrupted because it is adaptive, allowing pigment cell precursors to be shunted into other, more beneficial differentiation pathways. Some of these possibilities are testable and predict a bright future for the Astyanax system in addressing why, as well as how, developmental changes have occurred during evolution.
Figure 6.
Summary of early and late events in cavefish eye degeneration and consequences on craniofacial development.
Acknowledgements
The research from the Jeffery laboratory described in this article was supported by grants from NIH (R01-EY014619) and NSF (IBN-0542384).
References
- Alunni A, Menuet A, Candal E, Pénigault J-B, Jeffery WR, Rétaux S. Developmental mechanisms for retinal degeneration in the blind cavefish Astyanax mexicanus. J. Comp. Neurol. 2007;505:221–233. doi: 10.1002/cne.21488. [DOI] [PubMed] [Google Scholar]
- Alvarez J. Descripción de Anoptichthys hubbsi caracinindo ceigo de La Cueva de Los Sabinos. S. L. P. Soc. Mex. Hist. Nat. 1947;8:215–219. [Google Scholar]
- Barr T. Cave ecology and the evolution of troglobites. Evol. Biol. 1968;2:35–102. [Google Scholar]
- Baxter LI, Pavan WJ. The oculocutaneous albinism type IV gene Matp is a new marker of pigment cell precursors during mouse embryonic development. Mech. Dev. 2002;116:209–212. doi: 10.1016/s0925-4773(02)00130-2. [DOI] [PubMed] [Google Scholar]
- Behrens M, Wilkens H, Schmale H. Cloning of the αA-crystallin genes of the blind cave form and the epigean form of Astyanax fasciatus: a comparative analysis of structure, expression and evolutionary conservation. Gene. 1998;216:319–326. doi: 10.1016/s0378-1119(98)00346-1. [DOI] [PubMed] [Google Scholar]
- Berti R, Durand JP, Becchi S, Brizzi R, Keller N, Ruffat G. Eye degeneration in the blind cave-dwelling fish Phreatichys andruzzi. Can. J. Zool. 2001;79:1278–1285. [Google Scholar]
- Borowsky R. Restoring sight in blind cavefish. Curr. Biol. 2008;18:R23–R24. doi: 10.1016/j.cub.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Borowsky R, Wilkens H. Mapping a cave fish genome. Polygenic systems and regressive evolution. J. Hered. 2002;93:19–21. doi: 10.1093/jhered/93.1.19. [DOI] [PubMed] [Google Scholar]
- Brilliant MH. The mouse p (pink-eyed dilution) and human P genes, oculocutaneous albinism type 2 (OCA2), and melanosomal pH. Pig. Cell Res. 2001;14:86–93. doi: 10.1034/j.1600-0749.2001.140203.x. [DOI] [PubMed] [Google Scholar]
- Brilliant MH, King R, Francke U, Schuffenhauer S, Meitinger T, Gardner JM, Durham-Pierre D, Nakatsu Y. The mouse pink-eyed dilution gene: association with hypopigmentation in Prader-Willi and Angelman syndromes and with human OCA2. Pig. Cell Res. 1994;7:398–402. doi: 10.1111/j.1600-0749.1994.tb00068.x. [DOI] [PubMed] [Google Scholar]
- Cahn PH. Comparative optic development in Astyanax mexicanus and in two of its blind cave derivatives. Bull. Am. Mus. Nat. Hist. 1958;115:73–112. [Google Scholar]
- Culver D. Cave Life. Evolution and Ecology. Cambridge MA: Harvard; 1982. [Google Scholar]
- Dowling TE, Martasian DP, Jeffery WR. Evidence for multiple genetic lineages with similar eyeless phenotypes in the blind cavefish, Astyanax mexicanus. Mol. Biol. Evol. 2002;19:446–455. doi: 10.1093/oxfordjournals.molbev.a004100. [DOI] [PubMed] [Google Scholar]
- Durand JP. Ocular development and involution in the European cave salamander, Proteus anguinus Laurenti. Biol. Bull. 1976;151:450–466. doi: 10.2307/1540499. [DOI] [PubMed] [Google Scholar]
- Ekker SC, Ungar AR, von Greenstein P, Porter JA, Moon RT, Beachy P. Patterning activities of vertebrate hedgehog proteins in the developing eye and brain. Curr. Biol. 1995;5:944–955. doi: 10.1016/s0960-9822(95)00185-0. [DOI] [PubMed] [Google Scholar]
- Eigenmann CH. The eyes of the blind vertebrates of North America. V. The history of the eye of blind Amblyopsis from its appearance to its disintegration in old age. Contributions Zool. Lab. Indiana Univ. 1908:167–204. Mark Anniversary Volume. [Google Scholar]
- Erickson CA. From the crest to the periphery: control of pigment cell migration and lineage segregation. Pig. Cell Res. 1993;6:336–347. doi: 10.1111/j.1600-0749.1993.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Fernald RD. Teleost vision: seeing while growing. J. Exp. Zool. 1991;5:167–180. doi: 10.1002/jez.1402560521. [DOI] [PubMed] [Google Scholar]
- Fukamachi S, Asakawa S, Wakamatsu Y, Shimizu N, Mitanti H, Shima A. Conserved function of Medaka pink-eyed dilution in melanin synthesis and its divergent transcriptional regulation in gonads among vertebrates. Genetics. 2004;168:1519–1527. doi: 10.1534/genetics.104.030494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooven TA, Yamamoto Y, Jeffery WR. Blind cavefish and heat shock protein chaperones: A novel role for hsp90α in lens apoptosis. Int. J. Dev. Biol. 2004;48:731–738. doi: 10.1387/ijdb.041874th. [DOI] [PubMed] [Google Scholar]
- Jeffery WR. Cavefish as a model system in evolutionary developmental biology. Dev. Biol. 2001;231:1–12. doi: 10.1006/dbio.2000.0121. [DOI] [PubMed] [Google Scholar]
- Jeffery WR. Adaptive evolution of eye degeneration in the Mexican blind cavefish. J. Hered. 2005;96:185–196. doi: 10.1093/jhered/esi028. [DOI] [PubMed] [Google Scholar]
- Jeffery WR. Regressive evolution of pigmentation in the cavefish Astyanax. Is. J. Ecol. Evol. 2006;52:405–422. [Google Scholar]
- Jeffery WR. Emerging systems in Evo/Devo: cavefish and mechanisms of microevolution. Evol. Dev. 2008;10:265–272. doi: 10.1111/j.1525-142X.2008.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery WR, Martasian DP. Evolution of eye regression in the cavefish Astyanax: apoptosis and the Pax-6 gene. Amer Zool. 1998;38:685–696. [Google Scholar]
- Jeffery WR, Strickler AG, Guiney S, Heyser D, Tomarev SI. Prox1 in eye degeneration and sensory organ compensation during development and evolution of the cavefish Astyanax. Dev. Genes Evol. 2000;210:223–230. doi: 10.1007/s004270050308. [DOI] [PubMed] [Google Scholar]
- Jeffery WR, Strickler AG, Yamamoto Y. To see or not to see: Evolution of eye degeneration in Mexican blind cavefish. Comp. Int. Biol. 2003;43:531–541. doi: 10.1093/icb/43.4.531. [DOI] [PubMed] [Google Scholar]
- Krauss S, Johannsen T, Korzh V, Fijose A. Zebrafish pax[zf-a]: a paired box gene expressed in the neural tube. EMBO J. 1991;10:3609–3619. doi: 10.1002/j.1460-2075.1991.tb04927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langecker TG, Schmale H, Wilkens H. Transcription of the opsin gene in degenerate eyes of cave dwelling Astyanax fasciatus (Teleostei, Characidae) and its conspecific ancestor during early ontogeny. Cell Tiss. Res. 1993;273:183–192. [Google Scholar]
- Le Douarin NM, Kalcheim C. The Neural Crest. Second Edition. New York: Cambridge University Press; 1999. [Google Scholar]
- McCauley DW, Hixon E, Jeffery WR. Evolution of pigment cell regression in the cavefish Astyanax: A late step in melanogenesis. Evol. Dev. 2004;6:209–218. doi: 10.1111/j.1525-142X.2004.04026.x. [DOI] [PubMed] [Google Scholar]
- Macdonald R, Anukampa Barth K, Xu Q, Holder N, Mikkola I, Wilson S. Midline signalling is required for Pax6 gene regulation and patterning of the eyes. Development. 1995;121:3267–3278. doi: 10.1242/dev.121.10.3267. [DOI] [PubMed] [Google Scholar]
- Menuet A, Alunni A, Joly J-S, Jeffery WR, Rétaux S. Shh overexpression in Astyanax cavefish: multiple consequences on forebrain development and evolution. Development. 2007;134:845–855. doi: 10.1242/dev.02780. [DOI] [PubMed] [Google Scholar]
- Mitchell RW, Russell WH, Elliot WR. Mexican eyeless characin fishes, genus Astyanax: Environment, distribution, and evolution. Spec. Publ. Mus. Texas Tech Univ. 1977;12:1–89. [Google Scholar]
- Morales AV, Barbas JA, Nieto MA. How to become neural crest: from segregation to delamination. Semin. Cell Dev. Biol. 2005;16:655–662. doi: 10.1016/j.semcdb.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Newton JM, Cohen-Barak O, Hagiwara H, Gardner JM, Davisson MT, King RA, Brilliant MH. Mutations in the human orthologue of the mouse underwhite gene (uw) underlie a new form of oculocutaneous albinism, OCA4. Am. J. Human Genet. 2002;69:981–988. doi: 10.1086/324340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeting WS, King RA. Molecular basis of albinism: mutations and polymorphisims of pigmentation genes associated with albinism. Hum. Mut. 1999;13:99–113. doi: 10.1002/(SICI)1098-1004(1999)13:2<99::AID-HUMU2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Porter ML, Dittmar de la Cruz K, Pérez-Losada M. How long does evolution of the troglomorphic form take? Estimating divergence times in Astyanax mexicanus. Acta Carsologica. 2007;36:173–182. [Google Scholar]
- Protas ME, Hersey C, Kochanek D, Zhou Y, Wilkens H, Jeffery WR, Zon LT, Borowsky R, Tabin CJ. Genetic analysis of cavefish reveals molecular convergence in the evolution of albinism. Nature Genet. 2006;38:107–111. doi: 10.1038/ng1700. [DOI] [PubMed] [Google Scholar]
- Protas M, Conrad M, Gross JB, Tabin C, Borowsky R. Regressive evolution in the Mexican cave tetra, Astyanax mexicanus. Curr. Biol. 2007;17:452–454. doi: 10.1016/j.cub.2007.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Püschel AW, Gruss P, Westerfield M. Sequence and expression pattern of pax-6 are highly conserved between zebrafish and mice. Development. 1992;114:643–651. doi: 10.1242/dev.114.3.643. [DOI] [PubMed] [Google Scholar]
- Rasquin P. Progressive pigmentary regression in fishes associated with cave environments. Zoologia. 1947;32:35–44. [PubMed] [Google Scholar]
- Rinchik EM, Bultman SJ, Horsthemke B, Lee ST, Strunk KM, Spritz RA, Avidano KM, Jong MT, Nicholls RD. A gene for the mouse pink-eyed dilution locus and for human type II oculocutaneous albinism. Nature. 1993;361:72–76. doi: 10.1038/361072a0. [DOI] [PubMed] [Google Scholar]
- Rosenblatt S, Durham-Pierce D, Garner JM, Nakatsu Y, Brilliant MH, Orlow SJ. Identification of a melanosomal membrane protein encoded by the pink-eyed dilution (type II oculocutaneous albinism) gene. Proc. Nat. Acad. Sci. USA. 1994;91:12071–12075. doi: 10.1073/pnas.91.25.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoglu P. A Mendelian gene for albinism in natural cave fish. Experientia. 1957;13:394. [Google Scholar]
- Schwarz M, Cecconi F, Berneir G, Andrejewski N, Kammandel B, Wagner M, Gruss P. Spatial specification of mammalian eye territories by reciprocal transcriptional repression of Pax2 and Pax6. Development. 2000;127:4325–4334. doi: 10.1242/dev.127.20.4325. [DOI] [PubMed] [Google Scholar]
- Soares D, Yamamoto Y, Strickler AG, Jeffery WR. The lens has a specific influence on optic nerve and tectum development in the blind cavefish Astyanax. Dev Neuroscience. 2004;26:308–317. doi: 10.1159/000082272. [DOI] [PubMed] [Google Scholar]
- Strecker U, Bernachez L, Wilkens H. Genetic divergence between cave and surface populations of Astyanax in Mexico (Characidae, Teleostei) Mol. Ecol. 2003;12:699–710. doi: 10.1046/j.1365-294x.2003.01753.x. [DOI] [PubMed] [Google Scholar]
- Strecker U, Faúndez VH, Wilkens H. Phylogeography of surface and cave Astyanax (Teleostei) from Central and North America based on cytochrome b sequence data. Mol. Phylogenet. Evol. 2004;33:469–481. doi: 10.1016/j.ympev.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Strickler AG, Jeffery WR. Differentially expressed genes identified by cross species microarray in the blind cavefish Astyanax. Int. Zool. 2009 doi: 10.1111/j.1749-4877.2008.00139.x. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickler AG, Yamamoto Y, Jeffery WR. Early and late changes in Pax6 expression accompany eye degeneration during cavefish development. Dev. Genes Evol. 2001;211:138–144. doi: 10.1007/s004270000123. [DOI] [PubMed] [Google Scholar]
- Strickler AG, Famuditimi K, Jeffery WR. Retinal homeobox genes and the role of cell proliferation in cavefish eye degeneration. Int. J. Dev. Biol. 2002;46:285–294. [PubMed] [Google Scholar]
- Strickler AG, Yamamoto Y, Jeffery WR. The lens controls cell survival in the retina: evidence from the blind cavefish Astyanax. Dev. Biol. 2007a;311:512–523. doi: 10.1016/j.ydbio.2007.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickler AG, Byerly MS, Jeffery WR. Lens gene expression analysis reveals downregulation of the anti-apoptotic chaperone αA crystallin during cavefish eye degeneration. Dev. Genes Evol. 2007b;217:771–782. doi: 10.1007/s00427-007-0190-z. [DOI] [PubMed] [Google Scholar]
- Sviderskaya EV, Novak EK, Swank RT, Bennent DC. The murine misty mutation: phenotypic effects on melanocytes, platelets, and brown fat. Genetics. 1998;148:381–390. doi: 10.1093/genetics/148.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Take-uchi M, Clarke JD, Wilson SW. Hedgehog signalling maintains the optic stalk-retinal interface through the regulation of Vax gene activity. Development. 2003;130:955–968. doi: 10.1242/dev.00305. [DOI] [PubMed] [Google Scholar]
- Toyofuku K, Valencia JC, Kushimoto T, Costin G-E, Virador VM, Viera WD, Ferrans VJ, Hearing VJ. The etiology of oculocutaneous albinism (OCA) type II: The pink protein modulates the processing and transport of tyrosinase. Pig. Cell Res. 2002;15:217–224. doi: 10.1034/j.1600-0749.2002.02007.x. [DOI] [PubMed] [Google Scholar]
- Van Valen L. Festschrift. Science. 1973;180:488. [Google Scholar]
- Wilkens H. Genetic interpretation of regressive evolutionary processes: studies of hybrid eyes of two Astyanax cave populations (Characidae, Pisces) Evolution. 1971;25:530–544. doi: 10.1111/j.1558-5646.1971.tb01913.x. [DOI] [PubMed] [Google Scholar]
- Wilkens H. Evolution and genetics of epigean and cave Astyanax fasciatus (Characidae, Pisces) Evol. Biol. 1988;23:271–367. [Google Scholar]
- Wilkens H, Strecker U. Convergent evolution of the cavefish Astyanax (Characidae, Teleostei): genetic evidence from reduced eye-size and pigmentation. Biol. J. Linn. Soc. 2003;80:545–554. [Google Scholar]
- Yamamoto Y, Jeffery WR. Central role for the lens in cavefish eye degeneration. Science. 2000;289:631–633. doi: 10.1126/science.289.5479.631. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Espinasa L, Stock DW, Jeffery WR. Development and evolution of craniofacial patterning is mediated by eye-dependent and –independent processes in the cavefish Astyanax. Evol. Dev. 2003;5:435–446. doi: 10.1046/j.1525-142x.2003.03050.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Stock DW, Jeffery WR. Hedgehog signalling controls eye degeneration in blind cavefish. Nature. 2004;431:844–847. doi: 10.1038/nature02864. [DOI] [PubMed] [Google Scholar]