Abstract
Context
Understanding the relative contributions of genetic and environmental factors to trauma exposure, post-traumatic stress disorder (PTSD), and major depressive disorder (MDD) is critical to developing etiologic models of these conditions and their co-occurrence.
Objectives
To quantify heritable influences on low-risk trauma, high-risk trauma, PTSD, and MDD and to estimate the degree of overlap between genetic and environmental sources of variance in these 4 phenotypes.
Design
Adult twins and their siblings were ascertained from a large population-based sample of female and male twin pairs on the basis of screening items for childhood sexual abuse and physical abuse obtained in a previous assessment of this cohort.
Setting
Structured psychiatric telephone interviews.
Participants
Total sample size of 2591: 996 female and 536 male twins; 625 female and 434 male nontwin siblings.
Main Outcome Measure
Lifetime low- and high-risk trauma exposure, PTSD, and MDD.
Results
In the best-fitting genetic model, 47% of the variance in low-risk trauma exposure and 60% of the variance in high-risk trauma exposure was attributable to additive genetic factors. Heritable influences accounted for 46% of the variance in PTSD and 27% of the variance in MDD. An extremely high degree of genetic overlap was observed between high-risk trauma exposure and both PTSD (r =0.89; 95% CI, 0.78-0.99) and MDD (r =0.89; 95% CI, 0.77-0.98). Complete correlation of genetic factors contributing to PTSD and to MDD (r=1.00) was observed.
Conclusions
The evidence suggests that almost all the heritable influences on high-risk trauma exposure, PTSD, and MDD, can be traced to the same sources; that is, genetic risk is not disorder specific. Individuals with a positive family history of either PTSD or MDD are at elevated risk for both disorders and should be closely monitored after a traumatic experience for symptoms of PTSD and MDD.
Posttraumatic stress disorder (PTSD), unlike other psychiatric disorders, by definition involves exposure to a precipitating event. Most individuals experience 1 or more traumatic events during their lifetime1-3; a sizeable minority develop PTSD. Approximately 12% of women and 6% of men in the US population meet DSM-IV lifetime criteria.1,4,5 Significant risk for major depressive disorder (MDD) following a traumatic event has also been consistently demonstrated.6-9 Not surprisingly, MDD frequently co-occurs with PTSD.10-12 High rates of comorbidity have been found in a variety of populations. In an earlier study13 of young adult women by our group, 71% of PTSD-positive women met MDD criteria. Fu et al14 reported a 7-fold increase in the odds of developing PTSD for male veterans with preexisting MDD. Kessler et al4 found a 4-fold increase in the risk of MDD in individuals with PTSD in a nationally representative sample.
The high rates of co-occurrence of PTSD and MDD may be due in part to shared genetic liability. The contribution of genetic factors to both disorders is well established. Heritability estimates of MDD are typically 35% to 40%.15-17 In the case of PTSD, heritability in males is approximately 30%.18-21 Far fewer studies have examined genetic influences in females, but the evidence suggests that heritability is even higher in women.22 Although the relative contribution of genetic factors to each of these 2 disorders is modest, at least in men, a high degree of overlap exists between heritable factors that influence PTSD and those that influence MDD. In studies based on the Vietnam Era Twin Registry sample, Fu et al14 concluded that the common sources of risk for the 2 disorders were almost entirely genetic. Koenen et al20 reported that 58% of the genetic variance in PTSD could be attributed to heritable influences shared with MDD.
The present study expands on existing genetically informative research on the comorbidity of PTSD and MDD in 2 ways. First, we quantified genetic contributions to trauma exposure and their overlap with genetic contributions to PTSD and MDD. Heritable factors have been reported to have a role in exposure to certain types of traumatic events, such as combat23 and assaultive trauma,9 likely acting through heritable personality traits (eg, antisociality and openness to new experiences24,25) that influence the probability of experiencing traumatic events. There is also some evidence for common genetic sources of risk for trauma exposure and development of PTSD.22 Second, we differentiated low-risk from high-risk trauma empirically based on association with PTSD in the sample. Previous studies have found assaultive trauma to be associated with greater risk for PTSD1,2,5,26,27 and MDD9 compared with non-assaultive trauma, with evidence of genetic influences on trauma exposure limited to assaultive traumatic events.24
The present investigation uses families ascertained from a large population-based sample of twins on the basis of screening items for childhood sexual abuse (CSA) and physical abuse (CPA) obtained in a previous assessment of this cohort. The genetically informative nature of the twin and sibling sample allows us to examine genetic and environmental influences on trauma exposure, MDD, and PTSD in women and men in whom a broad range of traumatic events are represented. In addition, supplementary analyses of data on trauma exposure and MDD from the full population-based twin sample are included to demonstrate that these findings do not arise from ascertainment bias. The goal of this undertaking was to improve our understanding of the factors that contribute to the risk for experiencing traumatic events and the possible psychiatric sequelae of such exposures.
METHODS
The Childhood Trauma Study’s methods have been described in detail elsewhere28; a summary is provided herein. The composition and assessment of the volunteer twin panel from which families were drawn is briefly described, as data for supplemental analyses were derived from this twin panel.
SAMPLE
The Childhood Trauma Study sample includes twins from a large Australian National Health and Medical Research Council volunteer twin panel (cohort II, born between 1964 and 1971), their siblings, and their parents. The twins were initially registered with the panel by their parents between 1980 and 1982 in response to approaches through either school systems or mass media. From 1996 to 2000, a total of 6265 twins (2765 pairs and 735 singletons) completed a semistructured psychiatric diagnostic telephone assessment29 that included questions on childhood maltreatment that were used to ascertain families for the Childhood Trauma Study. The design initially involved interviewing all available twins, full siblings, and parents from 500 high-risk families and 500 control families. The inclusion criteria for high-risk families were endorsement by 1 or both twins of a screening question on childhood sexual abuse (5 questions) or childhood physical abuse (4 questions), permission to contact family members, a surviving parent, and at least 1 additional potentially available sibling (2 were required if only a single twin had participated in the cohort II assessment). The inclusion criteria for control families were identical other than the requirement that no interviewed twin had endorsed either form of abuse. A random-number generator program was used to select high-risk and control families frequency matched on the basis of age, sex, zygosity, and family structure. All high-risk families in which a male twin reported a history of CSA were preferentially recruited because of the lower prevalence of CSA in males.
Screening calls made to twins queried their willingness to consider participating and determined the number of surviving parents and siblings. If families met the inclusion criteria, study personnel requested permission to contact family members and update contact information. A summary of twin recruitment is provided in eTable 1 (http://www.archgenpsychiatry.com). Overall, recruitment was deemed reasonable given that the mean (SD) interval between interviews in cohort II and the present study is 7.2 (1.4) years. Verbal consent was obtained from each participant before starting the interview. Data collection began in 2003 and continued through 2008. Funding limitations necessitated scaling back enrollment; a decision was made to prioritize recruitment of high-risk family twins and reduce the number of targeted control families. Interviews were completed by 3434 respondents from 524 high-risk families and 373 control families. As an additional safeguard, respondents were required to provide written consent after the interview allowing use of the data obtained. Data from respondents who either returned consents requesting that their data not be used for analysis (n=17) or did not return their consents (n=10) were neither analyzed nor reported. Study procedures were approved by the ethics committees of the Queensland Institute of Medical Research and the Washington University School of Medicine. Parental data (n=813) are not included from the present study because of concerns that the advanced age of most parents (mean [SD] age: 66.7 [5.7] years) might be a source of bias (eg, secular trends, recall, or censoring). Respondents for whom data were missing for either MDD (n=12) or trauma exposure (n=23) (but not both [n=3]) were included in the analyses. The present study thus focuses on the 1532 twin (996 female, 536 male) and 1059 sibling (625 female, 434 male) respondents for whom trauma, PTSD, and/or MDD data are available. Respondents were almost all white and of European ancestry, but a wide range of educational backgrounds consistent with socioeconomic class diversity was represented in the sample (see eTable 2). Most respondents were married. The mean (SD) age at interview was 37.2 (2.3) years for twins and 40.6 (6.3) years for siblings. The mean (SD) number of twin and sibling participants per family was 2.9 (1.2). The breakdown of twins by zygosity and sex was as follows: 400 monozygotic (MZ) females (including 176 complete pairs), 177 MZ males (76 pairs), 367 dizygotic (DZ) females (157 pairs), 165 DZ males (66 pairs), and 423 DZ opposite-sex twins (169 pairs). Twins include 899 from high-risk families and 633 from control families.
ASSESSMENT
The Childhood Trauma Study’s computer-assisted diagnostic interview was completed via telephone. Interviewers completed an extensive training process supervised by an experienced clinical psychologist. Data reported herein on lifetime MDD were obtained using a modified section of the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA), for which reliability30 and validity31 are well established. The assessment of lifetime DSM-IV PTSD was modified from the National Comorbidity Survey4 interview, which itself was derived from the Revised Diagnostic Interview Schedule.32 The National Comorbidity Survey assessment, for which excellent psychometric properties have been reported,4 first asks respondents whether they had ever experienced a series of traumatic events (Table 1). To protect confidentiality, respondents looked at a numbered list of brief event descriptions contained in a booklet of materials mailed in advance of the interview. Each event was then queried by number (eg, “Did event number 1 ever happen to you?”). Respondents were then asked which event was most disturbing, and the assessment of lifetime PTSD focused on the identified event. Additional demographic information and DSM-IV diagnostic data were also obtained using interview sections modified from the SSAGA. To decrease respondent burden, sections of the interview (including the depression section) that were unchanged from a recent assessment were not readministered to twins or siblings interviewed in partially overlapping genetic studies (2001-2005) that focused on nicotine and alcohol.33,34 For the present study, the diagnosis of MDD was obtained from the Childhood Trauma Study for 2042 individuals; the previous assessment provided this diagnosis for the remaining 537 participants. The assessment used to obtain cohort II data, reported herein as supplementary results, included a similar SSAGA-based MDD section and the National Comorbidity Survey–derived traumatic event list without the items assessing the diagnostic criteria for PTSD.
Table 1.
Prevalence of Traumatic Events
| Event | Women, % (n = 1604) | Men, % (n = 964) |
|---|---|---|
| High risk | ||
| Rape | 10.9 | 2.8 |
| Sexual molestation | 24.2 | 11.0 |
| Physical attack or assault | 8.7 | 17.0 |
| Childhood physical abuse | 7.0 | 7.6 |
| Serious childhood neglect | 4.2 | 3.1 |
| Any high-risk traumatic event | 35.7 | 29.5 |
| Low risk | ||
| Combat exposure | 0.4 | 2.8 |
| Life-threatening accident | 18.8 | 28.9 |
| Fire, flood, or natural disaster | 11.9 | 28.1 |
| Witnessed injury or killing | 19.5 | 44.1 |
| Threatened with weapon/held captive | 8.5 | 20.0 |
| Any low-risk traumatic event | 40.9 | 66.7 |
OUTCOME VARIABLES
The binary lifetime DSM-IV diagnosis of MDD was derived from the respondent’s most recent assessment. The binary lifetime PTSD diagnosis was obtained from the Childhood Trauma Study interview. Individuals who did not report any lifetime trauma exposure were coded as missing for PTSD since the diagnosis of PTSD is contingent on prior trauma exposure. (See the article by Heath et al35 for the applicability of this approach to structural equation modeling with contingent phenotypes.) Because the nature of traumatic events experienced (eg, seasonal flooding vs a devastating tsunami), and how some events are perceived, can vary considerably between populations, traumatic events were classified empirically for the present study as either low or high risk based on the relative risk of PTSD associated with each specific event when nominated by respondents as most disturbing. Respondents were not categorized exclusively into low- vs high-risk trauma exposure groups; a given respondent could endorse both event types. The availability of these measures (other than the diagnosis of PTSD) in cohort II data enabled the same classification of low- and high-risk traumatic events to be applied in supplemental analyses examining the relationships of these variables and a diagnosis of MDD in a more general population–representative sample.
STATISTICAL METHODS
Descriptive and regression analyses were performed using a commercially available software program (SAS System for Windows, version 9.2). For regression analyses, robust variance estimators were used to adjust 95% CIs for the presence of multiple members of individual families. Preliminary insight into the most likely quantitative genetic model was obtained by examining the twin-pair and sib-pair correlations calculated separately for MZ twins, DZ twins, twin-sib pairs, and sib-sib pairs. Based on the tetrachoric correlations (eTable 3), a model with additive genetic (A), shared environmental (C), and nonshared environmental (E) influences was selected for the present analyses. All quantitative genetic analyses were conducted using a statistical package Mx.36 In the present analyses, we included up to 3 full siblings in addition to the twins (4 full siblings when data were available from only 1 twin). In families in which the number of available nontwin siblings exceeded these values, siblings closest in age to the twins were preferentially included.
The primary model tested was a quadrivariate Cholesky that assessed the nature and magnitude of influences on MDD, low-risk trauma exposure, high-risk trauma exposure, and PTSD. The Cholesky decomposition (see Neale and Cardon37) partitions the variance in the second variable, low-risk trauma exposure, into portions overlapping with the first variable, MDD (in eFigure 1, additive genetic and nonshared environmental paths to low-risk trauma from A1 and E1, respectively), and those specific to low-risk trauma exposure (in eFigure 1, additive genetic and nonshared environmental paths to low-risk trauma from A2 and E2, respectively). The third variable, high-risk trauma exposure, is then divided into portions overlapping with variable 1, those overlapping with variable 2, those specific to variable 3, etc. Given the well-established genetic contribution to MDD liability15-17 and reports of subsequently occurring trauma exposure associated with affective disorders,38-40 we included MDD as the first term in the model to enable its sharing of additive genetic variance with trauma exposure and PTSD to be easily calculated. The sample’s complex family structure (ie, containing variable numbers of same-sex and opposite-sex twins and siblings), coupled with the presence of fewer comparisons informative for calculating parameter estimates for PTSD (due to coding individuals not exposed to trauma as missing), limited the ability to calculate separate parameter estimates for women and men. We instead controlled for possible sex differences by including sex as a covariate in all the models. Models were fitted to raw data, allowing for maximal use of data and providing −2 times the log-likelihood as an index of fit. After the saturated model was tested, paths with very small loadings (<0.10) were removed. The difference between the log-likelihood of the saturated and reduced models can be interpreted as a χ2 with the degrees of freedom equal to the difference in the number of parameters estimated. Paths that were not significant were dropped from the model. The significance of paths with larger loadings was confirmed with calculation of CIs. A saturated trivariate Cholesky (ie, MDD, low-risk trauma, and high-risk trauma) was created for supplementary analyses of cohort II twin data (see eTables 5 and 6 and eFigure 2), and pathways were then dropped as described previously herein to determine the most parsimonious model.
RESULTS
The prevalence of self-reported exposure to traumatic events is given in Table 1. Some enrichment for childhood trauma (see eTable 4 for comparison), consistent with the sample’s ascertainment based on twins’ report of CSA or CPA, is evident, as is enrichment for psychiatric disorders (see eTable 2). The risk of PTSD associated with nomination of each specific event as the most disturbing (Table 2) exhibits a fairly consistent pattern in which higher risk is observed for assaultive and severe childhood trauma. One surprising exception is the relatively modest PTSD risk observed for combat exposure, the least prevalent event for both men and women.
Table 2.
PTSD Risk Associated With Events Nominated as Most Disturbing (n = 1643)a
| Event | Odds Ratio (95% CI) |
|---|---|
| High risk | |
| Rape | 6.74 (4.01-11.32) |
| Sexual molestation | 3.58 (2.28-5.63) |
| Physical attack or assault | 3.13 (1.78-5.51) |
| Childhood physical abuse | 3.48 (1.80-6.73) |
| Serious childhood neglect | 6.57 (2.76-15.63) |
| Low risk | |
| Combat exposure | 2.35 (0.49-11.18) |
| Life-threatening accident | 1.23 (0.69-2.18) |
| Fire, flood, or natural disaster | 0.26 (0.10-0.67) |
| Witnessed injury or killing | 1 [Reference]b |
| Threatened with weapon/held captive | 1.42 (0.74-2.71) |
| Overall | |
| Any high-risk event | 4.36 (3.25-5.85) |
| Any low-risk event | 1 [Reference] |
Abbreviation: PTSD, posttraumatic stress disorder.
Adjusted for sex.
Reference for all nominated events.
The prevalence of MDD in individuals exposed to low-risk traumatic events was significantly greater than that in individuals who reported no trauma exposure (36.8% vs 24.4%, , P < .001). The MDD prevalence in individuals exposed to high-risk trauma was more than double that in those without trauma exposure, with nearly half meeting the criteria for the disorder (49.4% vs 24.3%, , P < .001). The comorbidity of PTSD and MDD was also high: 67.9% of individuals who met criteria for PTSD also met MDD criteria compared with only 32.6% of those who had been exposed to trauma (either low or high risk) but did not develop PTSD ( , P < .001).
Within-individual cross-trait tetrachoric correlations for the 4 phenotypes are reported in Table 3. All correlations involving low-risk trauma were of modest magnitude; the somewhat counterintuitive negative correlation (r=−0.19) observed with PTSD can be attributed to classifying non–trauma-exposed individuals as missing for the disorder. In contrast, the correlations between high-risk trauma and PTSD and between high-risk trauma and MDD were 0.65 and 0.38, respectively.
Table 3.
Within-Individual Cross-Trait Phenotypic Tetrachoric Correlations (r) Among Low-Risk Trauma, High-Risk Trauma, PTSD, and MDD (n = 2554)
| Phenotype | Low-Risk Trauma | High-Risk Trauma | PTSD | MDD |
|---|---|---|---|---|
| High-risk trauma | 0.23a | |||
| PTSD | −0.19a | 0.65a | ||
| MDD | 0.11a | 0.38a | 0.48a |
Abbreviations: MDD, major depressive disorder; PTSD, posttraumatic stress disorder.
P < .001.
A full quadrivariate ACE Cholesky triangular decomposition was fitted to twin and sibling data. A full AE Cholesky (eFigure 1) was also fitted with no significant change observed in overall fit ( , P > .99). Additional models were fitted with a single pathway dropped in each instance and change in model fit determined to be nonsignificant. The best-fitting quadrivariate Cholesky was calculated in this manner. A nonsignificant change in fit was observed when this model was compared with the full AE Cholesky ( , P = .17). The final model is shown with standardized path coefficients in the Figure (95% CIs available on request) and is summarized with standardized variance components in Table 4.
Figure.
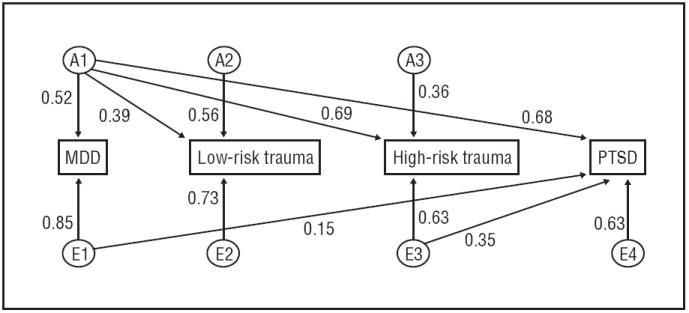
Final quadrivariate model of liability to major depressive disorder (MDD), trauma, and posttraumatic stress disorder (PTSD). Standardized parameter estimates are shown. A1 to A3 denote genetic factors; E1 to E4, nonshared environmental factors.
Table 4.
Magnitude (r) (95% CI) of Additive Genetic and Nonshared Environmental Influences on Low-Risk Trauma, High-Risk Trauma, PTSD, and MDD (n = 2545)
| Phenotype | Additive Genetic Influences | Nonshared Environmental Influences |
|---|---|---|
| Low-risk trauma | 0.47 (0.35-0.57) | 0.53 (0.43-0.65) |
| High-risk trauma | 0.60 (0.49-0.71) | 0.40 (0.29-0.51) |
| PTSD | 0.46 (0.31-0.62) | 0.54 (0.38-0.69) |
| MDD | 0.27 (0.21-0.37) | 0.73 (0.63-0.79) |
Abbreviations: MDD, major depressive disorder; PTSD, posttraumatic stress disorder.
In the final model, 47% of the variance in low-risk trauma exposure and 60% of the variance in high-risk trauma exposure was attributable to additive genetic factors. Heritable influences accounted for 46% of the variance in PTSD and 27% of the variance in MDD. Nonshared environmental influences accounted for the remaining variance in all 4 phenotypes. There was no evidence of significant shared environmental contributions to PTSD, MDD, or either high-or low-risk trauma exposure. Additive genetic and non-shared environmental correlations among the 4 phenotypes are reported in Table 5. Moderate to high genetic correlations were observed between low-risk trauma exposure and high-risk trauma exposure (r =0.50), PTSD (r=0.57), and MDD (r=0.57). An extremely high degree of genetic overlap was observed with high-risk trauma exposure and both MDD (r =0.89) and PTSD (r =0.89). Complete correlation of genetic factors contributing to MDD and to PTSD (r =1.0) was observed.
Table 5.
Correlations (r) Between Additive Genetic and Nonshared Environmental Influences on Low- and High-Risk Trauma, PTSD, and MDDa (n = 2545)
| Phenotype | High-Risk Trauma | PTSD | MDD |
|---|---|---|---|
| Low-risk trauma | 0.50 (0.39-0.62) | 0.57 (0.46-0.67) | 0.57 (0.46-0.69) |
| High-risk trauma | 0.89 (0.78-0.99) | 0.89 (0.77-0.98) | |
| PTSD | 0.48 (0.30-0.65) | 1.00 | |
| MDD | 0.20 (0.06-0.32) |
Abbreviations: MDD, major depressive disorder; PTSD, posttraumatic stress disorder.
Genetic correlations are shown in the unshaded area, and nonshared environmental correlations are shown in the darker shaded area.
COMMENT
The present study furthers our understanding of the links among trauma, MDD, and PTSD. These findings provide additional evidence of the substantial contribution of genetic factors to liability for MDD and PTSD and the overlap in heritable influences on the 2 disorders; in this study, all the genetic influences on PTSD are attributable to heritable factors shared with MDD. We extended this line of investigation by incorporating trauma phenotypes into genetic models of PTSD and MDD and differentiating low- from high-risk trauma, which allowed us to uncover distinctions by trauma severity in genetic overlap with PTSD and MDD. The heritability estimate for high-risk trauma is only slightly higher than that for low-risk trauma; however, these results indicate that inherited vulnerabilities to MDD and PTSD are shared to a much greater extent with heritable influences on high-than low-risk trauma exposures.
The estimated heritability of MDD in the present sample is 27%, somewhat below the 35% to 40% reported in the literature.15-17 The present heritability estimate of 46% for PTSD falls between the 30% estimate reported for the Vietnam Era Twin Registry sample (the all-male sample examined in most twin studies of PTSD) and the estimate of 71% reported for an all-female sample in earlier work by our group.22 The high degree of genetic overlap between MDD and PTSD observed in the present study is also consistent with previous studies based on the Vietnam Era Twin Registry sample.14,20
The finding that a significant proportion of the variance in high- and low-risk trauma can be traced to heritable sources is somewhat consistent with the study by Stein et al.24 They used a sample of 406 twin pairs to estimate the relative contributions of genetic factors to different trauma types, assaultive vs nonassaultive traumas, which, except for combat exposure, closely map onto the present high- and low-risk trauma categories. The present heritability estimates are considerably larger than the comparable values from the study by Stein et al24: high risk (60%) vs assaultive (20%) and low risk (47%) vs nonassaultive (0%). Substantial methodological differences may have contributed to the discrepancy in heritability estimates. Their sample was composed solely of twin pairs and opposite-sex pairs were excluded from their calculations of heritability estimates for trauma exposure. The intrapair correlations they reported, which include all pairs, suggest that higher heritability estimates would have been obtained if opposite-sex pairs were retained (approximated by twice the difference in MZ-DZ correlation as 30% for assaultive and 28% for nonassaultive trauma). The inclusion of data from both twins and siblings with control for sex could have contributed to the higher estimates in the present study (see the “Limitations” subsection). Their analyses used quantitative factor scores derived from ordinal scores for each trauma type, ranging from 0 (never occurred) to 3 (occurred at each age range assessed). The present analyses used separate binary composite measures for any lifetime occurrence of high- and low-risk trauma exposure that were derived from binary scores representing the presence or absence of each assessed trauma type. Although the present sample is several-fold larger, sample size is a limitation shared by both studies.
Differences could also be attributable to bias secondary to ascertainment of the sample on the basis of twins’ self-report of CSA or CPA. To address this possibility, we examined whether similar relationships between MDD and low- and high-risk trauma are found in the full community-ascertained cohort II sample (PTSD was not assessed) (see eTables 5 and 6 and eFigure 2). We found a somewhat higher estimated heritability of MDD (37%) and a slightly lower value for low-risk trauma (40%). The value for high-risk trauma was nearly identical (61%) to the value estimated in the main sample. A slightly lower genetic correlation for MDD and high-risk trauma was observed (0.72), whereas the genetic correlation for the 2 trauma types (0.56) was similar to that found in the main sample (0.52). Overall, the results of these supplementary analyses suggest that ascertainment bias had very limited effect on these findings. Thus, these findings provide evidence that low-risk trauma exposure is moderately heritable and that there is considerable overlap in genetic factors that influence high- and low-risk trauma exposure.
Although influenced by some of the same heritable factors, low-risk trauma exposures differed substantially from high-risk trauma exposures in the degree of genetic overlap with MDD and PTSD. The genetic correlation of low-risk trauma with MDD and PTSD was 0.50 compared with 0.89 for high-risk trauma. Together with the finding that all the genetic variance in PTSD was attributable to heritable factors common to PTSD and MDD, the evidence suggests that nearly all the genetic influences on PTSD, MDD, and high-risk trauma exposure can be traced to the same sources. Identification of these sources is beyond the scope of the present study, but the limited research in this area suggests that heritable traits associated with depression and PTSD, such as neuroticism3,41,42 and propensity to externalizing behaviors,14,43,44 are likely possibilities.
The results of the present study have important implications for the prevention of trauma-related psychopathology. These findings indicate that PTSD and MDD are different manifestations of common inherited vulnerabilities. Because genetic risk is not disorder specific, a family history of either disorder puts individuals at elevated risk for PTSD, MDD, or both. Those with a positive family history should be closely monitored following trauma exposure for signs of either disorder. It is important to remember, however, that although more susceptible to trauma-related psychopathology, those with high genetic liability are by no means destined to develop either PTSD or MDD, both of which are influenced to a greater extent by environment than by genes.
LIMITATIONS
Certain limitations should be kept in mind when interpreting findings from the present study. First, although the categorization of low- vs high-risk traumas closely parallels the assaultive vs nonassaultive distinctions used in the larger trauma literature, the finding that combat exposure did not confer high risk for PTSD is inconsistent with studies conducted with US samples. This inconsistency is likely due to differences in military experiences between these US samples and that of the present study (whose age precluded participation in Vietnam and for whom assessment largely preceded recent conflicts [ie, Afghanistan and Iraq]). It does make an important point that research using broad categories of traumatic events must be interpreted with consideration of the distribution of exposure severity represented and, perhaps, the population-specific perception of individual events. It is possible that the sample-specific empirical categorization of trauma risk may have contributed to the magnitude of the overall association observed between high-risk trauma and PTSD. Second, the design included oversampling of families in which twins reported childhood maltreatment. Thus, to the extent that the relationship between trauma and MDD in this high-risk group differs from that in the general population, generalizability of these findings may be limited. However, results of the analyses we conducted with the community-based full cohort II sample suggest that any ascertainment bias likely had a very limited impact. Third, the ordering of variables had some effect on the heritability estimates obtained from the final reduced model. Estimates of heritability robust to the order of the first 3 variables, derived from the saturated AE model (shown in eFigure 1), are 36% for MDD, 46% for low-risk trauma, and 58% for high-risk trauma. Thus, the heritability for MDD calculated from the reduced model may have been slightly reduced. Fourth, given the aim of characterizing heritability and overlapping genetic influences on trauma exposure, MDD, and PTSD, twins make up a large proportion of the sample. Although they compose a small minority of the general population and they differ from singletons with respect to certain health outcomes in childhood, these differences are not significant beyond age 5 years45,46 and are, therefore, unlikely to influence the outcomes of interest in this study. Fifth, although the DZ twin, sib-twin, and sib-sib correlations are similar, the models include an assumption of their overall equality. A reduction in resemblance for these phenotypes in nontwin siblings, vs that of DZ twin pairs, would reduce the estimate for comparison with MZ twin pairs and, consequently, inflate estimates of genetic effects. Sixth, given that PTSD and MDD have partially overlapping symptoms, diagnostic imprecision could have contributed to the estimates of shared vulnerability that we obtained. Seventh, the approach of coding individuals with no history of trauma exposure as missing for PTSD, consistent with DSM-IV Criterion A, clearly affected the findings. The alternative, coding them as not meeting criteria for the disorder, would have added solely to those cells containing individuals without PTSD and each type of trauma exposure, thus increasing the magnitude of correlations between each trauma type and PTSD and the respective estimates of their total shared variance. The present approach reduced the number of comparisons informative for calculating parameter estimates for PTSD and, thus, limited our ability to examine alternative models (eg, obtaining separate estimates for women and men). Eighth, given the sample’s complex family structure and sample size constraints, we used models that controlled for the main effect of sex. By doing so, we may have failed to detect underlying genotype × sex interactions.
FUTURE DIRECTIONS
There are a variety of possible directions for further investigation of common mechanisms underlying susceptibility to trauma exposure, MDD, and PTSD. First, given the relative lack of research on shared heritable influences on these phenotypes, replication in other community-based samples is necessary. Additional research is also needed to identify the heritable traits or behaviors that contribute to the heritability of trauma exposure. Given that personality traits, such as neuroticism and antisociality, have been reported16,25 to influence the likelihood of selecting environments in which risk of stressful events is higher, this line of research should include testing for mediating of genetic effects by these and related traits (eg, openness to experiences). In addition, the possibility that similar relationships to those observed herein exist among other disorders known to co-occur with PTSD and MDD and to begin or worsen following traumatic events (eg, substance use disorders) merits exploration. It is critical as well that we work toward identifying the specific genes and gene × environment interactions that contribute to the manifestation of PTSD and MDD following trauma exposure. These findings strongly suggest the importance of incorporating examination of genetic risks contributing to trauma exposure into such investigations.
Acknowledgments
Funding/Support: This work was primarily supported by grants AA013446 (Dr Nelson), AA011998_5978 (Dr Nelson), AA017921 (Dr Sartor), AA12640 (Dr Bucholz), AA10249 (Dr Heath), AA011998 (Dr Heath), and AA018146 (Dr McCutcheon) from the National Institute on Alcohol Abuse and Alcoholism and by grants DA14363 (Dr Bucholz), DA023696 (Dr Waldron), and DA012854 (Dr Madden) from the National Institute on Drug Abuse.
Role of the Sponsor: The funding sources had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr Nelson had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Online-Only Material: The eTables and eFigures are available at http://www.archgenpsychiatry.com.
Financial Disclosure: None reported.
References
- 1.Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J Consult Clin Psychol. 1993;61(6):984–991. doi: 10.1037//0022-006x.61.6.984. [DOI] [PubMed] [Google Scholar]
- 2.Creamer M, Burgess P, McFarlane AC. Post-traumatic stress disorder: findings from the Australian National Survey of Mental Health and Well-being. Psychol Med. 2001;31(7):1237–1247. doi: 10.1017/s0033291701004287. [DOI] [PubMed] [Google Scholar]
- 3.Breslau N. The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma Violence Abuse. 2009;10(3):198–210. doi: 10.1177/1524838009334448. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 5.Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998;55(7):626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- 6.Fergusson DM, Horwood LJ, Lynskey MT. Childhood sexual abuse and psychiatric disorder in young adulthood, II: psychiatric outcomes of childhood sexual abuse. J Am Acad Child Adolesc Psychiatry. 1996;35(10):1365–1374. doi: 10.1097/00004583-199610000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Nelson EC, Heath AC, Madden PAF, Cooper ML, Dinwiddie SH, Bucholz KK, Glowinski A, McLaughlin T, Dunne MP, Statham DJ, Martin NG. Association between self-reported childhood sexual abuse and adverse psychosocial outcomes: results from a twin study. Arch Gen Psychiatry. 2002;59(2):139–145. doi: 10.1001/archpsyc.59.2.139. [DOI] [PubMed] [Google Scholar]
- 8.Hedtke KA, Ruggiero KJ, Fitzgerald MM, Zinzow HM, Saunders BE, Resnick HS, Kilpatrick DG. A longitudinal investigation of interpersonal violence in relation to mental health and substance use. J Consult Clin Psychol. 2008;76(4):633–647. doi: 10.1037/0022-006X.76.4.633. [DOI] [PubMed] [Google Scholar]
- 9.McCutcheon VV, Heath AC, Nelson EC, Bucholz KK, Madden PAF, Martin NG. Accumulation of trauma over time and risk for depression in a twin sample. Psychol Med. 2009;39(3):431–441. doi: 10.1017/S0033291708003759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orsillo SM, Weathers FW, Litz BT, Steinberg HR, Huska JA, Keane TM. Current and lifetime psychiatric disorders among veterans with war zone-related posttraumatic stress disorder. J Nerv Ment Dis. 1996;184(5):307–313. doi: 10.1097/00005053-199605000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Breslau N, Davis GC, Peterson EL, Schultz LR. A second look at comorbidity in victims of trauma: the posttraumatic stress disorder-major depression connection. Biol Psychiatry. 2000;48(9):902–909. doi: 10.1016/s0006-3223(00)00933-1. [DOI] [PubMed] [Google Scholar]
- 12.Maercker A, Michael T, Fehm L, Becker ES, Margraf J. Age of traumatisation as a predictor of post-traumatic stress disorder or major depression in young women. Br J Psychiatry. 2004;184:482–487. doi: 10.1192/bjp.184.6.482. [DOI] [PubMed] [Google Scholar]
- 13.Sartor CE, McCutcheon VV, Pommer NE, Nelson EC, Duncan AE, Waldron M, Bucholz KK, Madden PAF, Heath AC. Posttraumatic stress disorder and alcohol dependence in young women. J Stud Alcohol Drugs. 2010;71(6):810–818. doi: 10.15288/jsad.2010.71.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu Q, Koenen KC, Miller MW, Heath AC, Bucholz KK, Lyons MJ, Eisen SA, True WR, Goldberg J, Tsuang MT. Differential etiology of posttraumatic stress disorder with conduct disorder and major depression in male veterans. Biol Psychiatry. 2007;62(10):1088–1094. doi: 10.1016/j.biopsych.2007.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, Meyer JM, Toomey R, Faraone SV, Merla-Ramos M, Tsuang MT. A registry-based twin study of depression in men. Arch Gen Psychiatry. 1998;55(5):468–472. doi: 10.1001/archpsyc.55.5.468. [DOI] [PubMed] [Google Scholar]
- 16.Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for major depression in women. Am J Psychiatry. 2002;159(7):1133–1145. doi: 10.1176/appi.ajp.159.7.1133. [DOI] [PubMed] [Google Scholar]
- 17.Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for major depression in men. Am J Psychiatry. 2006;163(1):115–124. doi: 10.1176/appi.ajp.163.1.115. [DOI] [PubMed] [Google Scholar]
- 18.True WR, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, Nowak J. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50(4):257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- 19.Xian H, Chantarujikapong SI, Scherrer JF, Eisen SA, Lyons MJ, Goldberg J, Tsuang M, True WR. Genetic and environmental influences on posttraumatic stress disorder, alcohol and drug dependence in twin pairs. Drug Alcohol Depend. 2000;61(1):95–102. doi: 10.1016/s0376-8716(00)00127-7. [DOI] [PubMed] [Google Scholar]
- 20.Koenen KC, Fu QJ, Ertel K, Lyons MJ, Eisen SA, True WR, Goldberg J, Tsuang MT. Common genetic liability to major depression and posttraumatic stress disorder in men. J Affect Disord. 2008;105(1-3):109–115. doi: 10.1016/j.jad.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tambs K, Czajkowsky N, Røysamb E, Neale MC, Reichborn-Kjennerud T, Aggen SH, Harris JR, Ørstavik RE, Kendler KS. Structure of genetic and environmental risk factors for dimensional representations of DSM-IV anxiety disorders. Br J Psychiatry. 2009;195(4):301–307. doi: 10.1192/bjp.bp.108.059485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sartor CE, McCutcheon VV, Pommer NE, Nelson EC, Grant JD, Duncan AE, Waldron M, Bucholz KK, Madden PAF, Heath AC. Common genetic and environmental contributions to post-traumatic stress disorder and alcohol dependence in young women. Psychol Med. 2011;41(7):1497–1505. doi: 10.1017/S0033291710002072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyons MJ, Goldberg J, Eisen SA, True W, Tsuang MT, Meyer JM, Henderson WG. Do genes influence exposure to trauma? a twin study of combat. Am J Med Genet. 1993;48(1):22–27. doi: 10.1002/ajmg.1320480107. [DOI] [PubMed] [Google Scholar]
- 24.Stein MB, Jang KL, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am J Psychiatry. 2002;159(10):1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- 25.Jang KL, Stein MB, Taylor S, Asmundson GJ, Livesley WJ. Exposure to traumatic events and experiences: aetiological relationships with personality function. Psychiatry Res. 2003;120(1):61–69. doi: 10.1016/s0165-1781(03)00172-0. [DOI] [PubMed] [Google Scholar]
- 26.Hapke U, Schumann A, Rumpf HJ, John U, Meyer C. Post-traumatic stress disorder: the role of trauma, pre-existing psychiatric disorders, and gender. Eur Arch Psychiatry Clin Neurosci. 2006;256(5):299–306. doi: 10.1007/s00406-006-0654-6. [DOI] [PubMed] [Google Scholar]
- 27.Gill JM, Page GG, Sharps P, Campbell JC. Experiences of traumatic events and associations with PTSD and depression development in urban health care-seeking women. J Urban Health. 2008;85(5):693–706. doi: 10.1007/s11524-008-9290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson EC, Lynskey MT, Heath AC, Madden PAF, Martin NG. A family study of adult twins with and without a history of childhood abuse: stability of retrospective reports of maltreatment and associated family measures. Twin Res Hum Genet. 2010;13(2):121–130. doi: 10.1375/twin.13.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heath AC, Howells W, Kirk KM, Madden PAF, Bucholz KK, Nelson EC, Slutske WS, Statham DJ, Martin NG. Predictors of non-response to a questionnaire survey of a volunteer twin panel: findings from the Australian 1989 twin cohort. Twin Res. 2001;4(2):73–80. doi: 10.1375/1369052012182. [DOI] [PubMed] [Google Scholar]
- 30.Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55(2):149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- 31.Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA: a comparison with the SCAN. Addiction. 1999;94(9):1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- 32.Breslau N, Davis GC, Andreski P, Peterson E. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch Gen Psychiatry. 1991;48(3):216–222. doi: 10.1001/archpsyc.1991.01810270028003. [DOI] [PubMed] [Google Scholar]
- 33.Saccone SF, Pergadia ML, Loukola A, Broms U, Montgomery GW, Wang JC, Agrawal A, Dick DM, Heath AC, Todorov AA, Maunu H, Heikkila K, Morley KI, Rice JP, Todd RD, Kaprio J, Peltonen L, Martin NG, Goate AM, Madden PAF. Genetic linkage to chromosome 22q12 for a heavy-smoking quantitative trait in two independent samples. Am J Hum Genet. 2007;80(5):856–866. doi: 10.1086/513703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agrawal A, Pergadia ML, Saccone SF, Lynskey MT, Wang JC, Martin NG, Statham D, Henders A, Campbell M, Garcia R, Broms U, Todd RD, Goate AM, Rice J, Kaprio J, Heath AC, Montgomery GW, Madden PAF. An autosomal linkage scan for cannabis use disorders in the nicotine addiction genetics project. Arch Gen Psychiatry. 2008;65(6):713–721. doi: 10.1001/archpsyc.65.6.713. [DOI] [PubMed] [Google Scholar]
- 35.Heath AC, Martin NG, Lynskey MT, Todorov AA, Madden PAF. Estimating two-stage models for genetic influences on alcohol, tobacco or drug use initiation and dependence vulnerability in twin and family data. Twin Res. 2002;5(2):113–124. doi: 10.1375/1369052022983. [DOI] [PubMed] [Google Scholar]
- 36.Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6. Richmond: Department of Psychiatry, Virginia Commonwealth University; 2003. [Google Scholar]
- 37.Neale MC, Cardon LR. Methodology for Genetic Studies for Twins and Families. Dordrecht, the Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- 38.Burnam MA, Stein JA, Golding JM, Siegel JM, Sorenson SB, Forsythe AB, Telles CA. Sexual assault and mental disorders in a community population. J Consult Clin Psychol. 1988;56(6):843–850. doi: 10.1037//0022-006x.56.6.843. [DOI] [PubMed] [Google Scholar]
- 39.Breslau N, Davis GC, Andreski P, Peterson EL, Schultz LR. Sex differences in posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54(11):1044–1048. doi: 10.1001/archpsyc.1997.01830230082012. [DOI] [PubMed] [Google Scholar]
- 40.Kendler KS. Anna-Monika-Prize paper: major depression and the environment: a psychiatric genetic perspective. Pharmacopsychiatry. 1998;31(1):5–9. doi: 10.1055/s-2007-979287. [DOI] [PubMed] [Google Scholar]
- 41.Cox BJ, MacPherson PS, Enns MW, McWilliams LA. Neuroticism and self-criticism associated with posttraumatic stress disorder in a nationally representative sample. Behav Res Ther. 2004;42(1):105–114. doi: 10.1016/s0005-7967(03)00105-0. [DOI] [PubMed] [Google Scholar]
- 42.Parslow RA, Jorm AF, Christensen H. Associations of pre-trauma attributes and trauma exposure with screening positive for PTSD: analysis of a community-based study of 2,085 young adults. Psychol Med. 2006;36(3):387–395. doi: 10.1017/S0033291705006306. [DOI] [PubMed] [Google Scholar]
- 43.Koenen KC, Fu QJ, Lyons MJ, Toomey R, Goldberg J, Eisen SA, True W, Tsuang M. Juvenile conduct disorder as a risk factor for trauma exposure and posttraumatic stress disorder. J Trauma Stress. 2005;18(1):23–32. doi: 10.1002/jts.20010. [DOI] [PubMed] [Google Scholar]
- 44.Wolf EJ, Miller MW, Krueger RF, Lyons MJ, Tsuang MT, Koenen KC. Posttraumatic stress disorder and the genetic structure of comorbidity. J Abnorm Psychol. 2010;119(2):320–330. doi: 10.1037/a0019035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christensen K, Vaupel JW, Holm NV, Yashin AI. Mortality among twins after age 6: fetal origins hypothesis versus twin method. BMJ. 1995;310(6977):432–436. doi: 10.1136/bmj.310.6977.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van den Oord EJ, Koot HM, Boomsma DI, Verhulst FC, Orlebeke JF. A twin-singleton comparison of problem behaviour in 2-3-year-olds. J Child Psychol Psychiatry. 1995;36(3):449–458. doi: 10.1111/j.1469-7610.1995.tb01302.x. [DOI] [PubMed] [Google Scholar]


