Abstract
Objective
To design a novel mobility training intervention incorporating infant motor learning and neurorehabilitation principles and investigate its feasibility, tolerability and effect on motor development in toddlers with cerebral palsy (CP).
Methods
A single-subject research design with repeated measures during 6-week baseline and intervention phases and after treatment withdrawal was used. Five participants attended therapy utilizing novel dynamic weight assistance technology, which allowed practice of motor skills beyond participants’ current abilities.
Results
Average attendance and engagement rates exceeded 90%. Gains in gross motor function were observed after treatment that exceeded the expected rate in four of the five participants. Rates of motor development during treatment were 10.8, 3.8, 7.0, 15.1, and 0.3 times greater than during baseline for the five participants, respectively.
Conclusions
This intervention was tolerated and demonstrated the potential to alter the trajectory of motor development in CP, providing proof of concept for further investigation.
Keywords: Cerebral palsy, rehabilitation, motor development, gross motor function, gait, infants, toddlers, weight support technology, mobility training
Introduction
Cerebral palsy (CP) is the most common cause of motor disability in children [1]. Participation restrictions experienced by individuals with CP are directly related to the degree of their motor impairment, including reduced involvement in community activities [2] and romantic relationships [3] and reduced likelihood of independent living [4] with increasing motor disability. Significantly reducing the degree of motor impairment in individuals with CP will impact their societal participation and inclusion.
The best opportunity to maximize motor ability throughout the lifespan may be early in development. Children with CP reach 90% of their motor potential by 4.8 years, 4.4 years, 3.7 years, 3.5 years and 2.7 years, for Gross Motor Function Classification System (GMFCS) Levels I–V [5], respectively, and maladaptive changes after central nervous injury are difficult to reverse once established [6]. In fact, an individual’s GMFCS classification remains relatively stable over the lifespan [7,8], regardless of treatment (whether therapeutic, medical, or surgical). However, the early years of life are an exception, with less stability in GMFCS classifications before the age of 2 years [9].
A broad look at motor development curves in CP [5] further underscores the fact that relatively small changes in motor function at a young age may alter the trajectory of motor development and influence the degree of motor impairment later in life. These observations suggest a window of opportunity to reduce motor disability during the toddler years, when there is variability in individual skill attainment and when the gaps in motor skill between functional levels are small. How best to optimize interventions during this window when the greatest impact may be possible remains unanswered.
Heathcock and colleagues [10, 11] demonstrated that brief amounts of motor training delivered prior to skill development can enhance reaching and kicking skills in pre-term infants at risk for neuromotor impairment. However, motor outcomes from existing rehabilitation strategies to improve walking in children with CP are largely discouraging and inconsistent. The efficacy of various regimented locomotor training programmes has been limited in young children at risk for neuromotor delay [12] as well as in older children with CP [13]. One reason for these findings may be that few of the participants from the studies included in those reviews received the locomotor training when their walking skills were newly emerging.
Another probable factor is that regimented gait training programmes, inherent to their objective of providing high repetition of normal stepping patterns, do not reflect typical infant motor learning. These regimented training approaches may be useful in retraining stepping with someone that has already learned but lost the ability to walk, but they do not allow the motor variability and error that characterize typical toddler movements when first learning to walk.
Typical toddler movement is characterized by a high degree of self-initiated motor exploration and movement variability, both critical factors in motor development [14]. Infants and toddlers with CP often have difficulty or are unable to create these types of experiences on their own and are therefore unable to continuously practice and refine their motor control like their typically-developing peers. Instead, young children with CP develop and repeatedly practice poorly controlled and less adaptable motor patterns. This repeated practice of abnormal movements during development leads to lost opportunities to learn more co-ordinated movements and to establish the associated neural pathways for skilled motor control. The results of a recent randomized trial comparing two different therapeutic approaches in high-risk infants support the hypothesis that encouraging self-initiated motor variability and challenge may be critical factors to enhance motor development in this population [15].
It is perhaps no coincidence that many principles of infant motor learning are also important factors in maximizing rehabilitation outcomes after neurological injury. Variability in movement patterns during training enhances motor outcomes [16] and reflects more complex movement skill [17]. The role of error is increasingly recognized as critical for enhancing motor performance [18, 19] and is clearly a part of the development of refined walking skill in infants, who typically fall four times (range 0–12) in every 16 minutes of free play when learning to walk [20]. Salience, the meaningfulness of a task to an individual, promotes active engagement and self-initiation. Salience facilitates neuroplasticity of motor control centres [21] and the degree of salience of a cue can determine whether an infant will reach toward that cue [22]. Finally, the most effective neurorehabilitation strategies include intensive and challenging motor practice [23, 24]. There has been minimal application of these overlapping principles of infant motor learning and effective neurorehabilitation to children with CP.
This study sought to design a training protocol that incorporated these key principles by enabling infants and toddlers with CP to have motor learning experiences through playful self-discovery and motor exploration similar to typically-developing children, with the primary goal of altering the trajectory of their motor development. To accomplish this goal, novel technology was used that provided weight support without movement constraint, which perhaps would allow participants to use motor strategies that otherwise would not be possible given their available physical and neurological resources.
The objectives of this pilot study were to design and implement a novel mobility training intervention incorporating key infant motor learning principles with neurorehabilitation principles and investigate its feasibility, tolerability, and effect on motor function in infants and toddlers with CP.
Methods
Study design
This pilot study used a single-subject research design, with repeated measures during 6-week baseline and treatment phases and a final assessment after a 6-week withdrawal phase. All procedures occurred at the National Institutes of Health Clinical Center and received ethics approval. Caregivers of all participants provided written, informed consent.
Participants
Intervention was targeted for young children with a diagnosis of, or with suspected, CP early in their development of walking skill. Five participants met the following inclusion criteria: 12–36 months of age (adjusted for pre-term birth below 2 years, if applicable), motor delay of at least 4 months [25], diagnosis of bilateral cerebral palsy or neurological evidence of spasticity or brain damage, ability to pull to stand at a surface and cognitive ability to follow one-step commands. Potential participants were excluded if they had a diagnosis of unilateral CP; a secondary orthopaedic, neuromuscular or cardiovascular condition unrelated to CP; greater than 6 months of independent walking experience; or history of lower extremity surgery or injury in the previous 6 months.
All participants maintained the therapy services they were already receiving throughout all phases of the study, except Participant 1 who was receiving physical therapy four times per week. He continued that frequency during the baseline and withdrawal phases but eliminated one or two of those sessions per week during the treatment phase.
Mobility training
Each of the principles identified from the literature as key components of infant motor development and effective neurorehabilitation was incorporated into the training programme and is described in Table I.
Table I.
Integration of each neurorehabilitation principle into the treatment programme [33].
| Rehabilitation/Learning principle | Integration into therapy |
|---|---|
| Early | Therapy is delivered at a young age and in the beginning stages of upright mobility skill development. |
| Variable | Many different motor activities are encouraged within each session by the environment and the therapists; Frequent transitions among activities. |
| Error experience | Participants are not prevented from falling or losing their balance by either the weight support system or the therapists; Encouragement of challenging tasks inherently encourages error. |
| Salient | Mimics typical toddler motor play with freedom of exploration to encourage self-discovery; Stimulating environment and age-appropriate toys. |
| Intensive | Three times per week for 30 minutes; 90 minutes per week exceeds the intensity of other interventions shown to have a positive effect on motor function in infants and toddlers with motor disabilities [11, 33] and exceeds the amount of time these infants spend practicing upright activities on their own because they are not independent with them. |
| Challenging | Weight support allows practice of activities that are beyond the participant’s current level of function; Environment also encourages motor activity just beyond the participant’s current level of function (tailored to each child and modified as they progress). |
Recent progress in rehabilitation technology has led to the development of an advanced weight support system that assists but, unlike traditional static body weight support systems, does not control the user’s vertical position and therefore does not constrain movement. This dynamic system (ZeroG™, Aretech LLC, Ashburn, VA) continuously provides the desired amount of weight assistance in real time, independent of where or how the user moves within the limits of an overhead track. This allows free and unconstrained movements, including but not limited to walking, squatting, climbing stairs, crawling, getting down to and up from the floor and falling. The system was used in a stimulating environment (salience) to enable activities to mimic typical toddler over-ground motor play and exploration (variability) while allowing postural error and falls (error experience) and encouraging motor tasks beyond the children’s current skill levels (challenging). See Figure 1 for examples.
Figure 1.

Examples of motor variability and error during therapy sessions. The dynamic weight support allows for a wide range of movements in all directions, various modes of toddler locomotion (crawling, knee walking, walking, climbing), attempts to jump and falling. The children are practicing skills they are not yet able to perform on their own.
Mobility training was delivered three times per week for 6 weeks. Tasks were tailored to individual skill level and interests. Weight support ranged from 10–40% of the child’s body weight initially, determined by the level that allowed walking and squatting with the least amount of assistance from the therapist for those tasks, and was gradually reduced as the child progressed. All sessions were videotaped for later activity coding.
Outcome measures
Feasibility
Completion rate was defined as the percentage of therapy sessions attended. Participation rate was the percentage of therapy time that participants were actively engaged in motor activity (i.e. not fussy or taking a snack break) and was an indicator of the children’s tolerance to the training.
Motor function
The Gross Motor Function Measure (GMFM-66) was administered every 2 weeks during the baseline and treatment phases and at the end of the withdrawal phase [26]. Three of the five GMFM sub-scales were also scored for evaluation with video coding results, including the crawling and kneeling (C), standing (D), and walking, running, and jumping (E) dimensions.
Data analysis
Motor development rates during each phase were calculated using the split middle technique for single-subject study data [27]. Briefly, celeration lines were constructed in each phase to account for multiple data points. Comparing the slopes of the celeration lines during treatment and withdrawal to that of the baseline indicates how participants responded to the intervention.
The magnitude of change attributed to the training was determined by calculating net change scores, subtracting baseline change from treatment change. Net change scores were evaluated in the context of published minimal clinically important difference values and the differences between adjacent GMFCS levels.
Videotaped therapy sessions for children with positive net changes were coded to quantify the participation rate (engagement) and the amount of practice in each skill domain (consistent with GMFM dimensions C, D and E). Correlation coefficients between net change scores for the individual dimensions and amount of therapy time spent in relevant activities were calculated to determine the amount of variance in motor change explained by the therapy.
Results
Feasibility
Demographic characteristics of the five participants are presented in Table II. Four of the five participants attended all 18 therapy sessions (100% completion rate) and all assessment sessions. Participant 3 was able to attend all assessment sessions, but only 13/18 therapy sessions (72% completion) due to unanticipated transportation issues. Participation rate averaged 91% (range 87–95%).
Table II.
Participant characteristics, GMFM-66 outcomes and video coding results. Motor development rates represent average GMFM-66 change per week. Relative rates of change compare rates during treatment and follow-up phases compared to baseline (with 1.0 representing equal rates of change). Video coding results include degree of motor engagement, average number of falls per session and variance explained (r2) between training time in each activity sub-scale (GMFM dimensions C, D and E) and motor change in those dimensions.
| ID | Age (months) | GMFCS level | Motor delay | Motor development rates
|
Relative rates of change
|
Video coding results
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Treatment | Withdrawal | Treatment/Baseline | Withdrawal/Baseline | Percentage engaged | Falls | Dimension change explained by therapy time (r2) | ||||
| 1 | 18 | III | 8 months | 0.05 | 0.55 | 0.04 | 10.8 | 0.7 | 91% | 11 | 0.80 |
| 2 | 27 | III | 17 months | 0.16 | 0.61 | 0.08 | 3.8 | 0.5 | 87% | 6 | 0.74 |
| 3 | 17 | II | 7 months | 0.30 | 2.05 | 0.20 | 7.0 | 0.7 | 95% | 17 | 0.98 |
| 4 | 13 | III | 5 months | 0.05 | 0.77 | 0.24 | 15.1 | 4.6 | 88% | 14 | 1.00 |
| 5 | 14 | I | 4 months | 1.56 | 0.46 | −0.06 | 0.3 | −0.04 | – | – | – |
GMFCS, Gross Motor Function Classification System.
Motor function
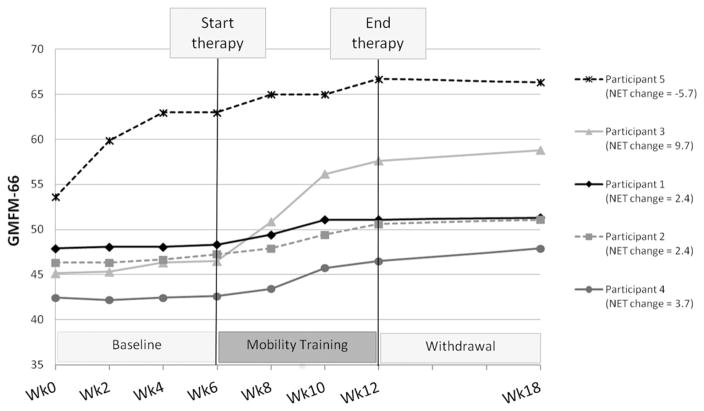
GMFM-66 results with treatment net change scores are presented in Figure 2. Net change scores of 0.0 represent no change beyond that predicted from the baseline phase. Table II presents motor development rates in each phase and relative rates of change during treatment (Treatment/Baseline) and withdrawal (Withdrawal/Baseline) compared to baseline. A value of 1.0 for relative rates of change represents no change in motor development rate. Values greater than 1.0 represent increased rates and values less than 1.0 represent decreased rates relative to baseline.
Figure 2.
GMFM-66 data for the five participants. Treatment net change scores were calculated by subtracting baseline change from treatment change and are included in the legend.
Participants 1–4 demonstrated motor development rates during treatment that were 3.8–15.1 times greater than their respective baseline rates. Although Participant 5 demonstrated improvement during treatment, the rate was less than her baseline (0.3). This child made large gains during baseline when she started walking on her own (at 16 months of age), and as a result there is no indication that the training improved her motor ability beyond what could be predicted.
Participant 3, who used a walker at the start of the study, began walking alone with the dynamic support during the first therapy session (with many falls). She did not start walking on her own at home or during assessment sessions (without the dynamic support), until approximately 3 weeks later. Participants 3 and 5 both transitioned from the floor to standing through the less mature modified plantigrade pattern using their arms, but completed this transition without coaxing through half-kneeling without their arms when using the dynamic support, a pattern that is difficult for children with CP to achieve. These examples suggest that the dynamic support allowed practice of motor strategies more advanced than the children’s current ability.
All participants maintained their treatment gains, but returned to slower rates of motor development during withdrawal. Participants 1–3 demonstrated rates of change that were less than but close to their baseline rates (0.5–0.7). Improvement for Participant 4 remained elevated after treatment relative to her baseline (4.6), but not as elevated as during treatment (15.1). She had the lowest level of motor function and demonstrated the greatest relative improvement during and after treatment. Participant 5 essentially did not change during the withdrawal phase.
The GMFM-66 net treatment change score for Participant 5 was −5.7 due to her large baseline improvement. Net change scores for the other participants ranged from 2.4–9.7, each exceeding the respective minimal clinically important difference value for treatments with a large effect size (1.5 for GMFCS II and 1.3 for GMFCS III) [28]. However, caution should be taken as these values were determined from a sample of children aged 4–18 years. It is unknown if the minimal clinically important differences would be the same earlier in development, but it is possible that they would be higher.
The GMFCS motor development curves offer another approach to evaluate the magnitude of observed changes [5]. Participant 3’s GMFM-66 net change (9.7) was sufficient for her to be re-classified as level I after training. The other three participants with positive net changes (Participants 1, 2 and 4) were level III. They made roughly half the amount of change needed to reach motor function at level II, with net changes of 2.4, 2.4 and 3.7, respectively, compared to changes of 4–7 points needed (depending on their age) to be classified as level II. This suggests that a training duration twice as long (12 weeks) may produce large enough changes to impact the classification level.
The amount of training time spent in each GMFM activity dimension (C, D and E) explained a high degree of variance in dimension net change scores for each of the four children with overall GMFM-66 positive net change scores (r2 range 0.74–1.00). This strongly suggests that changes were related to the training. Video coding also revealed high rates of participation and error (falls), as intended (see Table II, note: raw scores for activity dimensions are not shown). Also of note, the relationship between number of falls and total GMFM-66 change was quite strong (r =0.90), suggesting that falling and learning were coincident.
Discussion
This pilot study demonstrates the feasibility of developing and implementing an intervention that incorporates overlapping principles of infant motor learning and neurorehabilitation for toddlers with CP. The training was well-tolerated and encouraged a high level of engagement (practice) in varied motor activities with a high degree of error. Gains in motor function exceeded the predicted rate and the minimal clinically important difference in four of five participants. The observed gains appear to be sustainable in the short-term.
Research in typically-developing infants has identified that the rate-limiting factors in the development of independent walking are postural control and lower extremity strength [29]. Pervasive deficits in postural control and muscle strength are hallmarks of motor impairment in CP, explaining in large part why infants with CP have difficulties developing skilled walking [30, 31]. The intervention developed in this study may have addressed both of these impairments without compensating for them, allowing gradual development of more skilled postural control and strength and preventing repeated practice of stereotypical and poorly co-ordinated movements.
The provision of dynamic support during upright motor skill development reduces the demand on the lower extremities. This may allow children with CP to develop better co-ordination and motor control in spite of their weakness, instead of developing the compensatory strategies of posture and movement that are often required to simply support their bodies against gravity, but impede complex movement. This may also provide an opportunity for strength to gradually increase through progressive loading of anti-gravity muscles.
The motor variability allowed by the system and encouraged by the environment mimicked typical motor play and exploration. These experiences may facilitate the development of more skilled postural control that must continually adapt to the environment through small changes in trunk movement and position. This type of movement contrasts with the repetitive, consistent practice of one pattern while the trunk is essentially constrained as in many current locomotor training programmes (i.e. supported stepping on a treadmill). In fact, previous enthusiasm for regimented step training programmes has decreased, even for retraining walking in adults, in lieu of over-ground practice [32].
Participant 5 was the least affected (50th percentile of GMFCS level I at the start of the study and 97th percentile by the end) and likely not an ideal candidate for this training. She continued to demonstrate a 4-month motor delay after treatment, but likely did not benefit additionally from the dynamic weight support because she was already creating motor experiences with high variability and error on her own.
Conclusions made from these data are clearly limited by the small sample and lack of a control group. Additionally, despite the activity coding that supports the changes observed, it is well known that motor development is not linear, but rather is characterized by ‘fits and starts’ and, therefore, the baseline change may not have been an accurate predictor of expected change during treatment.
However, these outcomes are promising given the relatively small dose of training. The 90 minutes of training each week is only a fraction of the time these participants are able to practice motor skills, suggesting that their experiences during this training were different from those they were able to create on their own. The caregivers may also have altered their own interactions with their infants as a result of observing the training sessions, which could have contributed to the gains observed, but the rates of change would likely have remained elevated beyond the treatment phase if this were the case.
It appears that this intervention provided a unique opportunity for self-initiated movement that clearly differs from assistance provided by a caregiver or therapist. The findings suggest that there may be potential to alter the trajectory of motor development at a young age in CP and warrant further investigation to determine efficacy. A larger controlled clinical trial is planned, with slight modifications to the participant selection criteria and longer treatment and follow-up durations.
Acknowledgments
The authors acknowledge Cristiane Zampieri-Gallagher, PT, PhD for her assistance with data collection, and Joe Hidler, PhD for software development to allow use of the technology with small children. This research was supported by the Intramural Research Program of the National Institutes of Health Clinical Center.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Pakula AT, Van Naarden Braun K, Yeargin-Allsopp M. Cerebral palsy: Classification and epidemiology. Physical Medicine & Rehabilitation Clinics of North America. 2009;20:425–452. doi: 10.1016/j.pmr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Palisano RJ, Kang LJ, Chiarello LA, Orlin M, Oeffinger D, Maggs J. Social and community participation of children and youth with cerebral palsy is associated with age and gross motor function classification. Physical Therapy. 2009;89:1304–1314. doi: 10.2522/ptj.20090162. [DOI] [PubMed] [Google Scholar]
- 3.Wiegerink DJ, Roebroeck ME, van der Slot WM, Stam HJ, Cohen-Kettenis PT. Importance of peers and dating in the development of romantic relationships and sexual activity of young adults with cerebral palsy. Developmental Medicine & Child Neurology. 2010;52:576–582. doi: 10.1111/j.1469-8749.2010.03620.x. [DOI] [PubMed] [Google Scholar]
- 4.Michelsen SI, Uldall P, Hansen T, Madsen M. Social integration of adults with cerebral palsy. Developmental Medicine & Child Neurology. 2006;48:643–649. doi: 10.1017/S0012162206001368. [DOI] [PubMed] [Google Scholar]
- 5.Rosenbaum PL, Walter SD, Hanna SE, Palisano RJ, Russell DJ, Raina P, Wood E, Bartlett DJ, Galuppi BE. Prognosis for gross motor function in cerebral palsy: Creation of motor development curves. Journal of the American Medical Association. 2002;288:1357–1363. doi: 10.1001/jama.288.11.1357. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan RV. Relearning of locomotion in injured spinal cord: New directions for rehabilitation programs. International Journal of Neuroscience. 2003;113:1333–1351. doi: 10.1080/00207450390231446. [DOI] [PubMed] [Google Scholar]
- 7.Palisano RJ, Cameron D, Rosenbaum PL, Walter SD, Russell D. Stability of the gross motor function classification system. Developmental Medicine & Child Neurology. 2006;48:424–428. doi: 10.1017/S0012162206000934. [DOI] [PubMed] [Google Scholar]
- 8.McCormick A, Brien M, Plourde J, Wood E, Rosenbaum P, McLean J. Stability of the Gross Motor Function Classification System in adults with cerebral palsy. Developmental Medicine & Child Neurology. 2007;49:265–269. doi: 10.1111/j.1469-8749.2007.00265.x. [DOI] [PubMed] [Google Scholar]
- 9.Gorter JW, Ketelaar M, Rosenbaum P, Helders PJ, Palisano R. Use of the GMFCS in infants with CP: The need for reclassification at age 2 years or older. Developmental Medicine & Child Neurology. 2009;51:46–52. doi: 10.1111/j.1469-8749.2008.03117.x. [DOI] [PubMed] [Google Scholar]
- 10.Heathcock JC, Lobo M, Galloway JC. Movement training advances the emergence of reaching in infants born at less than 33 weeks of gestational age: A randomized clinical trial. Physical Therapy. 2008;88:310–322. doi: 10.2522/ptj.20070145. [DOI] [PubMed] [Google Scholar]
- 11.Heathcock JC, Galloway JC. Exploring objects with feet advances movement in infants born preterm: A randomized controlled trial. Physical Therapy. 2009;89:1027–1038. doi: 10.2522/ptj.20080278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valentin-Gudiol M, Mattern-Baxter K, Girabent-Farres M, Bagur-Calafat C, Hadders-Algra M, Angulo-Barroso RM. Treadmill interventions with partial body weight support in children under six years of age at risk of neuromotor delay. Cochrane Database of Systematic Reviews. 2011;12:CD009242. doi: 10.1002/14651858.CD009242.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Damiano DL, DeJong SL. A systematic review of the effectiveness of treadmill training and body weight support in pediatric rehabilitation. Journal of Neurologic Physical Therapy. 2009;33:27–44. doi: 10.1097/NPT.0b013e31819800e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adolph KE. Learning to move. Current Directions in Psychological Science. 2008;17:213–218. doi: 10.1111/j.1467-8721.2008.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blauw-Hospers CH, Dirks T, Hulshof LJ, Bos AF, Hadders-Algra M. Pediatric physical therapy in infancy: From nightmare to dream? A two-arm randomized trial. Physical Therapy. 2011;91:1323–1338. doi: 10.2522/ptj.20100205. [DOI] [PubMed] [Google Scholar]
- 16.Lewek MD, Cruz TH, Moore JL, Roth HR, Dhaher YY, Hornby TG. Allowing intralimb kinematic variability during locomotor training poststroke improves kinematic consistency: A subgroup analysis from a randomized clinical trial. Physical Therapy. 2009;89:829–839. doi: 10.2522/ptj.20080180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harbourne RT, Stergiou N. Movement variability and the use of nonlinear tools: Principles to guide physical therapist practice. Physical Therapy. 2009;89:267–282. doi: 10.2522/ptj.20080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torres-Oviedo G, Bastian AJ. Natural error patterns enable transfer of motor learning to novel contexts. Journal of Neurophysiology. 2012;107:346–356. doi: 10.1152/jn.00570.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornby TG, Campbell DD, Kahn JH, Demott T, Moore JL, Roth HR. Enhanced gait-related improvements after therapist- versus robotic-assisted locomotor training in subjects with chronic stroke: A randomized controlled study. Stroke. 2008;39:1786–1792. doi: 10.1161/STROKEAHA.107.504779. [DOI] [PubMed] [Google Scholar]
- 20.Joh AS, Adolph KE. Learning from falling. Child Development. 2006;77:89–102. doi: 10.1111/j.1467-8624.2006.00858.x. [DOI] [PubMed] [Google Scholar]
- 21.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. Journal of Speech, Language, and Hearing Research. 2008;51:S225–S239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- 22.Clearfield MW, Dineva E, Smith LB, Diedrich FJ, Thelen E. Cue salience and infant perseverative reaching: Tests of the dynamic field theory. Developmental Science. 2009;12:26–40. doi: 10.1111/j.1467-7687.2008.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: The EXCITE randomized clinical trial. Journal of the American Medical Association. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 24.Horn SD, DeJong G, Smout RJ, Gassaway J, James R, Conroy B. Stroke rehabilitation patients, practice, and outcomes: Is earlier and more aggressive therapy better? Archive of Physical Medicine & Rehabilitation. 2005;86:S101–S114. doi: 10.1016/j.apmr.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 25.Bayley N. Therapy Skill Builders. 2. Psychological Corporation; San Antonio, TX, USA: 2001. Bayley Scales of Infant Development, (BSID-II: Motor Scale Kit) [Google Scholar]
- 26.Russell DJ, Avery LM, Rosenbaum PL, Raina PS, Walter SD, Palisano RJ. Improved scaling of the gross motor function measure for children with cerebral palsy: Evidence of reliability and validity. Physical Therapy. 2000;80:873–885. [PubMed] [Google Scholar]
- 27.Portney L, Watkins M, editors. Foundations of clinical research, application to practice. Stamford, USA: Appleton & Lange; 1993. [Google Scholar]
- 28.Oeffinger D, Bagley A, Rogers S, Gorton G, Kryscio R, Abel M, Damiano D, Barnes D, Tylkowski C. Outcome tools used for ambulatory children with cerebral palsy: Responsiveness and minimum clinically important differences. Developmental Medicine & Child Neurology. 2008;50:918–925. doi: 10.1111/j.1469-8749.2008.03150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thelen E, Cooke DW. Relationship between newborn stepping and later walking: A new interpretation. Developmental Medicine & Child Neurology. 1987;29:380–393. doi: 10.1111/j.1469-8749.1987.tb02492.x. [DOI] [PubMed] [Google Scholar]
- 30.de Graaf-Peters VB, Blauw-Hospers CH, Dirks T, Bakker H, Bos AF, Hadders-Algra M. Development of postural control in typically developing children and children with cerebral palsy: Possibilities for intervention? Neuroscience & Biobehavioral Reviews. 2007;31:1191–1200. doi: 10.1016/j.neubiorev.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Wiley ME, Damiano DL. Lower-extremity strength profiles in spastic cerebral palsy. Developmental Medicine & Child Neurology. 1998;40:100–107. doi: 10.1111/j.1469-8749.1998.tb15369.x. [DOI] [PubMed] [Google Scholar]
- 32.Dobkin BH, Duncan PW. Should body weight-supported treadmill training and robotic-assistive steppers for locomotor training trot back to the starting gate? Neurorehabilitation & Neural Repair. 2012 May;26(4):308–317. doi: 10.1177/1545968312439687. (Epub 2012 Mar 12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ulrich DA, Ulrich BD, Angulo-Kinzler RM, Yun J. Treadmill training of infants with Down syndrome: Evidence-based developmental outcomes. Pediatrics. 2001;108:E84. doi: 10.1542/peds.108.5.e84. [DOI] [PubMed] [Google Scholar]