Abstract
Background
Whether long-term, low-level hydrogen sulfide (H2S) gas is a cause of health effects, including asthma, is uncertain. Rotorua city, New Zealand, has the largest population exposed, from geothermal sources, to relatively high ambient levels of H2S. In a cross-sectional study, the authors investigated associations with asthma in this population.
Methods
A total of 1,637 adults, aged 18-65 years, were enrolled during 2008-2010. Residences and workplaces were geocoded. H2S exposures at homes and workplaces were estimated using city-wide networks of passive H2S samplers and kriging to create exposure surfaces. Exposure metrics were based on (1) time-weighted exposures at home and work; and (2) the maximum exposure (home or work). Exposure estimates were entered as quartiles into log-binomial regression models, with covariate data.
Results
Neither exposure metric showed evidence of increased asthma risk from H2S. However, some suggestion of exposure-related reduced risks for diagnosed asthma and asthma symptoms, particularly wheezing during the last 12 months, emerged. With the maximum exposure metric, the prevalence ratio for wheeze in the highest exposure quartile was 0.80 (0.65, 0.99) and, for current asthma treatment, 0.75 (0.52, 1.08). There was no evidence that this was caused by a “survivor effect”.
Conclusions
The study provided no evidence that asthma risk increases with H2S exposure. Suggestions of a reduced risk in the higher exposure areas are consistent with recent evidence that H2S has signaling functions in the body, including induction of smooth muscle relaxation and reduction of inflammation. Study limitations, including possible confounding, preclude definitive conclusions.
Keywords: Hydrogen sulfide, Geothermal, New Zealand, Asthma, Wheeze
1. Introduction
Hydrogen sulfide (H2S) is an odorous and highly toxic gas, produced by a number of industries and facilities, including sewage treatment plants, paper mills, oil and gas refineries, and concentrated animal farming operations, as well as occurring naturally in geothermal or volcanic areas. In occupational situations, H2S has been responsible for many deaths, particularly when high concentrations arise in confined areas (Fuller and Suruda, 2000; Hendrickson et al., 2004). Communities surrounding H2S sources are often exposed to lower, but odorous, concentrations (Jaakkola et al., 1999; Legator et al., 2001; Wing et al., 2008). The odor threshold varies, but is reported to be in the range 0.5 to 30 ppb for most people (Schiffman and Williams, 2005). Whether there are any effects on health, other than a response to odor, of long-term lower level (<2000 ppb) exposures to H2S is unknown, as few epidemiologic studies have addressed this and results have been inconsistent.
Some air pollutants, such as ozone, nitrogen dioxide, and sulfur dioxide, have been associated with asthma outcomes (Di Giampaolo et al., 2011). However, the relationship between ambient levels of H2S and asthma is unclear and has not been extensively studied. Campagna et al. (Campagna et al., 2004) found an association between unscheduled or emergency outpatient hospital visits for asthma and H2S levels on the previous day, and indications of association with asthma-like symptoms have been reported in communities living near concentrated animal feeding operations (Schinasi et al., 2011). A recent study reported a small increase, after a 3-5 day lag, in dispensing of drugs for obstructive pulmonary disease associated with ambient H2S concentrations from geothermal sources in Iceland (Carlsen et al., 2012). Other studies have found no evidence of an H2S association with asthma (Jappinen et al., 1990). However, recent animal studies have shown that the H2S donor, NaHS, when administered intraperitoneally, can reduce the severity of asthma in experimental animals (Chen et al., 2009). In patients with asthma, the endogenous serum concentration of H2S has been shown to be significantly reduced, compared with serum H2S concentrations in healthy subjects (Wu et al., 2008). An array of other studies, in animals and humans, has shown that endogenously produced H2S has important signaling functions, with anti-inflammatory and cytoprotective roles, as well as induction of smooth muscle relaxation (Olson and Donald, 2009; Whiteman et al., 2011). These findings have led to suggestions of possible therapeutic benefits of H2S administration (Faller et al., 2010; King and Lefer, 2011; Wagner et al., 2009).
Rotorua city, in the North Island of New Zealand, sits on an active geothermal field at the southern end of Lake Rotorua, an old volcanic caldera. Vents emitting H2S are located in and around the city. The population of nearly 60 000, the largest anywhere with chronic ambient H2S exposures, has long been recognized as an ideal place to investigate whether long-term exposures to ambient levels of H2S are associated with health effects (IPCS, 1981). The Rotorua population has not been extensively studied, however, although several ecological studies have suggested that there may be health effects related to H2S exposure (Bates et al., 1997; Bates et al., 1998; Bates et al., 2002; Durand and Wilson, 2006). Taking advantage of this unique natural experiment and as part of a larger study, we investigated associations between H2S exposures and asthma symptoms or a diagnosis of asthma.
2. Materials and methods
Institutional Review Board approvals for study procedures were obtained at the University of California, Berkeley and from the Northern Ethics Committee in New Zealand. Written informed consent was obtained from all participants at the outset of their participation.
2.1 Participant recruitment
Participants were 1637 men and women, aged 18-65, resident in Rotorua for at least 3 years. Rotorua has a centralized patient register to which all local medical practitioners subscribe and which contains an estimated 98% of the Rotorua population. Patient data are stored in a common format and were used to randomly select potential participants. To ensure adequate representation in the study of people from the more highly exposed areas of the city, we first stratified the city into “high”, “medium” and “low’’ H2S-exposed areas, based on the Rotorua H2S exposure investigation of Horwell et al. (Horwell et al., 2005), and as previously used (Bates et al., 2002), and then identified potential study participants equally from these areas by randomly selecting, according to residential area, from the central patient register. Recruitment was carried out by first mailing a letter with information on the study, followed by repeated telephone calls until the possible study participant was either contacted, found to have moved elsewhere, or at least four calls were made at different times without making contact.
Ineligible for the study were persons who had not spent the last 3 years living in Rotorua, persons unable to speak and write English, persons who, by reason of disability, were unable to visit the study clinic, and people who were blind from birth, or became blind in childhood or through trauma. Women who reported they were pregnant were also excluded from the study because one part of the study involved administration of mydriatics.
Participants attended the study clinic, during April 2008 to December 2010, where they were administered a questionnaire and a series of clinical tests. The questionnaire obtained residential and workplace histories, inquired about doctor-diagnosed medical conditions, including asthma, and asked a series of questions about respiratory symptoms in the last 12 months. Current residential and workplace addresses were geocoded. Questions also covered the number of hours spent at each workplace.
2.2 Exposure estimation
Exposure data were obtained by setting out across Rotorua two networks of passive H2S samplers (Radiello, Sigma-Aldrich Co. LLC), each for 2 weeks, in the summer (February) and in the winter (July) of 2010. In the winter there were 50 sampling sites and 53 in the summer. The majority of the sampling sites were the same for the two monitoring periods. These provided average ambient concentrations at the exposure points across each of the 2-week periods. Concentration surfaces were then created across the Rotorua urban area with ArcGIS (v. 10), using ordinary kriging with a spherical semivariogram. The default value used employs the 12 closest sampling points as inputs to the interpolation between those points. Geocoded residential and workplace locations were allocated concentrations from these surfaces. For the analysis presented here, the average of the summer and winter H2S concentrations at each current residential and workplace location was used for calculation of exposure metrics.
2.3 Statistical analysis
Outcomes examined were yes/no responses to questions about breathing difficulties in the last 12 months. Specifically, these outcomes were:
wheezing or whistling in the chest
woken with a feeling of tightness in the chest
experienced an attack of shortness of breath that came on during the day when at rest
woken by an attack of shortness of breath
woken by an attack of coughing.
We also examined ‘current asthma’, defined as an affirmative response to the question “Have you ever been diagnosed by a doctor as having asthma?”, plus either wheeze in the last 12 months or current use of asthma medication.
Statistical analysis was carried out using SAS, version 9 and Stata, version 11. The main analysis involved log-binomial regression analysis, using quartiles of exposure, to generate prevalence ratios (PR) (Spiegelman and Hertzmark, 2005). However, when the log-binomial model did not converge, Poisson regression with the robust variance was used. All models were adjusted for gender, age-group, smoking, attained level of education, employment status and income. Quartiles of two exposure metrics were used: (i) The mean time-weighted average exposure based on hours at work, and assuming the remainder was spent at home; (ii) The maximum exposure, whether at work or home. Both metrics assumed that during the hours that a person was working within Rotorua, but not at a specific workplace, they were exposed to 10 ppb of H2S. This is based on the assumption that many of these people would have been working at residences. Most residences in Rotorua are in low H2S exposure areas and we assumed that the average exposure was at the top of the first exposure quartile in our study. All work outside of Rotorua city was assumed to have been associated with zero H2S exposure.
Tests for trend were carried out by assigning a midpoint H2S concentration to each quartile and treating H2S concentration as a continuous variable in log-binomial regressions.
3. Results
Attempts were made to contact 6,573 people using the last recorded telephone number in their medical records. Contact was made with 4,498 people, of whom 976 were ineligible for participation. Of the remaining 3,522 people, 1,927 (54.7%) agreed to participate. Because of study timeframe constraints, only 1,637 people actually participated and provided useable data. To investigate possible participation bias, we carried out analysis of data for the eligible group. For all these people we had information on whether they lived in an area that was designated ‘high’, ‘medium’ or ‘low’ H2S exposure, according to a previous study (Bates et al., 2002), their age, gender, and self-reported ethnic group (European, Maori, and ‘other’, which included Asians and Pacific Island people). A logistic regression model including all these as independent variables and treating those who agreed to participate as “cases” and those who declined as “controls” produced no evidence that participation was related to exposure status (Table 1). However, it did show that women were more likely to participate than men, younger people were less likely to participate than those who were older, and willingness to participate was highest in those who reported European ethnicity and least in those in the “other” category. Examination of interaction terms between H2S exposure status and age group, gender and ethnicity, produced no evidence of effect modification (not shown).
Table 1.
Multivariate logistic regression model of willingness to participate of potential study recruits (N = 3,522), Rotorua, New Zealand, 2008-2010.
| Non-participants (row %) | Participants (row %) | ORa | 95% CI | |
|---|---|---|---|---|
| Sex | ||||
| Male | 776 (50.6) | 759 (49.4) | 1.00 | |
| Female | 819 (41.2) | 1,168 (58.8) | 1.49 | 1.30, 1.71 |
| Age Group (yrs): | ||||
| 18-29 | 314 (53.6) | 272 (46.4) | 1.00 | |
| 30-39 | 381 (49.4) | 390 (50.6) | 1.17 | 0.94, 1.45 |
| 40-49 | 387 (40.5) | 569 (59.5) | 1.66 | 1.34, 2.05 |
| 50-59 | 365 (41.8) | 508 (58.2) | 1.55 | 1.25, 1.92 |
| 60+ | 148 (44.1) | 188 (55.9) | 1.35 | 1.02, 1.77 |
| Ethnic Group: | ||||
| European | 1011 (42.5) | 1370 (57.5) | 1.00 | |
| Maori | 467 (49.3) | 480 (50.7) | 0.77 | 0.66, 0.90 |
| Other | 117 (60.3) | 77 (39.7) | 0.49 | 0.36, 0. 66 |
| H2S exposure area | ||||
| Low | 537 (44.2) | 677 (55.8) | 1.00 | |
| Medium | 551 (46.0) | 646 (54.0) | 0.94 | 0.80, 1.11 |
| High | 507 (45.6) | 604 (54.4) | 0.96 | 0.81, 1.13 |
Abbreviations: OR, Odds ratio.
95% CI, 95% confidence interval.
Higher values indicate greater willingness to participate.
The median H2S concentration, averaged across summer and winter, for current residences was 20.3 ppb, with mean 20.8 ppb (standard deviation (SD), 15.5 ppb). For current workplaces, the median and mean (SD) were 26.4 and 27.0 (17.7) ppb, respectively. The range for both residences and workplaces was 0-64 ppb.
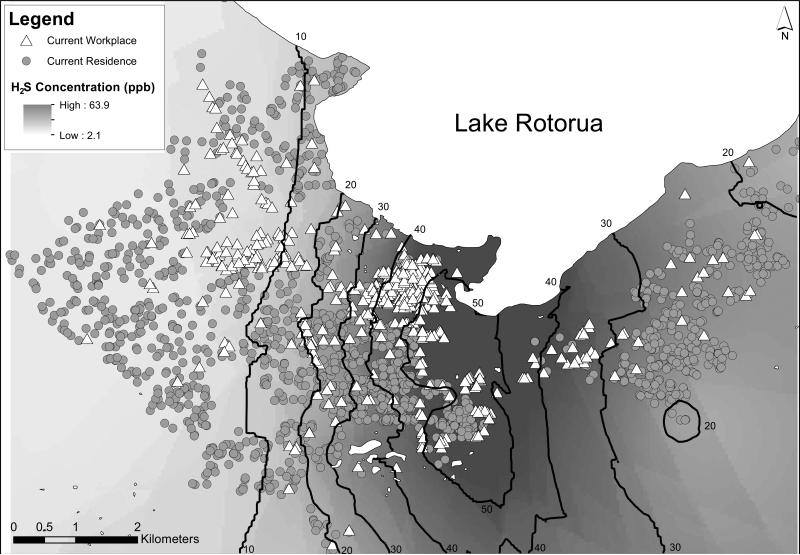
The highest H2S concentrations are located on an axis between the Whakarewarewa geothermal area and Lake Rotorua, down Fenton Street, the main Rotorua business street. The distributions of current residences and workplaces of study participants, superimposed on the year-averaged exposure surface, are shown in Figure 1. Many people who work in the highly exposed downtown area near Lake Rotorua, reside in relatively low-exposure residential areas.
Figure 1.
Locations of Residences and Workplaces at Time of Study Participation and Estimated H2S Concentration Surface, with Contours in Parts per Billion (ppb), Rotorua, New Zealand, 2008-2010.
Table 2 shows the distribution of covariates across our study population, stratified by quartile of time-weighted average exposure concentrations for current home and workplace. There is little in the way of major differences between quartiles. The distribution of covariates is similar if examined in terms of quartiles of highest exposure.
Table 2.
Description of the Study Population, by Quartiles (Q1 to Q4) of Estimated Time-weighted Average H2S Exposure Concentration for Current Home and Workplace of Participants, Rotorua, New Zealand, 2008-2010.
| N | Q1 (0-10 ppb) | Q2 (11-20 ppb) | Q3 (21-30 ppb) | Q4 (31-64 ppb) | |
|---|---|---|---|---|---|
| Sex: | |||||
| Female | 981 | 62.2% | 56.0% | 59.7% | 61.9% |
| Male | 656 | 37.8% | 44.0% | 40.3% | 38.1% |
| Tobacco Smoking Status: | |||||
| Never | 831 | 51.0% | 53.3% | 50.6% | 48.2% |
| Ex | 476 | 27.1% | 26.2% | 32.3% | 30.8% |
| Current | 330 | 22.0% | 20.5% | 17.1% | 21.0% |
| Age Group (years): | |||||
| 18-29 | 168 | 13.2% | 9.8% | 9.3% | 8.8% |
| 30-39 | 305 | 19.5% | 20.8% | 17.1% | 17.1% |
| 40-49 | 454 | 29.3% | 26.2% | 32.3% | 23.2% |
| 50-59 | 471 | 24.4% | 31.3% | 29.6% | 29.8% |
| 60+ | 239 | 13.7% | 12.0% | 11.7% | 21.0% |
| Ethnic Group: | |||||
| European | 1146 | 67.3% | 71.6% | 72.1% | 68.9% |
| Maori | 397 | 27.3% | 22.0% | 23.0% | 24.7% |
| Other | 94 | 5.4% | 6.4% | 4.9% | 6.4% |
| Education: | |||||
| No qualification earned | 215 | 17.8% | 13.0% | 7.8% | 13.9% |
| Secondary qualification | 374 | 20.2% | 23.0% | 23.5% | 24.7% |
| Tertiary Non-Degree | 744 | 47.3% | 45.5% | 48.4% | 40.6% |
| University Degree | 304 | 14.6% | 18.6% | 20.3% | 20.8% |
| Employment Status: | |||||
| Employed | 1273 | 67.1% | 88.0% | 79.7% | 76.3% |
| Homemaker/Student/Retired | 233 | 23.9% | 7.3% | 12.5% | 13.2% |
| Unemployed | 131 | 9.0% | 4.6% | 7.8% | 10.5% |
| Income (NZ$): | |||||
| $0-$20K | 357 | 30.7% | 13.2% | 21.0% | 22.2% |
| >$20K-$40K | 473 | 25.6% | 31.3% | 27.4% | 31.3% |
| >$40K-$60K | 339 | 21.2% | 24.7% | 18.1% | 18.8% |
| >$60K-$80K | 227 | 10.7% | 15.4% | 17.4% | 12.0% |
| >$80K | 192 | 8.3% | 13.2% | 13.7% | 11.7% |
| Don't Know/Refuse | 49 | 3.4% | 2.2% | 2.4% | 3.9% |
| TOTAL | 1637 | 100% | 100% | 100% | 100% |
Abbreviations:N, number of participants; NZ$, New Zealand dollars; ppb, parts per billion, Q, quartile.
Results of the main analysis for the entire study population, using the two exposure metrics, are shown in Tables 3 (time-weighted average exposure) and Table 4 (maximum exposure—home or work).
Table 3.
Adjusteda Prevalence Ratios and 95% Confidence Intervals for Asthma Outcomes by Quartile of Time-weighted Average H2S Exposure Concentrations for Current Home and Workplace for Study Participants, Rotorua, New Zealand, 2008-10.
| Outcome | % Overall | Q1 (0-10 ppb) | Q2 (11-20 ppb) | Q3 (21-30 ppb) | Q4 (31-64 ppb) | P-value Trend |
|---|---|---|---|---|---|---|
| Wheeze or whistling | 28.5% | 1.00 | 0.82 (0.67,1.00) | 0.96 (0.79, 1.16) | 0.74 (0.60, 0.91) | 0.02 |
| Woken with chest tightness | 14.7% | 1.00 | 0.64 (0.45, 0.89) | 0.84 (0.62, 1.13) | 0.69 (0.50, 0.96) | 0.08 |
| Shortness of breath at rest | 9.7% | 1.00 | 0.91 (0.59, 1.40) | 1.07 (0.71, 1.62) | 1.02 (0.68, 1.54) | 0.75 |
| Woken by shortness of breath* | 8.7% | 1.00 | 0.70 (0.44, 1.13) | 0.86 (0.55, 1.34) | 0.68 (0.42, 1.08) | 0.18 |
| Woken by coughing | 23.3% | 1.00 | 1.07 (0.83, 1.37) | 1.17 (0.92, 1.49) | 1.06 (0.83, 1.35) | 0.55 |
| Ever asthma diagnosis | 24.2% | 1.00 | 0.79 (0.62, 1.00) | 0.87 (0.69, 1.10) | 0.82 (0.65, 1.04) | 0.19 |
| Current asthma treatment | 13.1% | 1.00 | 0.78 (0.55, 1.10) | 0.92 (0.66, 1.28) | 0.72 (0.51, 1.03) | 0.15 |
| Current asthma | 16.7% | 1.00 | 0.77 (0.56, 1.04) | 0.88 (0.66, 1.18) | 0.82 (0.61, 1.11) | 0.34 |
Abbreviations: Q, quartile
Binomial model did not converge therefore Poisson model used to estimate PR
Adjusted for variables in Table 2
Table 4.
Adjusteda Prevalence Ratios and 95% Confidence Intervals for Asthma Outcomes by Quartile of Maximum H2S Exposure Concentrations at Either Current Home or Workplace for Study Participants, Rotorua, New Zealand, 2008-10.
| Outcome | % Overall | Q1 (0-17 ppb) | Q2 (18-29 ppb) | Q3 (30-44 ppb) | Q4 (45-64 ppb) | P-value Trend |
|---|---|---|---|---|---|---|
| Wheeze or whistling | 28.5% | 1.00 | 0.98 (0.81, 1.19) | 0.87 (0.71, 1.08) | 0.80 (0.65, 0.99) | 0.02 |
| Woken with chest tightness | 14.7% | 1.00 | 0.91 (0.67, 1.25) | 0.90 (0.65, 1.25) | 0.88 (0.63, 1.22) | 0.42 |
| Shortness of breath at rest | 9.7% | 1.00 | 0.96 (0.64, 1.42) | 0.73 (0.47, 1.15) | 0.92 (0.61, 1.38) | 0.45 |
| Woken by shortness of breath | 8.7% | 1.00 | 0.78 (0.51, 1.19) | 0.80 (0.52, 1.24) | 0.64 (0.41, 1.00) | 0.06 |
| Woken by coughing | 23.3% | 1.00 | 1.09 (0.86, 1.38) | 0.85 (0.65, 1.11) | 1.11 (0.88, 1.40) | 0.79 |
| Ever asthma diagnosis | 24.2% | 1.00 | 0.90 (0.71, 1.14) | 0.88 (0.69, 1.12) | 0.83 (0.65, 1.05) | 0.12 |
| Current asthma treatment | 13.1% | 1.00 | 1.01 (0.72, 1.42) | 0.87 (0.61, 1.24) | 0.75 (0.52, 1.08) | 0.09 |
| Current asthma | 16.7% | 1.00 | 1.00 (0.74, 1.34) | 0.93 (0.68, 1.27) | 0.83 (0.60, 1.13) | 0.22 |
Abbreviations: Q, quartile
Adjusted for variables in Table 2
There is some consistency in the results produced with the two exposure metrics, although trends are a little more apparent with the maximum exposure metric (Table 4). In general, the results suggest that higher H2S exposures at home or work are associated with lower rates of current asthma and respiratory symptoms, particularly wheeze or whistling. There is little evidence for an increasing risk trend for any of the outcomes.
Broadly, the pattern of covariates in models showed little difference by gender; current smokers had the highest PRs, followed by ex-smokers; older age-groups had lower risks, as did wealthier people. A higher PR for Maori is consistent with what has been found in other studies (Lewis et al., 1997).
One conceivable explanation for the lower prevalence ratios for the high exposure area is a “survivor effect”, by which people with asthma would have been more likely to move away from the areas with higher ambient H2S concentrations. To investigate this, we examined data on length of residence at current homes for people with and without previous diagnoses of asthma. Table 5 shows the mean length of residences by age group and by quartile of residential level of H2S exposure.
Table 5.
Mean length of residence (years) in current home, by age-group, quartile of residential H2S exposure, and whether ever diagnosed as having asthma, for study participants, Rotorua, New Zealand, 2008-10.
| Age group in years | Quartile of residential H2S exposure |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of participants (N) | Never diagnosed with asthma |
Ever diagnosed with asthma |
||||||||
| 1 | 2 | 3 | 4 | Total | 1 | 2 | 3 | 4 | Total | |
| 18 to <25 | 4.3 | 8.0 | 5.7 | 9.0 | 6.1 | 8.0 | 9.2 | 6.7 | 4.9 | 7.6 |
| N | 23 | 11 | 13 | 8 | 55 | 12 | 10 | 10 | 5 | 37 |
| 25 to <35 | 3.5 | 5.8 | 5.1 | 5.6 | 5.0 | 3.4 | 3.2 | 3.4 | 5.3 | 3.7 |
| N | 32 | 37 | 35 | 27 | 131 | 21 | 13 | 15 | 11 | 60 |
| 35 to <45 | 6.6 | 6.1 | 7.0 | 7.2 | 6.7 | 6.6 | 6.0 | 5.9 | 3.3 | 5.5 |
| N | 73 | 78 | 65 | 72 | 288 | 30 | 28 | 24 | 25 | 107 |
| 45 to <55 | 10.6 | 12.5 | 8.3 | 7.5 | 9.6 | 8.8 | 11.5 | 8.5 | 7.2 | 9.0 |
| N | 91 | 88 | 104 | 92 | 375 | 30 | 24 | 31 | 21 | 106 |
| 55-65 | 12.8 | 13.1 | 12.3 | 11.1 | 12.2 | 15.0 | 12.0 | 14.3 | 12.4 | 13.1 |
| N | 83 | 92 | 95 | 120 | 390 | 15 | 27 | 17 | 28 | 87 |
| Total | 9.0 | 10.1 | 8.8 | 8.7 | 9.1 | 7.9 | 8.8 | 7.9 | 7.4 | 8.0 |
| N | 302 | 306 | 312 | 319 | 1,239 | 108 | 102 | 97 | 90 | 397 |
The table shows that, as would be expected, mean length of residence increases with older age group, but there is no consistent trend with either quartile of exposure or asthma diagnosis status. Three-way analysis of variance confirmed this, showing a strong age association (P < 0.0001), a possible association with exposure quartile (P = 0.06) and no association with asthma (P = 0.43) or the interaction term of asthma diagnosis and exposure quartile (P = 0.89).
4. Discussion
This is by far the largest study of associations between ambient H2S exposures and respiratory health effects ever conducted. It has several advantages over most other epidemiologic studies of H2S-exposed populations, including: (i) an absence of other co-emitted, potentially confounding exposures, as occur with other H2S sources, such as oil and natural gas refineries, paper mills, sewage treatment plants and concentrated animal feeding operations; (ii) relatively high ambient H2S exposures that are reasonably constant over time and quantifiable; (iii) information on where people live and where they work and how long they have been at these places; and (iv) a population that, based on the authors’ experience, is generally unworried about the H2S exposures, reducing the likelihood of over-reporting of symptoms.
Studies that have examined asthma and its characteristic symptoms in relation to H2S have produced inconsistent results (Campagna et al., 2004; Carlsen et al., 2012; Jappinen et al., 1990). A previous study in Rotorua, which involved analysis of hospital discharge data, reported evidence of increasing respiratory disease risk with residence in higher H2S exposure areas (Bates et al., 2002). The relative risk estimate for combined ICD-9 codes 490-496 (chronic obstructive pulmonary disease and allied conditions) was 1.57 (95% CI: 1.32, 1.86) in the high exposure area relative to the rest of New Zealand, not including Rotorua. This grouping contains asthma (ICD-9 code 493). A later similarly conducted study in Rotorua also used hospital discharge data, finding relative risk estimates of 7.6 and 10.5 for asthma, depending on the covariates used to adjust the models (Durand and Wilson, 2006). However, this study was unable to detect or exclude multiple hospital admissions of the same patient. This could have biased relative risks upward. The earlier study (Bates et al., 2002) was able to exclude repeat admissions. There are many other possible reasons for the differences between the present study and the ecologic studies, including lack of information on possible confounding factors and workplace exposures in the ecologic studies, and possibly less complete information from private hospitals. It is likely that hospitalized cases would be more serious than those treated by family medical practitioners. Nonetheless, we would expect the distribution of more serious cases to reflect the distribution of asthma diagnoses generally.
The ambient H2S exposure levels found in this study, with an estimated mean concentration across homes and workplaces of about 20 ppb, are comparable with or higher than levels found in other studies of such exposures. In a study of communities surrounding concentrated animal feeding operations in North Carolina, at 15 out of 16 measuring sites more than 90% of H2S measurements were < 2 ppb(Wing et al., 2008); an annual average concentration of 7-27 ppb H2S was reported for a community in Texas exposed to emissions from industrial wastewater (Legator et al., 2001); in the most polluted communities exposed to odorous emissions from wood pulp mills in South Karelia, Finland, the annual average H2S concentration was 8 μg/m3 (5.3 ppb)(Jaakkola et al., 1999); and the annual mean 24-hour H2S concentration at a single monitoring station in Reykjavik, Iceland, was 7.2 μg/m3 (4.8 ppb) (Carlsen et al., 2012).
Overall, the results of the present analysis are reassuring—there is no evidence of increased asthma risk and there are some indications of asthma and symptom reduction in those who live or work in higher H2S exposure areas. The one possible exception is “woken by coughing” in relation to the time-weighted average H2S concentration (Table 3). This shows a slightly elevated prevalence ratio for the third quartile. However, the confidence interval contains the null, there is no monotonic trend, and the corresponding measure is below the null for the other exposure metric (Table 4). We think, therefore, that this result probably reflects random variation.
Although the symptoms are non-specific, there are highly statistically significant (P < 0.0001) correlation coefficients, ranging from 0.19 to 0.40, of all the respiratory symptoms examined with reporting a doctor's diagnosis of asthma. The correlation coefficients are even stronger with current asthma, ranging from 0.26 to 0.59. The correlation coefficient between doctor's diagnosis of asthma and current asthma treatment was 0.69.
There is some plausibility to the suggestions of prevalence reductions with increasing exposure, as recent animal studies have shown H2S to be an important endogenously produced signaling molecule, whose effects include smooth muscle relaxation and reduced inflammation (Calvert et al., 2010), both of which might contribute to a protective effect against asthma. It has been shown that endogenous H2S is down-regulated in an asthmatic rat model (Chen et al., 2009) and, consistent with this, serum H2S concentrations are reduced in asthma patients, and more so with severe asthma (Wu et al., 2008). It has also been shown that inhaled H2S (80 ppm) reduced ventilator-induced lung injury in mice (Faller et al., 2010). However, to our knowledge, no published epidemiologic studies, experimental or observational, have examined whether inhalation of low, ambient H2S concentrations could have a beneficial effect on asthma in exposed human populations.
There are several considerations before inferences may be drawn from the present study. First, there is the possibility of selection bias. The participation rate was 55%, raising the concern that those who refused to participate may have in some way, related to both H2S exposure and health outcome, been systematically different to those who did participate. That women and older people were generally more willing to participate is consistent with what epidemiologic studies have often found (Galea and Tracy, 2007). Other studies in New Zealand have also found Maori and Pacific Island people to have lower participation rates than people of self-reported European ethnicity (Fink et al., 2011; Mannetje et al., 2011). We have no reason to think that the lower response rates for younger people, and Maori and Pacific Island people have any particular implications for interpretation of our study. Most reassuring, however, is the complete lack of suggestion that participation was in any way differential according to the H2S exposure status of the current residence (Table 1).
It is conceivable that a “survivor effect” could have biased ORs downward in the high exposure quartile. In other words, people who have asthma may be more likely to move away from the high exposure areas. We investigated this by examining the length of residence in current homes (Table 5). This produced no evidence that people who had ever been diagnosed with asthma were resident in the higher exposure areas for a shorter time than those who had never been diagnosed with asthma. If a survivor effect were in play we would expect to see that asthmatics had a shorter residential time in the high exposure area, reflecting a faster cycling in and out of that area.
Second, the possibility of residual confounding from incomplete adjustment for confounding factors must be considered. One possibility is negative confounding by particulate matter. The area of high H2S concentrations in Rotorua runs in a North-South corridor through the center of the city, including the central business district. On either side of that corridor, particularly to the West, are residential areas in which wood-burning stoves and fireplaces are a means of heating residences in winter. Modeling studies predict high winter PM10 concentrations in these areas (Fisher et al., 2007), implying that there may be an approximately inverse relationship between concentrations of H2S and concentrations of particulate matter, at least in the winter.
Whether this inverse relationship could be confounding the observed associations with H2S depends on whether particulate matter is a risk factor for asthma or asthma symptoms. Recent reviews of the relationship between wood smoke and asthma provide, at most, weak evidence that this is the case. Of four studies of indoor air pollution from woodstoves reviewed by Belanger and Triche (Belanger and Triche, 2008), three found no association with asthma. A meta-analysis of respiratory illnesses in women and children in relation to solid fuel use obtained a summary odds ratio of 0.50 (95% CI: 0.12, 1.98) for asthma in children and 1.34 (95% CI: 0.93-1.93) for women (Po et al., 2011). Since the studies in this meta-analysis were carried out in developing countries, exposure to particulate matter is likely to have been much higher than in Rotorua, where peak PM10 concentrations will generally be less than 100 μg/m3 (Fisher et al., 2007).
Nitrogen oxides (NOx) have also been associated with asthma. The major source of NOx in Rotorua is traffic. This source has been modeled in Rotorua, showing that the peak 1-hour NOx concentration was less than 20 μg/m3, compared to the 1-hour standard of 200 μg/m3 (Fisher et al., 2007). The downtown area where traffic is highest in the city is also in the area of Rotorua with highest H2S concentrations. Therefore any confounding by NOx would be likely to inflate the prevalence ratio for the high H2S exposure quartile. As such, it could not account for the trends seen.
A limitation of our study was that we did not study areas of New Zealand outside of Rotorua, making it difficult or impossible to infer directly from our data whether the observed inverse associations of H2S with asthma and asthma symptoms would hold up if examined in the broader New Zealand context. However, a New Zealand national survey of asthma prevalence, involving nearly 26,000 respondents, carried out in 1991-93, showed that, out of the 93 parliamentary electorates at the time, Rotorua had the fifth lowest 12-month asthma prevalence in New Zealand (Lewis et al., 1997). The Rotorua prevalence was 10.3%, compared to the overall prevalence of 15.3% and a range across electorates of 5.5 to 23%. No other national surveys of asthma in adults have been carried out in New Zealand. The relatively low asthma prevalence in Rotorua is more notable when considering that the city population has a high proportion of Maori people (approximately 30% compared with an average of 15% across New Zealand). The prevalence of asthma in Maori in the national survey was 22.1% compared to 14.1% in other ethnic groups (excluding Pacific Island people who had a prevalence of 20.6%).
The third area to consider is information bias, particularly with estimates of H2S exposure levels. These were based on data from networks of passive monitors set out during two 2-week periods in 2010, extrapolated to current homes and workplaces. We know, however, from occasional spot measurements that we have made, that in some of the more highly exposed places around Rotorua H2S concentrations may, at least briefly, rise as high as 1 to 2 ppm. Also, we made assumptions about the relative amounts of time people spent both at home and at work. We expect the resulting exposure estimation errors would most likely have biased prevalence ratios towards the null and attenuated observation of any true trends. Despite these limitations, we believe that, because of the fairly constant nature of the geothermal sources, the exposure estimates in our study compare favorably with other studies, such as that of Campagna (2004), in which H2S exposure sources are likely to have been more intermittent.
Although we used self-reported doctor-diagnosed asthma and asthmatic symptoms as outcome measures, we believe they have good validity. The respiratory questions in the questionnaire were based on those in the widely used and well-validated European Community Respiratory Health Survey II questionnaire (www.ecrhs.org/Quests.htm; last accessed Dec 16, 2012).
5. Conclusions
Our study has found no evidence that H2S exposure at levels found in Rotorua is a risk factor for asthma or asthma symptoms. This is reassuring not only for the residents of Rotorua, but also for those with asthma who are exposed to lower levels of H2S in industrial or workplace situations. Our results suggest that at the ambient H2S levels found in Rotorua there may be a reduced risk of asthma and its symptoms. This has consistency with findings from some animal studies. However, more work, including studies in other settings, would be needed to confirm such an effect. It is important also to appreciate that H2S is very toxic at higher concentrations and, without appropriate supporting data from exposed populations, no conclusions should be drawn about higher concentrations. That “hot spots” of H2S can occur in Rotorua is well-known and deaths are sometimes attributed to them (Bassindale and Hosking, 2011). Irrespective of the relationship between H2S and asthma, there are other potential health outcomes from hydrogen sulfide exposure, including possible neurologic and neuropsychologic effects (Kilburn et al., 2010) and effects on the eye, particularly cataract (Bates et al., 2002). These also need to be investigated. Despite these caveats, the present study potentially opens up a fruitful area for further investigation.
Highlights.
Largest epidemiologic study of hydrogen sulfide (H2S) exposure and asthma risk.
No evidence was found for an association of H2S with increased asthma risk.
Some indication that higher H2S exposures might decrease asthma risk.
Inverse association of H2S and asthma is consistent with recent animal study results.
Possible residual confounding and selection bias limit direct inferences.
Acknowledgements
Thanks to Melinda Sando, Dr Sandra Linenberger, Fiona Paignton, Kataraina George, Nerene Lynskey and John McKeogh for data collection and management.
Assistance with participant recruitment and data management was provided by the Rotorua Area Public Health Service (RAPHS). Thanks to Kirsten Stone, David Bradford, Chris Walmsley, Jennifer Anastasi and Miranda Whitwell.
Funding sources:
This work was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health [grant number R01ES014038] and the Regional Councils Environment Bay of Plenty and Environment Waikato. The authors thank Shane Iremonger (Environment Bay of Plenty) and Nick Kim (Environment Waikato) for their assistance.
Funders played no role in study design, data collection, data analysis, interpretation of results, or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bassindale T, Hosking M. Deaths in Rotorua's geothermal hot pools: hydrogen sulphide poisoning. Forensic Sci Int. 2011;207:e28–9. doi: 10.1016/j.forsciint.2010.11.025. [DOI] [PubMed] [Google Scholar]
- Bates MN, Garrett N, Graham B, Read D. Air pollution and mortality in the Rotorua geothermal area. Aust N Z J Public Health. 1997;21:581–6. doi: 10.1111/j.1467-842x.1997.tb01759.x. [DOI] [PubMed] [Google Scholar]
- Bates MN, Garrett N, Graham B, Read D. Cancer incidence, morbidity and geothermal air pollution in Rotorua, New Zealand. Int J Epidemiol. 1998;27:10–4. doi: 10.1093/ije/27.1.10. [DOI] [PubMed] [Google Scholar]
- Bates MN, Garrett N, Shoemack P. Investigation of health effects of hydrogen sulfide from a geothermal source. Arch Environ Health. 2002;57:405–11. doi: 10.1080/00039890209601428. [DOI] [PubMed] [Google Scholar]
- Belanger K, Triche EW. Indoor combustion and asthma. Immunol Allergy Clin North Am. 2008;28:507–19. vii. doi: 10.1016/j.iac.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert JW, Coetzee WA, Lefer DJ. Novel insights into hydrogen sulfide--mediated cytoprotection. Antioxid Redox Signal. 2010;12:1203–17. doi: 10.1089/ars.2009.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagna D, Kathman SJ, Pierson R, Inserra SG, Phifer BL, Middleton DC, Zarus GM, White MC. Ambient hydrogen sulfide, total reduced sulfur, and hospital visits for respiratory diseases in northeast Nebraska, 1998-2000. J Expo Anal Environ Epidemiol. 2004;14:180–7. doi: 10.1038/sj.jea.7500313. [DOI] [PubMed] [Google Scholar]
- Carlsen HK, Zoega H, Valdimarsdottir U, Gislason T, Hrafnkelsson B. Hydrogen sulfide and particle matter levels associated with increased dispensing of anti-asthma drugs in Iceland's capital. Environ Res. 2012 doi: 10.1016/j.envres.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Chen YH, Wu R, Geng B, Qi YF, Wang PP, Yao WZ, Tang CS. Endogenous hydrogen sulfide reduces airway inflammation and remodeling in a rat model of asthma. Cytokine. 2009;45:117–23. doi: 10.1016/j.cyto.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Di Giampaolo L, Quecchia C, Schiavone C, Cavallucci E, Renzetti A, Braga M, Di Gioacchino M. Environmental pollution and asthma. Int J Immunopathol Pharmacol. 2011;24:31S–38S. [PubMed] [Google Scholar]
- Durand M, Wilson JG. Spatial analysis of respiratory disease on an urbanized geothermal field. Environ Res. 2006;101:238–45. doi: 10.1016/j.envres.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Faller S, Ryter SW, Choi AM, Loop T, Schmidt R, Hoetzel A. Inhaled hydrogen sulfide protects against ventilator-induced lung injury. Anesthesiology. 2010;113:104–15. doi: 10.1097/ALN.0b013e3181de7107. [DOI] [PubMed] [Google Scholar]
- Fink JW, Paine SJ, Gander PH, Harris RB, Purdie G. Changing response rates from Maori and non-Maori in national sleep health surveys. N Z Med J. 2011;124:52–63. [PubMed] [Google Scholar]
- Fisher G, Thornton D, Godfrey J. Rotorua Airshed Modelling Investigation: Final Report. Endpoint Ltd.; Auckland, New Zealand: 2007. [Google Scholar]
- Fuller DC, Suruda AJ. Occupationally related hydrogen sulfide deaths in the United States from 1984 to 1994. J Occup Environ Med. 2000;42:939–42. doi: 10.1097/00043764-200009000-00019. [DOI] [PubMed] [Google Scholar]
- Galea S, Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol. 2007;17:643–53. doi: 10.1016/j.annepidem.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Hendrickson RG, Chang A, Hamilton RJ. Co-worker fatalities from hydrogen sulfide. Am J Ind Med. 2004;45:346–50. doi: 10.1002/ajim.10355. [DOI] [PubMed] [Google Scholar]
- Horwell C, Patterson J, Gamble J, Allen A. Monitoring and mapping of hydrogen sulphide emissions across an active geothermal field: Rotorua, New Zealand. Journal of Volcanology and Geothermal Research. 2005;139:259–269. [Google Scholar]
- IPCS . Hydrogen Sulfide. Vol. 19. World Health Organization; Geneva: 1981. [Google Scholar]
- Jaakkola JJ, Partti-Pellinen K, Marttila O, Miettinen P, Vilkka V, Haahtela T. The South Karelia Air Pollution Study: changes in respiratory health in relation to emission reduction of malodorous sulfur compounds from pulp mills. Arch Environ Health. 1999;54:254–63. doi: 10.1080/00039899909602483. [DOI] [PubMed] [Google Scholar]
- Jappinen P, Vilkka V, Marttila O, Haahtela T. Exposure to hydrogen sulphide and respiratory function. Br J Ind Med. 1990;47:824–8. doi: 10.1136/oem.47.12.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilburn KH, Thrasher JD, Gray MR. Low-level hydrogen sulfide and central nervous system dysfunction. Toxicol Ind Health. 2010;26:387–405. doi: 10.1177/0748233710369126. [DOI] [PubMed] [Google Scholar]
- King AL, Lefer DJ. Cytoprotective actions of hydrogen sulfide in ischaemia-reperfusion injury. Exp Physiol. 2011;96:840–6. doi: 10.1113/expphysiol.2011.059725. [DOI] [PubMed] [Google Scholar]
- Legator MS, Singleton CR, Morris DL, Philips DL. Health effects from chronic low-level exposure to hydrogen sulfide. Arch Environ Health. 2001;56:123–31. doi: 10.1080/00039890109604063. [DOI] [PubMed] [Google Scholar]
- Lewis S, Hales S, Slater T, Pearce N, Crane J, Beasley R. Geographical variation in the prevalence of asthma symptoms in New Zealand. N Z Med J. 1997;110:286–9. [PubMed] [Google Scholar]
- Mannetje A, Eng A, Douwes J, Ellison-Loschmann L, McLean D, Pearce N. Determinants of non-response in an occupational exposure and health survey in New Zealand. Aust N Z J Public Health. 2011;35:256–63. doi: 10.1111/j.1753-6405.2011.00703.x. [DOI] [PubMed] [Google Scholar]
- Olson KR, Donald JA. Nervous control of circulation--the role of gasotransmitters, NO, CO, and H2S. Acta Histochem. 2009;111:244–56. doi: 10.1016/j.acthis.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Po JY, FitzGerald JM, Carlsten C. Respiratory disease associated with solid biomass fuel exposure in rural women and children: systematic review and meta-analysis. Thorax. 2011;66:232–9. doi: 10.1136/thx.2010.147884. [DOI] [PubMed] [Google Scholar]
- Schiffman SS, Williams CM. Science of odor as a potential health issue. J Environ Qual. 2005;34:129–38. [PubMed] [Google Scholar]
- Schinasi L, Horton RA, Guidry VT, Wing S, Marshall SW, Morland KB. Air pollution, lung function, and physical symptoms in communities near concentrated Swine feeding operations. Epidemiology. 2011;22:208–15. doi: 10.1097/EDE.0b013e3182093c8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner F, Asfar P, Calzia E, Radermacher P, Szabo C. Bench-to-bedside review: Hydrogen sulfide--the third gaseous transmitter: applications for critical care. Crit Care. 2009;13:213. doi: 10.1186/cc7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman M, Le Trionnaire S, Chopra M, Fox B, Whatmore J. Emerging role of hydrogen sulfide in health and disease: critical appraisal of biomarkers and pharmacological tools. Clin Sci (Lond) 2011;121:459–88. doi: 10.1042/CS20110267. [DOI] [PubMed] [Google Scholar]
- Wing S, Horton RA, Marshall SW, Thu K, Tajik M, Schinasi L, Schiffman SS. Air pollution and odor in communities near industrial swine operations. Environ Health Perspect. 2008;116:1362–8. doi: 10.1289/ehp.11250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R, Yao WZ, Chen YH, Geng B, Tang CS. [Plasma level of endogenous hydrogen sulfide in patients with acute asthma]. Beijing Da Xue Xue Bao. 2008;40:505–8. [PubMed] [Google Scholar]