Abstract
Depression is one of the widespread diseases whose etiology is still unclear. Dysregulation of the hypothalamic–pituitary–adrenal axis can be the cause of this illness which is concomitant with a high level of cortisol. For this reason, the purpose of the study was to estimate the influence of the selective serotonin reuptake inhibitors (SSRIs) therapy used in monotherapy and polypragmasy on cortisol level in saliva of depressed women. Cortisol was determined in saliva collected from 40 depressed patients treated with SSRIs. HPLC with UV detection was used for quantification of cortisol after its extraction with dichloromethane. For statistical evaluation of the data, the cluster analysis and principal components analysis were used. Results of the study have shown that the SSRIs treatment reduces the cortisol level in saliva. The therapy with sertraline and polypragmasy had a strong influence on suppressing the cortisol secretion. Besides, the amplitude of changes of the cortisol level during the treatment had an impact on the duration of hospitalization. In conclusion, it can be stet that the process of reduction of the cortisol level is multiphasic and that the combination treatment had a stronger influence on suppressing the cortisol secretion than did antidepressants used in monotherapy.
Keywords: Saliva, Cortisol, Selective serotonin reuptake inhibitors, HPLC, Major depressive disorder
Introduction
Cortisol is one of the most important steroid hormones produced by the adrenal cortex, which controls the majority of body metabolism. An increase in its level is concomitant in many diseases, such as depression, which according to WHO (2012), ranks fourth among common diseases in the world. It is suggested that depression appears predominantly in women population, and it can be caused by hormonal changes in females’ organisms. Relation between depression and hormonal changes is evident during pregnancy and postpartum. It is shown that postpartum period is the time of greatest risk for development of major depressive disorder (MDD), and more than 15 % young mothers are susceptible to depression (Brummelte and Galea 2010).
The pathophysiology of the depression is unknown, but there are some theories about it. The oldest one is based on the role played by catecholamines, especially noradrenaline and serotonin. The etiology of this theory is closely related to first drugs, imipramine and iproniazid, used in the treatment of depression. Nowadays, there are few groups of antidepressant drugs. They can be classified based on their influence on neurotransmitters. The most popular group of antidepressants encompasses selective serotonin reuptake inhibitors (SSRIs) which proved effective in the treatment of MDD and also of obsessive compulsive disorder as well as some other anxiety disorders (Humble 2000).
Several studies have revealed a close relation between MDD and dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis, such as a high cortisol level, non-suppression of cortisol release by dexamethasone, or abnormal 24-h cortisol secretion reported in patients with MDD (Plotsky et al. 1998; Schüle 2006). On the other hand, the HPA axis dysfunction can also be manifested as a mild hypocortisolism during chronic fatigue syndrome (CFS). In both cases, MDD and CFS, treatment with psychotropic medications can correct HPA axis dysfunction (Papadopoulos et al. 2009, 2011).
The administration of antidepressants which potentiate the serotonin (5HT) and noradrenaline reuptake potentiates secretion of the cortisol in healthy subjects as well. This stimulation of the HPA activity can be caused by administration of SSRIs, especially of citalopram and escitalopram. The endocrine responses to administration of these drugs are probably due to modulation of the serotonergic system and the HPA axis by way of their effect on the sensitivity of either the 5HT1A autoreceptor or the 5HT2 receptor (Attenburrow et al. 2001; Harmer et al. 2003; Mondelli et al. 2006).
The desensitization of the presynaptic 5HT1A receptor by citalopram and escitalopram is one of the hypotheses of the antidepressant action (Sargent et al. 1998; Navinés et al. 2007; Papakostas et al. 2010). Moreover, some studies have also shown that antidepressant treatment might normalize activity function of the HPA axis by increasing the expression of glucocorticoid receptors and in this way enhancing HPA feedback mechanism (Pariante and Miller 2001; Okugawa et al. 1999).
In the majority of papers, the effects of antidepressant drugs on the cortisol secretion have been studied mainly by monitoring its level at the beginning and the end of the therapy (Harmer et al. 2003; Navinés et al. 2007; Papakostas et al. 2010; Aihara et al. 2007), but only in few papers there is information that the cortisol level was repeatedly determined during the therapy (Mück-Šeler et al. 2002; Rota et al. 2005). The literature data have also shown that the treatment of depression with SSRIs, such as citalopram (Mondelli et al. 2006; Nikisch et al. 2005), paroxetine (Mück-Šeler et al. 2002), and fluvoxamine (Aihara et al. 2007), mostly resulted in the reduction of cortisol secretion. On the other hand, in some studies changes in cortisol levels following the administration of SSRIs, such as sertraline, but also citalopram, escitalopram, and paroxetine, have not been noticed at all (Papakostas et al. 2010; Tucker et al. 2004a, b).
In summary, there is a paucity of unequivocal information about the influence of the treatment of depression with SSRIs on cortisol secretion. The purpose of the present study was to learn whether or not the response to the treatment of depression is reflected by changes in saliva cortisol level during the SSRIs therapy. The purpose was realised by the day-by-day determination of the cortisol level in saliva of the depressed women and by using pattern recognition methods, cluster analysis (CA) and principal components analysis (PCA), for interpretation of the results (Hill and Lewicki 2006; Otto 1999).
Methods
Participants
Subjects with MDD defined according to the International Classification of Diseases (ICD-10) (http://apps.who.int/classifications/apps/icd/icd10online/) criteria were recruited at the Hospital for Nervous and Mental Diseases in Starogard Gdanski (Poland). The diagnoses were made by a psychiatrist using the clinical interview. All the subjects were informed in detail about the purpose of the study and gave their written consent to participate in it, and they were informed that they could discontinue the course at any time, if desired.
Some of the subjects were excluded if they were unable to understand the nature of the study after discussion with research nurse, if their medical condition precluded administration of SSRIs or if they had serious health problems, such as adrenal function disorders. Some other subjects were also excluded because of pregnancy and breastfeeding and if their participation in the course could be detrimental to their well-being.
Finally, 40 patients aged 26–69 with MDD satisfying inclusion criteria were entered into the study and enrolled in medication treatment. Table 1 summarizes the demographic and clinical characterization of the subjects and a detailed information on the antidepressants used and the doses of drugs. The study had been approved by the ethical committee of the Medical University of Gdansk, Poland.
Table 1.
Characteristics of the patients, the antidepressants used, and their influence on the cortisol level in saliva
| No. | Age of subjects (years) | Multiplicity of hospitalization | Antidepressants used (dose) | Time of hospitalization (days) | Cortisol concentration in saliva (ng/ml) | Highest and lowest cortisol concentration in successive periods of hospitalization | |||
|---|---|---|---|---|---|---|---|---|---|
| Initial | Final | 0–30 % | 31–60 % | 61–90 % | |||||
| 1 | 63 | 2 | Sertraline (50 mg) | 31 | 190.00 | 65.00 | 6.25–190.00 | 0.75–55.00 | 1.25–65.00 |
| 2 | 38 | 1 | Paroxetine (20 mg) | 29 | 47.50 | 195.00 | 17.50–47.50 | 9.50–35.00 | 3.75–195.00 |
| 3 | 55 | 1 | Sertraline (50 mg) | 24 | 45.00 | 8.75 | 5.00–45.00 | 2.50–7.50 | 2.50–8,75 |
| 4 | 36 | 1 | Sertraline (50 mg) | 31 | 23.50 | 6.75 | 3.75–23.50 | 4.25–17.50 | 2.25–6.94 |
| 5 | 38 | 3 | Sertraline (50 mg) | 84 | 4.62 | 3.25 | 3.75–10.87 | 3.25–12.37 | 1.25–45.00 |
| 6 | 45 | 4 | Citalopram (40 mg) | 41 | 22.45 | 0.62 | 2.50–22.45 | 1.50–18.50 | 0.62–8.75 |
| 7 | 48 | 5 | Escitalopram (20 mg) | 49 | 98.50 | 3.75 | 1.25–98.50 | 1.25–10.75 | 10.12–1.25 |
| 8 | 51 | 1 | CT | 52 | 6.25 | 3.75 | 1.25–6.25 | 1.25–6.25 | 2.50–3.75 |
| 9 | 54 | 3 | Citalopram (40 mg) | 39 | 88.75 | 5.00 | 2.50–88.75 | 1.25–15.00 | 2.50–70.00 |
| 10 | 59 | 1 | CT | 61 | 16.25 | 3.75 | 2.87–22.50 | 1.25–10.00 | 1.25–3.75 |
| 11 | 56 | 1 | Sertraline (50 mg) | 78 | 15.75 | 4.75 | 1.25–15.75 | 1.75–42.00 | 2.00–7.62 |
| 12 | 53 | 2 | CT | 49 | 45.75 | 2.37 | 2.50–45.75 | 1.75–4.25 | 2.12–9.00 |
| 13 | 58 | 2 | Citalopram (40 mg) | 72 | 82.24 | 15.00 | 2.50–28.75 | 1.25–48.75 | 1.25–15.00 |
| 14 | 48 | 7 | CT | 36 | 31.25 | 2.75 | 2.50–31.25 | 3.12–5.25 | 2.75–7.87 |
| 15 | 51 | 1 | Escitalopram (20 mg) | 18 | 21.75 | 8.00 | 19.70–21.75 | 9.00–14.50 | 5.50–10.00 |
| 16 | 55 | 2 | Escitalopram (20 mg) | 34 | 21.25 | 2.50 | 3.25–21.25 | 2.37–14.50 | 2.50–12.25 |
| 17 | 52 | 2 | Escitalopram (20 mg) | 34 | 23.25 | 7.12 | 10.75–23.25 | 6.12–20.00 | 2.87–8.00 |
| 18 | 51 | 1 | Citalopram (40 mg) | 32 | 42.75 | 16.25 | 26.25–42.75 | 8.00–28.75 | 6.75–16.25 |
| 19 | 36 | 5 | Paroxetine (20 mg) | 28 | 89.00 | 2.87 | 6.00–95.00 | 4.62–12.62 | 2.50–10.00 |
| 20 | 31 | 4 | Fluvoxamine (100 mg) | 14 | 49.75 | 9.75 | 11.25–49.75 | 7.34–45.00 | 9.75–16.62 |
| 21 | 47 | 4 | Fluvoxamine (100 mg) | 33 | 31.50 | 10.00 | 5.25–31.50 | 3.12–26.25 | 6.87–16.25 |
| 22 | 47 | 5 | Fluvoxamine (100 mg) | 29 | 49.37 | 42.50 | 6.62–49.75 | 2.75–18.75 | 5.62–42.50 |
| 23 | 42 | 2 | CT | 11 | 46.25 | 9.00 | 7.12–46.25 | 7.50–16.12 | 1.75–9.00 |
| 24 | 59 | 1 | CT | 52 | 95.00 | 11.37 | 4.87–95.00 | 4.75–32.50 | 2.50–11.37 |
| 25 | 39 | 1 | Escitalopram (20 mg) | 58 | 72.52 | 2.50 | 3.75–72.52 | 1.25–13.12 | 1.25–58.75 |
| 26 | 46 | 1 | CT | 46 | 39.00 | 10.75 | 6.75–39.00 | 3.25–13.75 | 4.00–23.75 |
| 27 | 69 | 2 | CT | 8 | 61.00 | 11.75 | 20.00–61.00 | 10.62–46.25 | 6.12–11.75 |
| 28 | 44 | 3 | Sertraline (50 mg) | 32 | 13.75 | 156.25 | 3.00–21.25 | 0.25–31.87 | 0.30–156.25 |
| 29 | 49 | 5 | Sertraline (50 mg) | 82 | 69.50 | 75.00 | 1.25–69.50 | 2.50–51.25 | 1.25–75.00 |
| 30 | 53 | 2 | Citalopram (40 mg) | 79 | 34.25 | 2.50 | 1.25–34.25 | 2.50–16.00 | 1.87–12.12 |
| 31 | 26 | 1 | Sertraline (50 mg) | 12 | 8.25 | 0.25 | 2.50–8.25 | 3.12–10.00 | 0.25–10.00 |
| 32 | 48 | 3 | Citalopram (40 mg) | 29 | 93.25 | 3.87 | 15.00–93.25 | 3.87–33.75 | 1.25–10.00 |
| 33 | 58 | 3 | Citalopram (40 mg) | 40 | 52.50 | 5.00 | 3.75–52.00 | 2.50–49.50 | 4.37–5.00 |
| 34 | 59 | 12 | CT | 76 | 72.00 | 5.50 | 3.75–72.00 | 3.00–31.25 | 2.62–11.75 |
| 35 | 60 | 6 | Fluoxetine (20 mg) | 31 | 2.50 | 3.75 | 2.50–3.75 | 2.50–3.75 | 2.50–3.75 |
| 36 | 66 | 2 | Sertraline (50 mg) | 76 | 10.00 | 2.50 | 2.25–19.62 | 3.25–18.62 | 2.37–27.12 |
| 37 | 31 | 1 | CT | 21 | 42.00 | 5.62 | 8.00–42.00 | 1.37–27.5 | 2.50–17.50 |
| 38 | 53 | 6 | CT | 47 | 44.80 | 2.37 | 5.50–44.80 | 3.00–17.87 | 2.37–12.12 |
| 39 | 44 | 2 | Sertraline (50 mg) | 25 | 14.00 | 2.62 | 8.25–14.00 | 2.00–13.75 | 2.50–5.75 |
| 40 | 53 | 1 | CT | 29 | 13.50 | 2.00 | 3.37–13.50 | 3.37–11.25 | 2.00–12.25 |
CT combination therapy (SSRIs in polypragmasy with venlafaxine 75 mg, trazodone 300 mg, or mianserin 60 mg)
Hormone assay
The salivary samples for determination of cortisol levels were collected from depressed women every day during the whole hospitalization period, including the first day. The samples were collected into plastic tubes without any stimulation at 10 a.m. and frozen. The subjects were instructed to rinse their mouths with water and not to eat or drink 30 min before the samples were collected. A control group for evaluation of the cortisol concentration consisted of ten healthy women. They were recruited from students, who were free from psychotropic medication. The mean age of volunteers was 23.1 (±1.7), the mean weight was 54.3 kg (±6.0), and the mean BMI was 21.5 (±1.8). The sampling was carried out according to the same procedure as that for the depressed subjects.
A new procedure has been elaborated for the determination of the cortisol level in saliva (Dziurkowska and Wesolowski 2009). HPLC with UV detection at 240 nm was used to quantify cortisol. The saliva samples obtained from the depressed patients were refined by extraction with dichloromethane. An acetonitrile/water (30:70 v/v) mixture as a mobile phase and a chromatographic column with reverse-phase C18 packing material as a stationary phase were used. Carbamazepine was used as an internal standard for calibration. The calibration curve was constructed by plotting the ratio of peak height of cortisol to peak height of internal standard vs. the cortisol concentration in saliva.
The stability of cortisol was checked by three freezing and thawing cycles. The efficiency of extraction for two concentrations (15 and 125 ng/mL) was checked, and the recovery exceeded 94 % in both cases. Validation of the method has shown that the coefficient of variation for the intra-assay study varied from 1.1 to 6.5 %, and for the inter-assay study, it did not exceed 6.8 %. The recovery fell in the range 93.6 to 100.8 %.
Statistical methods
Two chemometric techniques, CA and PCA, were used for data analysis (Hill and Lewicki 2006; Otto 1999). PCA involved a mathematical procedure that transformed the number of possibly correlated variables into a smaller number of orthogonal variables, called the principal components (PCs), with the minimum loss of original information. A two-dimensional score plot of PC1 vs. PC2 enabled classification of the studied subjects. On the other hand, CA measured similarities or dissimilarities between the subjects and classified these into groups. The distances between groups of the subjects were calculated using Ward’s method and the Euclidean distance.
In both cases (CA and PCA), the same matrix with 22 variables (age, hospitalization period and its multiplicity, initial and final cortisol levels, the highest and lowest cortisol levels and difference between them, medium concentration, median of concentration, stabilization of cortisol secretion at 8, 10, 15 ng/mL during the whole hospitalization period and at different hospitalization phases) characterizing all the studied subjects was used. Calculations were accomplished using the Statistica 7.1 package (StatSoft, Cracow, Poland).
Results
The results of the study are compiled in Table 1 and presented graphically in Fig. 1. As seen, 40 patients with mean age of 49.3 ± 9.7 were enrolled in the study. As many as 28 patients received SSRIs in the monotherapy (MT) and 12 in the combination therapy (CT). All subjects completed the study with a mean duration of hospitalization of 41.3 ± 21.1 days and the mean multiplicity of 2.8 ± 2.1. Mean initial and final cortisol levels in saliva were 45.76 ± 36.37 and 18.30 ± 39.99 ng/mL, respectively.
Fig. 1.

Changes of cortisol concentration in saliva of patients treated with: a sertraline (patient no. 39), b escitalopram (patient no. 25), c citalopram (patient no. 30), d CT, sertraline with trazodone (patient no. 10)
Based on the performed studies, it can be stated that in the case of:
Patient’s organism responded to treatment, the cortisol level dropped very quickly to attain the reference value of 1–8 ng/mL and did not increase up to the end of hospitalization (Fig. 1a). The difference between cortisol levels as determined day-by-day did not exceed few nanograms per milliliter. Fifteen patients treated with SSRIs had just that shape of the profile of cortisol secretion. It was independent of the course of the antidepressant therapy;
Patients treated with SSRIs in the MT course, who stayed at the hospital for more than 30 days, an increase in the secretion of cortisol on about 30th day of the therapy was noticed. After the next few days, the level of the hormone declined (Fig. 1b). In the majority of subjects, the cortisol level on those days was lower than the initial one. This shape of the profile of cortisol secretion was recorded in nine patients. In these cases, the amplitude of the cortisol level, as determined day-by-day, was large, especially at the beginning of the hospitalization. Moreover, the difference between the cortisol levels determined day-by-day fell in the range of several or several dozen nanograms per milliliter;
Patients with a large fluctuation of the cortisol level, the hospitalization period of those patients was longer, and they had already been repeatedly hospitalized because of the depression (Fig. 1c). The patients treated with citalopram and escitalopram represented this group in particular. When the CT-treated patients were excluded, in spite of prolonged hospitalization, the amplitude of cortisol level did not exceed 10 ng/mL;
Patients who underwent combined treatment and their hospitalization period was longer than 30 days, the secondary rise in the cortisol level was missing (Fig. 1d);
The therapy with fluvoxamine, the cortisol level attained stabilization at a characteristic for the patient level in response to the treatment;
Almost all of the patients, the final cortisol level was lower than that at the beginning of the therapy.
To verify these results, they were examined using cluster analysis and principal component analysis. A CA dendrogram presented in Fig. 2a shows that 22 variables characterizing the patients were grouped into three clusters at a level of 1/3 of the maximum distance. In this way, the results of CA calculation allowed to find a strong correlation between stabilization of the cortisol secretion at different levels during the whole hospitalization period and at different phases of the treatment, and also between the hospitalization period and the age of the patient. These variables formed cluster I. The strong correlations among the multiplicity of hospitalization, the lowest, medium, and final cortisol levels, and median of concentration were found. These variables formed cluster II. Furthermore, cluster III was formed by three other variables: initial cortisol level at the beginning of hospitalization, the highest level during the hospitalization, and the difference between the highest and the lowest cortisol levels. These variables are grouped with those contained in cluster II at a level of 1/2 of the maximum distance.
Fig. 2.

Dendrograms illustrating clustering of 22 variables (a) and 40 patients (b), based on the data on Table 1
As shown in Fig. 2b, all the patients were grouped into separate clusters based on variations of the amplitude of the cortisol level in saliva. At a level of 1/3 of the maximum distance there are four clusters. The first cluster (Ia) encompasses patients with a small amplitude in the hormone level, a few nanograms per milliliter, matching that of the references values. The majority of these subjects were hospitalized for the first time. This cluster represents patients with cortisol changes profiles shown in Fig. 1a, d. The opposite group IV is formed by subjects with very high initial or final concentrations of cortisol, above 140 ng/mL. In an exceptional case, the level of the cortisol secretion was very high, and there was no stabilization of the hormone secretion during the whole time of hospitalization. This cluster encompassed patients with a cortisol changes profile represented in Fig. 2b.
Clusters Ib–III are formed by those subjects who had some periods of suppression of the cortisol secretion during the hospitalization, and they were clustered in accordance with a period of time over which the depressed secretion of the cortisol has been noticed. Cluster Ib grouped patients with an enhanced fluctuation of cortisol secretion, in particular at the beginning of therapy. Cluster II was formed by patients with a very short time of stabilization of the cortisol secretion. This group encompasses the subjects whose secretion of the cortisol was higher than in the healthy ones, but attained stabilization at the characteristic level in response to the treatment. Almost all of the subjects were hospitalized because of the depression more than twice. As shown in Fig. 1c, patients with a large amplitude of cortisol level, especially at the beginning of therapy and at its end, formed cluster III. The mean hospitalization period in this group was 53.9 days.
The results of PCA calculations are presented in Fig. 3a, b as two-dimensional plots of PC1 vs. PC2. The first two principal components occupied together 66.5 % of the variance. This multivariate approach demonstrates that the variable with the most significant impact on the classification of the subjects according to the PC1 axis was the hospitalization period, at which the cortisol level was similar to the reference value. The medium concentration and median of concentration were important as well. According to the PC2 axis, the highest and the lowest cortisol levels and the difference between them were the most important factors for classification of the patients.
Fig. 3.
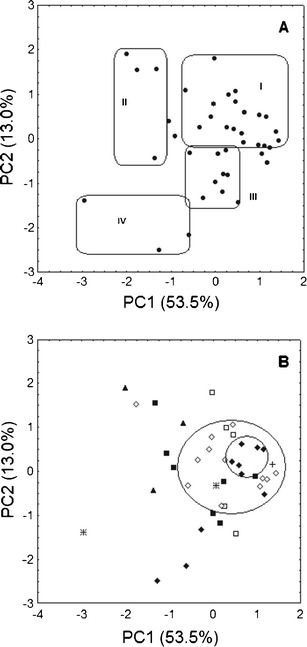
PCA score plots for the first two principal components showing the grouping of the patients mainly due to the hospitalization period (a) and the therapy used (b). Antidepressants used in the therapy: black diamond sertraline, white diamond CT, black square citalopram, white square escitalopram, asterisk paroxetine, plus sign fluoxetine, black triangle fluvoxamine
As shown in Fig. 3a, PCA confirms the results obtained by CA. Group I was formed by patients with a slightly elevated cortisol level and with small fluctuation or normal secretion of the hormone. Patients who were repeatedly hospitalized due to depression and had a fluctuating cortisol level with a short period of stabilization of the cortisol secretion fall in cluster II. Moreover, cluster III contains patients with a large amplitude of cortisol secretion, especially at the beginning and at the end of the therapy, whereas cluster IV is formed by the same patients as those grouped in cluster IV in the CA dendrogram (Fig. 2b).
PCA has also shown that patients can be grouped according to the antidepressants used for the treatment of depression. As shown in Fig. 3b, patients treated with sertraline and undergoing the combined treatment formed characteristic concentrations. The subjects were characterized by a smaller fluctuation of the cortisol level during the hospitalization than those with normal secretion of cortisol. These patients fall in cluster I in Fig. 3a. These patients also formed cluster I in the CA dendrogram, which contains 23 patients, 16 of them were treated with sertraline and CT. The profiles of cortisol changes in these patients are shown in Fig. 1a, d.
Discussion
An increase in cortisol secretion, as a result of dysregulation of the HPA axis, can be one of the symptoms of depression. The literature data have also shown that cortisol can be one of the direct factors involved in the pathogenesis of depression (Plotsky et al. 1998; Schüle 2006). Following these information, the influence of SSRIs used in the MT and CT on cortisol secretion which is reflected by its concentration in saliva collected from patients hospitalized because of the depression was examined.
Cortisol is secreted in diurnal cycle. The highest level of this hormone in the blood is observed at about 8 a.m. and falls down during the day. Hence, the cortisol concentration is the lowest in the evening. Taking into account that in a healthy adult only 1 % of the cortisol is excreted with urine and saliva (Chrousos 2011), the highest cortisol level in saliva occurs between 9 and 10 a.m. For this reason, it was decided that the sampling of the patients’ saliva will take place at 10 a.m.
To reduce the possibility that anxiety related to the blood sampling might affect cortisol level, the saliva was chosen as the diagnostic material. Moreover, in the case of depression, apathy of the patients could remarkably influence collaboration between patient and the research nurse. According to the literature data, there is a strong correlation between the cortisol level in the blood and in saliva (Baghai et al. 2002; Bhagwagar et al. 2002; Lilliecreutz et al. 2011). In these diagnostic materials, cortisol can be quantified by using either non-separation methods, such as radioimmunoassay (Tucker et al. 2004a; Rota et al. 2005) and enzyme immunoassay (Masharani et al. 2005) or separation methods, such as liquid chromatography with tandem mass spectrometry (Gröschl and Rauh 2006). The non-separation methods are quite frequently used owing to their rapidity; however, sometimes a liquid–liquid extraction before the analysis is preferable. Furthermore, the limits of detection and quantification are overall lower than those obtained for separation methods. On the other hand, the separation methods are more accurate due to lack of cross-reactions. The cross-reactions are crucial for steroids quantitation, but they are undesirable because the same antibody can react with steroid hormones having similar structure, and in consequence, the result of determination becomes overestimated. For these reasons, the separation method based on HPLC with UV detection was used for cortisol quantitation in saliva (Dziurkowska and Wesolowski 2009) because it prevented even a slight overstatement of the analysis result.
A literature survey shows that in most cases, cortisol was determined before and after the therapy. In the majority of studies, the decreasing cortisol secretion was reported as an effect of the SSRIs therapy (Mondelli et al. 2006; Aihara et al. 2007; Rota et al. 2005; Nikisch et al. 2005), as has also been found in this study. In most cases, the drop in cortisol level occurred during the antidepressant treatment, independently of the kind of the applied SSRI drug. In some cases, the reference values of cortisol level (1–8 ng/mL) were not attained, but the amplitude of the cortisol fluctuation determined day-by-day was smaller. This reflected a positive reaction of the subject’s organism to the antidepressant therapy and contributed to shortening of hospitalization.
The results of the investigation have shown that in the case of patients who were hospitalized for more than 30 days, a secondary rise in the cortisol secretion occurred on about the 30th day of the therapy. However, the cortisol level found on those days was lower than the initial one and after next few days it was declining. Moreover, in the case of citalopram treatment, after the secondary increase in cortisol secretion, the fluctuation of the hormone level became larger. Patients treated with CT (SSRIs in polypragmasy with venlafaxine, trazodone, or mianserin) were the exceptions. In spite of the prolonged hospitalization, the secondary rise in the cortisol level was missing.
According to the literature data, the cortisol level was determined only before and after the therapy, and its fluctuation during the treatment was not monitored. Only a few papers report that the cortisol level during the therapy was also determined (Mück-Šeler et al. 2002; Rota et al. 2005). This study has shown that the frequency of determination of the cortisol concentration is crucial because even a several-day interval in the assays can make that impact of the therapy on the cortisol secretion and the amplitude of the hormone level could not be noticed. The day-by-day determination of the level is important especially in the case of the therapy with a drug which may modulate the activity of 5HT receptors, e.g., citalopram (Papakostas et al. 2010; Tucker et al. 2004a, b). Otherwise, the actual changes of the cortisol level during the therapy could not be found, as has also been confirmed in the literature (Rota et al. 2005).
The performed study on the influence of SSRIs used in the MT and CT on the cortisol secretion has also shown that the antidepressant therapy with SSRIs reduces the symptoms of depression and suppresses cortisol secretion. Furthermore, patients treated with CT achieved a quicker normalization of the cortisol secretion, as has also been reported in other studies. However, CT has been found to prevent the occurrence of an enhanced night salivary cortisol (Yang et al. 2009). Besides, the study confirmed that CT has a significant influence on the cortisol secretion, as shown by PCA and CA. The majority of patients treated with CT were grouped in cluster I, which contains patients whose cortisol secretion and the amplitude of the hormone level decreased very quickly.
CA and PCA also demonstrated that sertraline used in the MT and CT, and escitalopram and citalopram used in CT, are the most effective drugs in the reduction of cortisol secretion during the treatment. Moreover, it was found that among patients good reacting to the therapy with antidepressants, independently of the mechanism of action of these medicines, the recovery was quick. It was manifested by a decrease in the cortisol concentration and a short period of hospitalization, which did not exceed 20 days.
In conclusion, this study enabled monitoring of the changes in cortisol level in saliva collected from hospitalized depressed patients treated with SSRIs in the MT and CT. The cortisol level was determined every morning during the whole period of hospitalization. Based on the results and their interpretation with the aid of multivariate statistical analysis, it can be stated that SSRIs therapy suppresses the cortisol secretion, but the process is complex and multiphasic. The amplitude of cortisol secretion determined day-by-day is the most important factor that has an impact on hospitalization period of depressed patients. The study has also demonstrated that CT, in particular the polypragmasy with sertraline and trazodone, has a stronger impact on suppressing the cortisol secretion than do antidepressants used in MT.
References
- Aihara M, Ida I, Yuuki N, Oshima A, Kumano H, Takahashi K, Fukuda M, Oriuchi N, Endo K, Matsuda H, Mikuni M. HPA axis dysfunction in unmedicated major depressive disorder and its normalization by pharmacotherapy correlates with alteration of neural activity in prefrontal cortex and limbic/paralimbic regions. Psychiatry Res. 2007;155:245–256. doi: 10.1016/j.pscychresns.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Attenburrow M-J, Mitter PR, Whale R, Terao T, Cowen PJ. Low-dose citalopram as a 5-HT neuroendocrine probe. Psychopharmacology. 2001;155:323–326. doi: 10.1007/s002130100729. [DOI] [PubMed] [Google Scholar]
- Baghai TC, Schule C, Zwanzger P, Minov C, Holme C, Padberg F, Bidlingmaier M, Strasburger CJ, Rupprecht R. Evaluation of a salivary based combined dexamethasone/CRH test in patients with major depression. Psychoneuroendocrinology. 2002;27:385–399. doi: 10.1016/S0306-4530(01)00060-9. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Hafizi S, Cowen PJ. Acute citalopram administration produces correlated increases in plasma and salivary cortisol. Psychopharmacology. 2002;163:118–120. doi: 10.1007/s00213-002-1149-4. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Galea LAM. Depression during pregnancy and postpartum: contribution of stress and ovarian hormones. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:766–776. doi: 10.1016/j.pnpbp.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Adrenocorticosteroids & adrenocortical antagonists. In: Katzung BG, Masters SB, Trevor AJ, editors. Basic and clinical pharmacology. 11. New York: McGraw-Hill; 2011. [Google Scholar]
- Dziurkowska E, Wesolowski M. Evaluation of two techniques for extraction of cortisol from human saliva. Chromatographia. 2009;70:769–774. doi: 10.1365/s10337-009-1239-0. [DOI] [Google Scholar]
- Gröschl M, Rauh M. Influence of commercial collection devices for saliva on the reliability of salivary steroids analysis. Steroids. 2006;71:1097–1100. doi: 10.1016/j.steroids.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Bhagwagar Z, Shelley N, Cowen PJ. Contrasting effects of citalopram and reboxetine on waking salivary cortisol. Psychopharmacology. 2003;167:112–114. doi: 10.1007/s00213-003-1417-y. [DOI] [PubMed] [Google Scholar]
- Hill T, Lewicki P. Statistic, methods and applications: a comprehensive reference for science, industry, and data mining. Tulsa: StatSoft, Inc.; 2006. [Google Scholar]
- Humble M. Noradrenaline and serotonin reuptake inhibition as clinical principles: a review of antidepressant efficacy. Acta Psychiatr Scand. 2000;101:28–36. doi: 10.1034/j.1600-0447.2000.02605.x. [DOI] [PubMed] [Google Scholar]
- Lilliecreutz C, Theodorsson E, Sydsjö G, Josefsson A. Salivary cortisol in pregnant women suffering from blood and injection phobia. Arch Womens Ment Health. 2011;14:405–411. doi: 10.1007/s00737-011-0234-2. [DOI] [PubMed] [Google Scholar]
- Masharani U, Shiboski S, Eisner MD, Katz PP, Janson SL, Granger DA, Blanc PD. Impact of exogenous glucocorticoids use on salivary cortisol measurement among adults with asthma and rhinitis. Psychoneuroendocrinology. 2005;30:744–752. doi: 10.1016/j.psyneuen.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Mondelli V, Gianotti L, Picu A, Daga GA, Giordano R, Berardelli R, Pariante CM, Fassino S, Ghigo E, Arvat E. Neuroendocrine effects of citalopram infusion in anorexia nervosa. Psychoneuroendocrinology. 2006;31:1139–1148. doi: 10.1016/j.psyneuen.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Mück-Šeler D, Pivac N, Šagud M, Jakovljević M, Mihaljević-Peleš A. The effects of paroxetine and tianeptine on peripheral biochemical markers in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1235–1243. doi: 10.1016/S0278-5846(02)00259-2. [DOI] [PubMed] [Google Scholar]
- Navinés R, Martin-Santos R, Gomez-Gil E, Martinez de Osaba MJ, Imaz ML, Gasto C. Effects of citalopram treatment on hypothermic and hormonal responses to the 5-HT1A receptor agonist buspirone in patients with major depression and therapeutic response. Psychoneuroendocrinology. 2007;32:411–416. doi: 10.1016/j.psyneuen.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Nikisch G, Mathe AA, Czernik A, Thiele J, Bohner J, Eap CB, Agren H, Baumann P. Long-term citalopram administration reduces responsiveness of HPA axis in patients with major depression: relationship with S-citalopram concentrations in plasma and cerebrospinal fluid (CSF) and clinical response. Psychopharmacology. 2005;181:751–760. doi: 10.1007/s00213-005-0034-3. [DOI] [PubMed] [Google Scholar]
- Okugawa G, Omori K, Suzukawa J, Fujiseki Y, Kinoshita T, Inagaki C. Long-term treatment with antidepressants increases glucocorticoid receptor binding and gene expression in cultured rat hippocampal neurones. Neuroendocrinology. 1999;11:887–895. doi: 10.1046/j.1365-2826.1999.00405.x. [DOI] [PubMed] [Google Scholar]
- Otto M. Chemometrics: statistics and computer application in analytical chemistry. New York: Wiley; 1999. [Google Scholar]
- Papadopoulos AS, Cleare AJ. Hypothalamic–pituitary–adrenal axis dysfunction in chronic fatigue syndrome. Nat Rev Endocrinol. 2011;8:22–32. doi: 10.1038/nrendo.2011.153. [DOI] [PubMed] [Google Scholar]
- Papadopoulos A, Ebrecht M, Roberts ADL, Poon L, Rohleder N, Cleare AJ. Glucocorticoid receptor mediated negative feedback in chronic fatigue syndrome using the low dose (0.5 mg) dexamethasone suppression test. J Affect Disord. 2009;112:289–294. doi: 10.1016/j.jad.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Chuzi SE, Sousa JL, Fava M. 5-HT1A-mediated stimulation of cortisol release in major depression: use of non-invasive cortisol measurements to predict clinical response. Eur Arch Psychiatry Clin Neurosci. 2010;260:175–180. doi: 10.1007/s00406-009-0035-z. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/S0006-3223(00)01088-X. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Owens MH, Nemeroff CB. Psychoneuroendocrinology of depression: hypothalamic–pituitary–adrenal axis. Psychiatr Clin North Am. 1998;21:293–307. doi: 10.1016/S0193-953X(05)70006-X. [DOI] [PubMed] [Google Scholar]
- Rota E, Broda R, Cangemi L, Migliaretti G, Paccotti P, Rosso C, Torre E, Zeppegno P, Portaleone P. Neuroendocrine (HPA axis) and clinical correlates during fluvoxamine and amitriptyline treatment. Psychiatry Res. 2005;133:281–284. doi: 10.1016/j.psychres.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Sargent PA, Quested DJ, Cowen PJ. Clomipramine enhances the cortisol response to 5-HTP: implications for the therapeutic role of 5-HT2 receptors. Psychopharmacology. 1998;140:120–122. doi: 10.1007/s002130050747. [DOI] [PubMed] [Google Scholar]
- Schüle C. Neuroendocrinological mechanisms of actions of antidepressant drugs. J Neuroendocrinol. 2006;19:213–226. doi: 10.1111/j.1365-2826.2006.01516.x. [DOI] [PubMed] [Google Scholar]
- Tucker P, Beebe KL, Burgin C, Wyatt DB, Parker DE, Masters BK, Nawar O. Paroxetine treatment of depression with PTSD: effects on autonomic reactivity and cortisol secretion. J Clin Psychopharmacol. 2004;24:131–140. doi: 10.1097/01.jcp.0000116649.91923.cb. [DOI] [PubMed] [Google Scholar]
- Tucker P, Ruwe WD, Masters B, Parker DE, Hossain A, Trautman RP, Wyatt DB. Neuroimmune and cortisol changes in selective serotonin reuptake inhibitor and placebo treatment of chronic posttraumatic stress disorder. Biol Psychiatry. 2004;56:121–128. doi: 10.1016/j.biopsych.2004.03.009. [DOI] [PubMed] [Google Scholar]
- WHO (2012) http://apps.who.int/classifications/apps/icd/icd10online/ Accessed 25 Jul 2012
- Yang T-T, Hsiao F-H, Wang K-C, Ng S-M, Ho RTH, Chan CLW, Lai Y-M, Chen Y-T. The effect of psychotherapy added to pharmacotherapy on cortisol responses in outpatients with major depressive disorder. J Nerv Ment Dis. 2009;197:401–406. doi: 10.1097/NMD.0b013e3181a61594. [DOI] [PubMed] [Google Scholar]


