Abstract
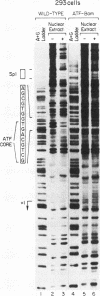
The adenovirus E1A oncoproteins interfere with the normal regulation of cellular proliferation through interactions with cell cycle regulatory proteins. In view of the essential role of proliferating-cell nuclear antigen (PCNA) in DNA replication, we performed a mutational analysis of the minimal human PCNA promoter (nucleotides -87 to +62) to define sequence elements which mediate transactivation by the 243-residue E1A protein (E1A 243R). Linker-scanning and site-directed mutants were examined for basal and E1A-induced expression of chloramphenicol acetyltransferase (CAT) from PCNA promoter-CAT reporter constructs transiently expressed in HeLa cells. The results define the cis-acting element required for induction of PCNA by E1A 243R as a region between -59 and -45 relative to the transcription initiation site. This PCNA E1A-responsive element (PERE), which is protected from DNase I digestion by nuclear extracts from 293 cells, includes the sequence AGCGTGG immediately upstream of the ATF binding site previously shown to be important for activation of PCNA by E1A 243R (G. F. Morris and M. B. Mathews, J. Virol. 65:6397-6406, 1991). Mutation of either the upstream component or the ATF site within the PERE diminishes basal promoter activity and abrogates transactivation by E1A 243R. This novel cis-acting element is also essential for both basal and E1A-induced expression in the context of the full-length PCNA promoter.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almendral J. M., Huebsch D., Blundell P. A., Macdonald-Bravo H., Bravo R. Cloning and sequence of the human nuclear protein cyclin: homology with DNA-binding proteins. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1575–1579. doi: 10.1073/pnas.84.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi S., Raychaudhuri P., Nevins J. R. Adenovirus E1A proteins can dissociate heteromeric complexes involving the E2F transcription factor: a novel mechanism for E1A trans-activation. Cell. 1990 Aug 24;62(4):659–669. doi: 10.1016/0092-8674(90)90112-r. [DOI] [PubMed] [Google Scholar]
- Bakker O., Parker M. G. CAAT/enhancer binding protein is able to bind to ATF/CRE elements. Nucleic Acids Res. 1991 Mar 25;19(6):1213–1217. doi: 10.1093/nar/19.6.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandara L. R., La Thangue N. B. Adenovirus E1a prevents the retinoblastoma gene product from complexing with a cellular transcription factor. Nature. 1991 Jun 6;351(6326):494–497. doi: 10.1038/351494a0. [DOI] [PubMed] [Google Scholar]
- Bauer G. A., Burgers P. M. Molecular cloning, structure and expression of the yeast proliferating cell nuclear antigen gene. Nucleic Acids Res. 1990 Jan 25;18(2):261–265. doi: 10.1093/nar/18.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. doi: 10.1146/annurev.ge.20.120186.000401. [DOI] [PubMed] [Google Scholar]
- Boshart M., Weber F., Jahn G., Dorsch-Häsler K., Fleckenstein B., Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Bravo R., Frank R., Blundell P. A., Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987 Apr 2;326(6112):515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Bravo R., Macdonald-Bravo H. Induction of the nuclear protein 'cyclin' in quiescent mouse 3T3 cells stimulated by serum and growth factors. Correlation with DNA synthesis. EMBO J. 1984 Dec 20;3(13):3177–3181. doi: 10.1002/j.1460-2075.1984.tb02276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcamo J., Buckbinder L., Reinberg D. The initiator directs the assembly of a transcription factor IID-dependent transcription complex. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8052–8056. doi: 10.1073/pnas.88.18.8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. S., Shi Y., Shenk T. Adeno-associated virus P5 promoter contains an adenovirus E1A-inducible element and a binding site for the major late transcription factor. J Virol. 1989 Aug;63(8):3479–3488. doi: 10.1128/jvi.63.8.3479-3488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappan S. P., Nevins J. R. DNA octamer element can confer E1A trans-activation, and adenovirus infection results in a stimulation of the DNA-binding activity of OTF-1/NFIII factor. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5878–5882. doi: 10.1073/pnas.87.15.5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes P., Buckbinder L., Leza M. A., Rak N., Hearing P., Merino A., Reinberg D. EivF, a factor required for transcription of the adenovirus EIV promoter, binds to an element involved in EIa-dependent activation and cAMP induction. Genes Dev. 1988 Aug;2(8):975–990. doi: 10.1101/gad.2.8.975. [DOI] [PubMed] [Google Scholar]
- Dery C. V., Herrmann C. H., Mathews M. B. Response of individual adenovirus promoters to the products of the E1A gene. Oncogene. 1987;2(1):15–23. [PubMed] [Google Scholar]
- Engel D. A., Muller U., Gedrich R. W., Eubanks J. S., Shenk T. Induction of c-fos mRNA and AP-1 DNA-binding activity by cAMP in cooperation with either the adenovirus 243- or the adenovirus 289-amino acid E1A protein. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3957–3961. doi: 10.1073/pnas.88.9.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch T., Nurse P. Coupling M phase and S phase: controls maintaining the dependence of mitosis on chromosome replication. Cell. 1991 Jun 14;65(6):921–923. doi: 10.1016/0092-8674(91)90542-7. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Xing Y. G., Lawrence J. B., Livingston D. M. Molecular cloning, chromosomal mapping, and expression of the cDNA for p107, a retinoblastoma gene product-related protein. Cell. 1991 Sep 20;66(6):1155–1164. doi: 10.1016/0092-8674(91)90038-z. [DOI] [PubMed] [Google Scholar]
- Ferguson B., Krippl B., Andrisani O., Jones N., Westphal H., Rosenberg M. E1A 13S and 12S mRNA products made in Escherichia coli both function as nucleus-localized transcription activators but do not directly bind DNA. Mol Cell Biol. 1985 Oct;5(10):2653–2661. doi: 10.1128/mcb.5.10.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai T. W., Horikoshi M., Roeder R. G., Green M. R. Analysis of the role of the transcription factor ATF in the assembly of a functional preinitiation complex. Cell. 1988 Sep 23;54(7):1043–1051. doi: 10.1016/0092-8674(88)90119-5. [DOI] [PubMed] [Google Scholar]
- Hai T. W., Liu F., Coukos W. J., Green M. R. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 1989 Dec;3(12B):2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- Hai T., Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Whyte P., Franza B. R., Jr, Schley C. Association of adenovirus early-region 1A proteins with cellular polypeptides. Mol Cell Biol. 1986 May;6(5):1579–1589. doi: 10.1128/mcb.6.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Herrmann C. H., Mathews M. B. The adenovirus E1B 19-kilodalton protein stimulates gene expression by increasing DNA levels. Mol Cell Biol. 1989 Dec;9(12):5412–5423. doi: 10.1128/mcb.9.12.5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert S. W., Chellappan S. P., Horowitz J. M., Nevins J. R. The interaction of RB with E2F coincides with an inhibition of the transcriptional activity of E2F. Genes Dev. 1992 Feb;6(2):177–185. doi: 10.1101/gad.6.2.177. [DOI] [PubMed] [Google Scholar]
- Hoeffler J. P., Deutsch P. J., Lin J., Habener J. F. Distinct adenosine 3',5'-monophosphate and phorbol ester-responsive signal transduction pathways converge at the level of transcriptional activation by the interactions of DNA-binding proteins. Mol Endocrinol. 1989 May;3(5):868–880. doi: 10.1210/mend-3-5-868. [DOI] [PubMed] [Google Scholar]
- Horikoshi M., Hai T., Lin Y. S., Green M. R., Roeder R. G. Transcription factor ATF interacts with the TATA factor to facilitate establishment of a preinitiation complex. Cell. 1988 Sep 23;54(7):1033–1042. doi: 10.1016/0092-8674(88)90118-3. [DOI] [PubMed] [Google Scholar]
- Horikoshi N., Maguire K., Kralli A., Maldonado E., Reinberg D., Weinmann R. Direct interaction between adenovirus E1A protein and the TATA box binding transcription factor IID. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5124–5128. doi: 10.1073/pnas.88.12.5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houweling A., van den Elsen P. J., van der Eb A. J. Partial transformation of primary rat cells by the leftmost 4.5% fragment of adenovirus 5 DNA. Virology. 1980 Sep;105(2):537–550. doi: 10.1016/0042-6822(80)90054-9. [DOI] [PubMed] [Google Scholar]
- Jaskulski D., Gatti C., Travali S., Calabretta B., Baserga R. Regulation of the proliferating cell nuclear antigen cyclin and thymidine kinase mRNA levels by growth factors. J Biol Chem. 1988 Jul 25;263(21):10175–10179. [PubMed] [Google Scholar]
- Jaskulski D., deRiel J. K., Mercer W. E., Calabretta B., Baserga R. Inhibition of cellular proliferation by antisense oligodeoxynucleotides to PCNA cyclin. Science. 1988 Jun 10;240(4858):1544–1546. doi: 10.1126/science.2897717. [DOI] [PubMed] [Google Scholar]
- Kannabiran C., Morris G. F., Labrie C., Mathews M. B. The adenovirus E1A 12S product displays functional redundancy in activating the human proliferating cell nuclear antigen promoter. J Virol. 1993 Jan;67(1):507–515. doi: 10.1128/jvi.67.1.507-515.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitabayashi I., Kawakami Z., Chiu R., Ozawa K., Matsuoka T., Toyoshima S., Umesono K., Evans R. M., Gachelin G., Yokoyama K. Transcriptional regulation of the c-jun gene by retinoic acid and E1A during differentiation of F9 cells. EMBO J. 1992 Jan;11(1):167–175. doi: 10.1002/j.1460-2075.1992.tb05039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus V. B., Moran E., Nevins J. R. Promoter-specific trans-activation by the adenovirus E1A12S product involves separate E1A domains. Mol Cell Biol. 1992 Oct;12(10):4391–4399. doi: 10.1128/mcb.12.10.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. A., Hai T. Y., SivaRaman L., Thimmappaya B., Hurst H. C., Jones N. C., Green M. R. A cellular protein, activating transcription factor, activates transcription of multiple E1A-inducible adenovirus early promoters. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8355–8359. doi: 10.1073/pnas.84.23.8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. S., Kao C. C., Bryant G. O., Liu X., Berk A. J. Adenovirus E1A activation domain binds the basic repeat in the TATA box transcription factor. Cell. 1991 Oct 18;67(2):365–376. doi: 10.1016/0092-8674(91)90188-5. [DOI] [PubMed] [Google Scholar]
- Lin Y. S., Green M. R. Interaction of a common cellular transcription factor, ATF, with regulatory elements in both E1a- and cyclic AMP-inducible promoters. Proc Natl Acad Sci U S A. 1988 May;85(10):3396–3400. doi: 10.1073/pnas.85.10.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Green M. R. A specific member of the ATF transcription factor family can mediate transcription activation by the adenovirus E1a protein. Cell. 1990 Jun 29;61(7):1217–1224. doi: 10.1016/0092-8674(90)90686-9. [DOI] [PubMed] [Google Scholar]
- Liu Y. C., Marraccino R. L., Keng P. C., Bambara R. A., Lord E. M., Chou W. G., Zain S. B. Requirement for proliferating cell nuclear antigen expression during stages of the Chinese hamster ovary cell cycle. Biochemistry. 1989 Apr 4;28(7):2967–2974. doi: 10.1021/bi00433a034. [DOI] [PubMed] [Google Scholar]
- Loeken M. R., Brady J. The adenovirus EIIA enhancer. Analysis of regulatory sequences and changes in binding activity of ATF and EIIF following adenovirus infection. J Biol Chem. 1989 Apr 15;264(11):6572–6579. [PubMed] [Google Scholar]
- Matsumoto K., Moriuchi T., Koji T., Nakane P. K. Molecular cloning of cDNA coding for rat proliferating cell nuclear antigen (PCNA)/cyclin. EMBO J. 1987 Mar;6(3):637–642. doi: 10.1002/j.1460-2075.1987.tb04802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran E., Mathews M. B. Multiple functional domains in the adenovirus E1A gene. Cell. 1987 Jan 30;48(2):177–178. doi: 10.1016/0092-8674(87)90418-1. [DOI] [PubMed] [Google Scholar]
- Morris G. F., Mathews M. B. Analysis of the proliferating cell nuclear antigen promoter and its response to adenovirus early region 1. J Biol Chem. 1990 Sep 25;265(27):16116–16125. [PubMed] [Google Scholar]
- Morris G. F., Mathews M. B. The adenovirus E1A transforming protein activates the proliferating cell nuclear antigen promoter via an activating transcription factor site. J Virol. 1991 Dec;65(12):6397–6406. doi: 10.1128/jvi.65.12.6397-6406.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. Mechanisms of viral-mediated trans-activation of transcription. Adv Virus Res. 1989;37:35–83. doi: 10.1016/s0065-3527(08)60832-5. [DOI] [PubMed] [Google Scholar]
- Ohashi Y., Sawada Y., Moriuchi T., Fujinaga K. Analysis of the 5' flanking region of the rat proliferating cell nuclear antigen (PCNA) gene. Biochim Biophys Acta. 1992 Mar 24;1130(2):175–181. doi: 10.1016/0167-4781(92)90525-5. [DOI] [PubMed] [Google Scholar]
- Phelps W. C., Bagchi S., Barnes J. A., Raychaudhuri P., Kraus V., Münger K., Howley P. M., Nevins J. R. Analysis of trans activation by human papillomavirus type 16 E7 and adenovirus 12S E1A suggests a common mechanism. J Virol. 1991 Dec;65(12):6922–6930. doi: 10.1128/jvi.65.12.6922-6930.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzkowski Z., Alder H., Chang C. D., Ku D. H., Baserga R. Characterization of an enhancer-like structure in the promoter region of the proliferating cell nuclear antigen (PCNA) gene. Exp Cell Res. 1991 Apr;193(2):283–290. doi: 10.1016/0014-4827(91)90098-f. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature. 1990 Aug 23;346(6286):760–763. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- Prelich G., Kostura M., Marshak D. R., Mathews M. B., Stillman B. The cell-cycle regulated proliferating cell nuclear antigen is required for SV40 DNA replication in vitro. Nature. 1987 Apr 2;326(6112):471–475. doi: 10.1038/326471a0. [DOI] [PubMed] [Google Scholar]
- Prelich G., Tan C. K., Kostura M., Mathews M. B., So A. G., Downey K. M., Stillman B. Functional identity of proliferating cell nuclear antigen and a DNA polymerase-delta auxiliary protein. Nature. 1987 Apr 2;326(6112):517–520. doi: 10.1038/326517a0. [DOI] [PubMed] [Google Scholar]
- Pugh B. F., Tjian R. Transcription from a TATA-less promoter requires a multisubunit TFIID complex. Genes Dev. 1991 Nov;5(11):1935–1945. doi: 10.1101/gad.5.11.1935. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri P., Bagchi S., Devoto S. H., Kraus V. B., Moran E., Nevins J. R. Domains of the adenovirus E1A protein required for oncogenic activity are also required for dissociation of E2F transcription factor complexes. Genes Dev. 1991 Jul;5(7):1200–1211. doi: 10.1101/gad.5.7.1200. [DOI] [PubMed] [Google Scholar]
- Rooney R. J., Raychaudhuri P., Nevins J. R. E4F and ATF, two transcription factors that recognize the same site, can be distinguished both physically and functionally: a role for E4F in E1A trans activation. Mol Cell Biol. 1990 Oct;10(10):5138–5149. doi: 10.1128/mcb.10.10.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P., Ransone L. J., Verma I. M. Cross-talk in signal transduction: TPA-inducible factor jun/AP-1 activates cAMP-responsive enhancer elements. Oncogene. 1990 Mar;5(3):427–431. [PubMed] [Google Scholar]
- Seto E., Shi Y., Shenk T. YY1 is an initiator sequence-binding protein that directs and activates transcription in vitro. Nature. 1991 Nov 21;354(6350):241–245. doi: 10.1038/354241a0. [DOI] [PubMed] [Google Scholar]
- Shenk T., Flint J. Transcriptional and transforming activities of the adenovirus E1A proteins. Adv Cancer Res. 1991;57:47–85. doi: 10.1016/s0065-230x(08)60995-1. [DOI] [PubMed] [Google Scholar]
- Shi Y., Seto E., Chang L. S., Shenk T. Transcriptional repression by YY1, a human GLI-Krüppel-related protein, and relief of repression by adenovirus E1A protein. Cell. 1991 Oct 18;67(2):377–388. doi: 10.1016/0092-8674(91)90189-6. [DOI] [PubMed] [Google Scholar]
- Shivji K. K., Kenny M. K., Wood R. D. Proliferating cell nuclear antigen is required for DNA excision repair. Cell. 1992 Apr 17;69(2):367–374. doi: 10.1016/0092-8674(92)90416-a. [DOI] [PubMed] [Google Scholar]
- Simon M. C., Fisch T. M., Benecke B. J., Nevins J. R., Heintz N. Definition of multiple, functionally distinct TATA elements, one of which is a target in the hsp70 promoter for E1A regulation. Cell. 1988 Mar 11;52(5):723–729. doi: 10.1016/0092-8674(88)90410-2. [DOI] [PubMed] [Google Scholar]
- Simon M. C., Kitchener K., Kao H. T., Hickey E., Weber L., Voellmy R., Heintz N., Nevins J. R. Selective induction of human heat shock gene transcription by the adenovirus E1A gene products, including the 12S E1A product. Mol Cell Biol. 1987 Aug;7(8):2884–2890. doi: 10.1128/mcb.7.8.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. C., Rooney R. J., Fisch T. M., Heintz N., Nevins J. R. E1A-dependent trans-activation of the c-fos promoter requires the TATAA sequence. Proc Natl Acad Sci U S A. 1990 Jan;87(2):513–517. doi: 10.1073/pnas.87.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale S. T., Baltimore D. The "initiator" as a transcription control element. Cell. 1989 Apr 7;57(1):103–113. doi: 10.1016/0092-8674(89)90176-1. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Schmidt M. C., Berk A. J., Baltimore D. Transcriptional activation by Sp1 as directed through TATA or initiator: specific requirement for mammalian transcription factor IID. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4509–4513. doi: 10.1073/pnas.87.12.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaete R. R., Mocarski E. S. Regulation of cytomegalovirus gene expression: alpha and beta promoters are trans activated by viral functions in permissive human fibroblasts. J Virol. 1985 Oct;56(1):135–143. doi: 10.1128/jvi.56.1.135-143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor I. C., Kingston R. E. E1a transactivation of human HSP70 gene promoter substitution mutants is independent of the composition of upstream and TATA elements. Mol Cell Biol. 1990 Jan;10(1):176–183. doi: 10.1128/mcb.10.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalmeier K., Synovzik H., Mertz R., Winnacker E. L., Lipp M. Nuclear factor E2F mediates basic transcription and trans-activation by E1a of the human MYC promoter. Genes Dev. 1989 Apr;3(4):527–536. doi: 10.1101/gad.3.4.527. [DOI] [PubMed] [Google Scholar]
- Travali S., Ku D. H., Rizzo M. G., Ottavio L., Baserga R., Calabretta B. Structure of the human gene for the proliferating cell nuclear antigen. J Biol Chem. 1989 May 5;264(13):7466–7472. [PubMed] [Google Scholar]
- Tsai L. H., Harlow E., Meyerson M. Isolation of the human cdk2 gene that encodes the cyclin A- and adenovirus E1A-associated p33 kinase. Nature. 1991 Sep 12;353(6340):174–177. doi: 10.1038/353174a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Gray A., Goeddel D. V., Dull T. J. Nucleotide sequence of a portion of human chromosome 9 containing a leukocyte interferon gene cluster. J Mol Biol. 1982 Apr 15;156(3):467–486. doi: 10.1016/0022-2836(82)90261-3. [DOI] [PubMed] [Google Scholar]
- White E., Cipriani R. Role of adenovirus E1B proteins in transformation: altered organization of intermediate filaments in transformed cells that express the 19-kilodalton protein. Mol Cell Biol. 1990 Jan;10(1):120–130. doi: 10.1128/mcb.10.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P., Buchkovich K. J., Horowitz J. M., Friend S. H., Raybuck M., Weinberg R. A., Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- Widen S. G., Kedar P., Wilson S. H. Human beta-polymerase gene. Structure of the 5'-flanking region and active promoter. J Biol Chem. 1988 Nov 15;263(32):16992–16998. [PubMed] [Google Scholar]
- Wiley S. R., Kraus R. J., Mertz J. E. Functional binding of the "TATA" box binding component of transcription factor TFIID to the -30 region of TATA-less promoters. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5814–5818. doi: 10.1073/pnas.89.13.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. T., McClanahan T. K., Morimoto R. I. E1a transactivation of the human HSP70 promoter is mediated through the basal transcriptional complex. Mol Cell Biol. 1989 Jun;9(6):2574–2587. doi: 10.1128/mcb.9.6.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., Hayashi Y., Hirose F., Matsuoka S., Moriuchi T., Shiroishi T., Moriwaki K., Matsukage A. Molecular cloning and structural analysis of mouse gene and pseudogenes for proliferating cell nuclear antigen. Nucleic Acids Res. 1991 May 11;19(9):2403–2410. doi: 10.1093/nar/19.9.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerler B., Roberts R. J., Mathews M. B., Moran E. Different functional domains of the adenovirus E1A gene are involved in regulation of host cell cycle products. Mol Cell Biol. 1987 Feb;7(2):821–829. doi: 10.1128/mcb.7.2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot R., Foulkes N., Mulder M., Kruijer W., Sassone-Corsi P. Positive regulation of jun/AP-1 by E1A. Mol Cell Biol. 1991 Jan;11(1):192–201. doi: 10.1128/mcb.11.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]