Abstract
The Otsuka Long-Evans Tokushima Fatty (OLETF) rat, an outbred strain of Long Evans Tokushima Otsuka rat (LETO) that lacks CCK-1 receptor expression, is hyperphagic and develops obesity and type-2 diabetes. The present study sought to assess how OLETF rats alter intake, preference, and conditioned preference of palatable solutions after acute food deprivation. Our results show that after 24 hr chow restriction LETO rats increase both sucrose intake and two-bottle sucrose preference relative to their free-fed baseline, whereas OLETF rats do not increase sucrose intake (0.3M or 1.0M sucrose) or preference (1.0M vs. 0.3M sucrose) when food-deprived. In contrast, OLETF rats exhibit a higher conditioned flavor preference when sucrose is used as unconditioned stimulus (US) relative to LETO rats, whether overnight food-restricted (81% vs. 71% for OLETF and LETO rats, respectively) or free-fed (82% vs. 54% for OLETF and LETO rats, respectively) on test. When a non-caloric saccharin solution is used as US, OLETF rats show a higher preference for the saccharin-associated flavor relative to LETO rats when non-deprived (76% vs. 58% for OLETF and LETO rats, respectively), however neither strain shows differential conditioned flavor preference for saccharin in the deprivation state on test. These findings suggest that OLETF rats fail to integrate post-absorptive and orosensory effects of sucrose in a conditioning setting to influence intake. Thus, it appears that OLETF rats form preferences for sucrose based largely on orosensory and hedonic properties of the solution, rather than caloric value.
Keywords: food intake, CCK-1 receptor, hyperphagia, diet-induced obesity, palatability
INTRODUCTION
The Otsuka Long-Evans Tokushima Fatty rat (OLETF) is an outbred strain of Long Evans Tokushima Otsuka rat (LETO) that has been previously characterized by the lack of CCK-1 receptor expression due to a spontaneous mutation spanning the promoter and first two exon regions of the CCK-1 receptor gene (45). In addition to the use of these animals as a model of insulin resistance, due to their natural manifestation of hyperglycemia and Non-Insulin Dependent Diabetes Mellitus (NIDDM) relative to age-matched LETO rats (28), OLETF rats are also currently under investigation as a model of obesity. OLETF rats exhibit an increased rate of weight gain relative to controls across their life span, with a marked elevation in body weight seen as early as 2–4 postnatal day (5, 34, 38). It is also known that OLETF rats are hyperphagic via increased meal size. This behavior has been attributed not only to deficits in CCK-related satiation mechanisms (34), but also to gastric mechano-detection (16), intestinal nutrient satiation signaling (12, 17, 38) and more recently, enhanced oral responsiveness to palatable stimuli (15).
It is well known that amount consumed within a meal is a function of both oral and post-oral nutrient properties of foods (42). Heightened orosensory responses to preferred tastants in OLETF rats (17, 21) suggest that altered oral or taste sensation may additionally compound hyperphagia attributed to known peripheral satiation defects in these animals. In this context, learned associations between the flavor of food and its post-ingestive consequences can influence food intake and preference (27, 39). Compelling evidence exists that learned preferences can be mediated by associations of nutritive feedback. Indeed, rats can acquire preference from odors and tastes that have been paired with intragastric or intraduodenal infusions of sugars (1, 18), as well as when given prior access to an arbitrary cue flavor solution paired with a specific palatable food (10).
It has also been established that rats subjected to periods of acute food deprivation increase subsequent consumption of palatable foods (11, 44). The exact mechanism responsible for this increase, however, is not entirely clear. For instance, food restricted rats can show heightened intake of both caloric sucrose and non-caloric saccharin solutions (44). It appears that an increase in motivation to eat due to caloric deprivation does not comprise the sole impetus for increased consumption under restrictive conditions, but rather increases the overall responsiveness to gustatory properties of the food. Indeed, there are reports of enhanced hedonic response (2), as well as lower taste thresholds for sweet solutions under deprived conditions (6, 51) that support this notion.
Bi and Moran have shown that in addition to overconsuming more chow over 24 hrs under ad libitum- fed conditions, OLETF rats also overconsume chow to a higher degree than LETO rats following a 24 hr fast (4). The effect of food restriction on the intake of preferred liquid solutions in these animals has not been tested. Therefore, the first aim of the present study was to test food deprivation effects on intake and preference for normally preferred sucrose solutions in OLETF and LETO rats.
Conditioning a flavor preference has been used to dissociate positive orosensory vs. post-ingestive effects in the consumption or preference for a particular food. Several studies have provided evidence that the formation and expression of flavor-taste and flavor-calorie associations are regulated differentially by levels of hunger (10, 19, 24). For example, hungry rats show increased preference for a conditioned stimulus (CS) flavor associated with sucrose relative to sated animals. In contrast, there appears to be no differential effect of hunger on flavor preference for a flavor associated with saccharin (8, 19, 24). Furthermore, more recent data suggest that food deprivation affects the expression, but not acquisition, of conditioned flavor preference (48, 49).
We have previously shown that OLETF rats exhibit increased intake as well as preference for palatable sucrose in both real and sham feeding paradigms (17, 21). This increased sensitivity to oral stimulation by sweet tastes suggests that orosensory components of sucrose solutions impart enhanced contributions to sucrose intake relative to LETO controls. To investigate effects of food deprivation on learned preferences for sweet solutions, we employed a conditioned flavor-calorie procedure using palatable sucrose or saccharin solutions as unconditioned stimuli (US) and tested intake of paired conditioned stimulus (CS) flavors under both deprived and free-fed conditions. If OLETF rats are more sensitive to the orosensory properties of sweet solutions and less sensitive to the post-absorptive effects, we would expect these rats to be less responsive to potential differences in expression of conditioned sucrose flavor preference induced by acute food restriction relative to LETO rats, when tested in a conditioned sweet preference paradigm. We also hypothesized that if OLETF rats are indeed more sensitive to orosensory associations of sweet solutions, then saccharin should produce a greater conditioned flavor preference in OLETF rats compared to LETO rats when animals are non-deprived. Therefore, our second aim was to assess possible alterations in the expression of conditioned flavor preferences for both caloric and non-caloric sweet solutions in OLETF and LETO rats under food deprived and ad libitum fed conditions.
MATERIALS AND METHODS
Subjects
Male OLETF and LETO rats were obtained as a generous gift of the Tokushima Research Institute, Otsuka Pharmaceutical, Tokushima, Japan. All rats were housed individually in mesh-floored, stainless-steel hanging cages and maintained in a temperature-controlled vivarium on a constant 12:12-h light-dark cycle (lights on at 0600). Animals were handled daily for a minimum of one week prior to the onset of experiments. Tap water and pelleted rat chow (Purina 5001) were available ad libitum throughout experiments, except where indicated otherwise. All protocols used were approved by The Pennsylvania State University Institutional Animal Care and Use Committee.
Intake and Preference of 0.3M and 1.0M sucrose in OLETF and LETO rats under ad libitum and food-deprived conditions
Twenty-four week old OLETF and LETO rats (n=5 per strain, weighing 537 ± 29.6 g and 435 ± 6.7 g, respectively) were used for the following experiments. In sucrose intake studies, all rats received daily, 1 hr access to calibrated glass drinking burettes containing 0.3M sucrose between 1000 and 1100 hr everyday. Prior to daily sucrose presentation, 24 hr chow intake and spillage was measured. After the establishment of a stable baseline sucrose intake under ad libitum fed conditions (6–8 days), rats were food deprived across two levels of chow restriction. In the low restriction condition, animals were tested 24 hrs after presentation of a food ration limited to 75% baseline daily ad libitum chow intake. Given the well-documented differences in daily chow intake between OLETF and LETO rats, the low restriction was rationed in this manner in order to provide comparable degrees of food restriction as a function of daily intake within strain. In the high restriction condition, rats had no access to food for 24h prior to testing (i.e., both strains were deprived of 100% ad libitum daily chow intake). One hour daily sucrose intake under either ad libitum or food restricted conditions was compared to test how differences in caloric motivation would affect intake of a palatable sucrose solution. Daily sucrose access time and duration remained unaltered throughout experimentation.
Food restriction conditions were imposed a minimum of 2 days apart, to both allow for compensatory chow intake, and to assess possible shifting in non-deprived sucrose baseline intake as a result of intermittent access. Every deprivation condition was repeated a minimum of two occasions. This study was first completed using 0.3M sucrose as test solution, followed by an identical experiment using a more concentrated 1.0M sucrose solution in the same groups of rats. Sucrose intake was measured to the nearest 0.1 ml.
In a further experiment, using the same deprivation schedule and sucrose access periods, daily 1 hr two-bottle choice tests were performed using 0.3M versus 1.0M sucrose to assess sucrose concentration preference changes as a function of motivational state. To control for side-preference, the position of each sucrose burette was alternated daily and counter-balanced within animals on the same testing day.
Conditioned preference for caloric and non-caloric sweet solutions in food deprived or non-deprived OLETF and LETO rats
Twenty, 10 wk old, naïve pre-diabetic OLETF and LETO rats were used in this experiment (n=10 per strain, weighing 287.2 ± 6.8 g and 220.4 ± 3.8 g, respectively). A two-day habituation period preceded experimentation during which all animals were given brief access to 3 ml of an unflavored 0.2% saccharin solution at 1000hr to familiarize the animals with one bottle acceptance. As shown in Figure 1, over the next eight calendar days all rats were exposed to a 3 ml volume of a cue flavored solution (conditioned stimulus, CS) of either grape or cherry flavored saccharin (0.2% saccharin (w/v), and 0.05% Kool Aid (w/v)) immediately preceding 30 min presentation of an assigned unconditioned stimulus (US) solution. One group of OLETF and LETO rats (n=5 per strain) received 3 ml grape saccharin (CS+) paired with 30 min ad libitum exposure to 0.3M unflavored sucrose (US+) and 3 ml cherry flavored saccharin (CS−) with 30 min access to plain water (US−), while an additional group of rats (n=5 per strain) were conditioned using the opposite CS−US pairings. All animals were given CS+/US+ and CS−/US− pairings on alternate calendar days for a total of 4 exposures to each pairing prior to CS preference testing. All solutions were presented in standard drinking bottles attached to the front of the home cage. Pelleted rat chow was continuously available except during conditioning procedures. To account for the possibility of inducing stronger preferences in OLETF due to greater reinforcement as a results of higher US+ intakes during training, in a separate group of rats (OLETF, n=13; LETO, n=10) OLETF sucrose intake (0.3M) during conditioning training was clamped to the amount consumed by LETO. This was done in both deprived and non-deprived conditions.
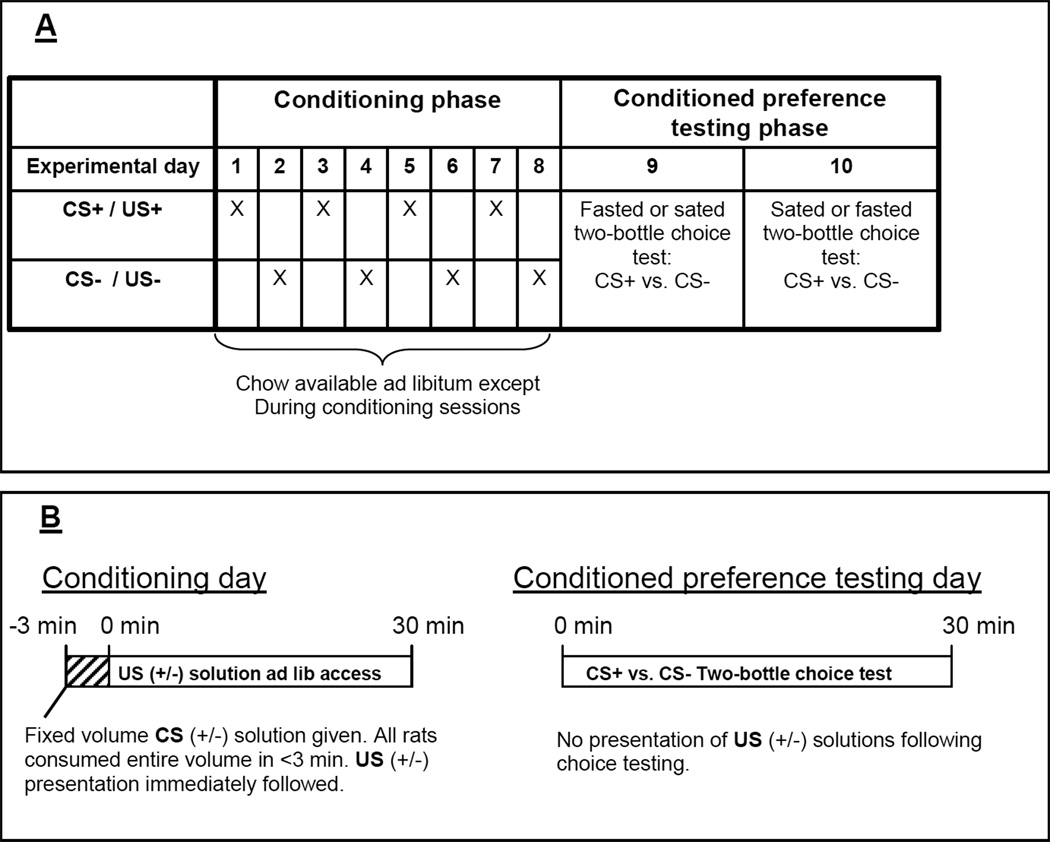
Fig. 1.
Experimental schedule for conditioned preference testing for both study duration (A) and within day design (B). All three conditioned preference studies conformed to identical procedures, with only US(+/−) solutions altered between studies. Over eight calendar days all rats were exposed to a fixed volume CS solutions immediately preceding 30 min presentation of an assigned US. All animals were given CS+/US+ and CS−/US− pairings on alternate calendar days for a total of 4 exposures to each pairing. Chow was continuously available except during conditioning procedures. In food-restricted conditioned preference tests, chow was removed at 1800 hr prior to testing the following day, while in sated tests, no prior food-deprivation was imposed. On days 9 and 10, thirty min two-bottle choice tests between CS+ and CS− flavors were conducted to assess conditioned preference for the CS solutions.
On days 9 and 10, thirty min two-bottle choice tests between CS+ and CS− flavors were conducted in food-deprived or ad libitum fed OLETF and LETO rats to assess conditioned preference for the CS solutions in the absence of US(+/−) presentation. Food restricted and ad libitum fed choice tests were counterbalanced between groups such that, on day 8, the two experimental groups within each strain were split randomly: half of the animals within each group having chow removed at 1800hr prior to testing on day 9, while the other half of the animals within each group were not food-restricted prior to day 9 preference testing. After testing on day 9, plain water and rat chow were returned. Chow was then removed at 1800hr from rats not food-restricted prior to two-bottle choice testing on day 9. On day 10, two-bottle choice testing was performed in rats under the opposite restriction conditions imposed prior to Day 9 choice testing. The left and right side orientation of the two CS flavor solutions was counterbalanced equally between animals. In the experiment where OLETF rats’ intake during conditioning was limited to the amount consumed by LETO rats, clamping did not occur during preference test, thus rats had ad libitum access to 0.3M sucrose solution.
A parallel experiment was conducted simultaneously with the experiment described above in a separate group of animals (twenty, 10 wk old, naïve rats, weighing 275.4 ± 5.0 g and 228.7 ± 6.2 g, respectively) in which 0.3M sucrose was replaced with 0.2% non-caloric saccharin solution as the US+ solution. All other protocols were identical. Our final experiment expanded upon the previous two conditioning studies by using both saccharin and sucrose solutions as US solutions. Therefore, in this last experiment, another group of rats (twenty, 10 wk old, naïve rats, weighing 265.9 ± 3.3 g and 222.5 ± 3.7 g, respectively) were conditioned to associate a CS with both a caloric and non-caloric sweet tasting US (0.3M sucrose: US+; 0.2% Saccharin: US−). All daily access and testing regimens were the same as described in the previous two studies.
Statistical Analysis
For sucrose intake studies, one-way repeated measures analysis of variance (rmANOVA) was performed to test within strain effects of food deprivation on subsequent sucrose intake and preference in OLETF or LETO rats. These results were analyzed post-hoc by Tukey's honestly significant difference (HSD) test when necessary. For sucrose preference testing, a two-way rmANOVA was performed with strain and chow deprivation as main effects. Total intake of both solutions (0.3M and 1.0M sucrose) was used to calculate preference percentages according to the following formula: Preference percentage = [volume of 1.0M sucrose solution (ml) × 100/total volume of 0.3M sucrose and 1.0M sucrose (ml)].
For all conditioned preference studies, US intake across the conditioning period was analyzed separately according to US solutions using two way rmANOVA with strain and time as main effects. Conditioned preference testing results were analyzed by appropriate one way ANOVA planned comparisons with strain or deprivation state on test as main factor.
All data were expressed as means ± SEM. Differences were considered statistically significant if P<0.05. Statistical analyses were computed with PC-SAS (version 8.02, SAS Institute, Carey, NC).
RESULTS
Intake of 0.3M and 1.0M sucrose in OLETF and LETO rats under food-deprived conditions
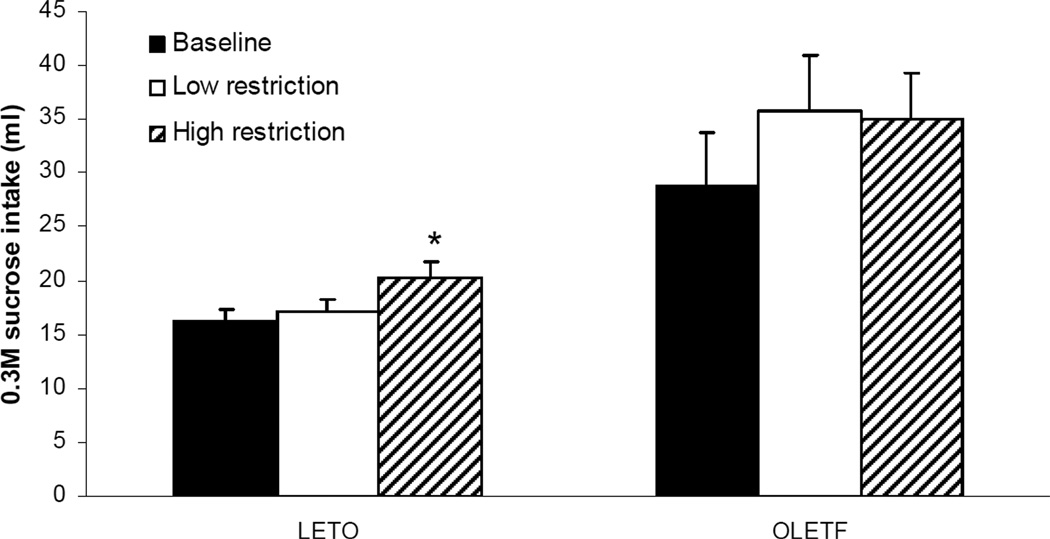
Results of ANOVA testing for a food deprivation effect on 0.3M sucrose intake revealed a significant increase in sucrose intake in LETO rats [F(2,12)=12.94; P<0.05], while OLETF rats showed no such effect (P=0.124). Figure 2 depicts results of post-hoc analyses showing that after high food restriction, LETO rats significantly increased their 0.3M sucrose intake relative to non-deprived sucrose intake (16.3 ± 1.1 vs. 20.4 ± 1.4 ml for baseline and low restriction intakes, respectively; P<0.05), while both OLETF and LETO rats did not increase intake when under low deprivation conditions (P=0.391). Similarly, when 1.0M sucrose was tested, LETO rats significantly increased their sucrose intake relative to non-deprived sucrose baseline after food deprivation [F(2,12)=18.42; P<0.05], while OLETF rats again did not (P=0.917). Post hoc results depicted in Figure 3 show an increase in consumption to have occurred only after high deprivation conditions (9.1 ± 0.9 vs. 12.5 ± 0.4 ml for baseline and low restriction intakes, respectively; P<0.01).
Fig. 2.
One hour, 0.3M sucrose intake in OLETF and LETO rats after food restriction. When tested after high acute food restriction, LETO rats significantly increased their 0.3M sucrose intake relative to non-deprived sucrose intake, while OLETF rats did not. Neither OLETF nor LETO rats increased intake when under low deprivation. (*P<0.05; indicates significant differences within strain).
Fig. 3.
One hour, 1.0M sucrose intake in OLETF and LETO rats after food restriction. When 1.0M sucrose was the test solution, LETO rats significantly increased their sucrose intake relative to non-deprived sucrose intake after high deprivation, while OLETF rats did not. As with 0.3M sucrose, both OLETF and LETO rats did not increase intake when low restriction conditions were imposed (**P<0.01; indicates significant differences within strain).
Preference for 1.0M vs. 0.3M sucrose in two-bottle choice test in OLETF and LETO rats under food-deprived conditions
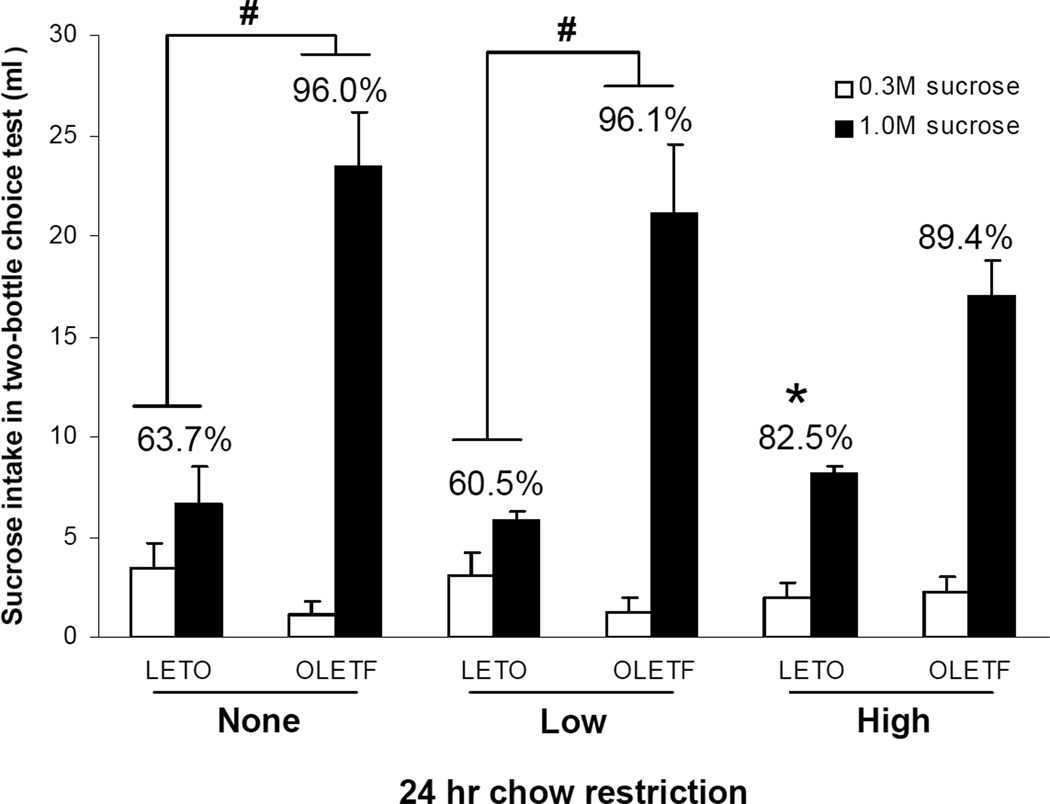
Both OLETF and LETO animals preferred 1.0M sucrose over 0.3M sucrose in two-bottle choice testing. One way ANOVA assessing strain effects on sucrose preference showed significantly higher preference for 1.0M sucrose in OLETF rats when compared to LETO animals [F(1,8)= 10.24; P<0.05]. As shown in Figure 4, OLETF rats showed higher preference for 1.0M sucrose than LETO rats when non-food deprived (96.0 ± 1.7 % vs. 63.7 ± 12.1 % for OLETF and LETO rats, respectively; P<0.05), or under low deprivation conditions (96.1 ± 1.2 % vs. 60.5 ± 12.4 % for OLETF and LETO rats, respectively; P<0.05), while no strain difference was noted under the higher restriction condition. Consistent with our preceding results indicating motivational state increases in sucrose intake in LETO rats, LETO rats also showed increased 1.0M sucrose preference [F(2,12)= 8.83; P<0.05] when food restricted, while no such response was detected in OLETF rats [Figure 4]. Post-hoc testing showed a significant increase in sucrose preference in LETO rats only after high food restriction (P<0.05).
Fig. 4.
1.0M sucrose preference in OLETF and LETO rats after food restriction. Non-food deprived OLETF rats showed higher preference for 1.0M sucrose as a percentage of total sucrose intakes than LETO rats when given 1 hr access to both 1.0M and 0.3M sucrose solutions. LETO rats showed increased 1.0M sucrose after high food restriction, while OLETF rats showed in difference in sucrose preference under the same restriction conditions. Low chow restriction did not alter sucrose preference in either strain. Numbers above SEM bars represent mean percent preference between 1.0M and 0.3M sucrose for each strain. (#P<0.05; indicates between strain preference differences within each restriction condition; *P<0.05; indicates within strain preference differences from non-deprived baseline).
Conditioned preference for sweet solutions in OLETF and LETO rats
There were no overall within group differences in US(+/−) intake during training, or CS(+/−) intake, or preference during testing, according to whether grape or cherry was used as CS+ flavor in all three conditioning studies. In all experiments, rats consumed all of the 3 ml clamped CS(+/) flavored solutions prior to US(+/−) access. Additionally, no significant differences between results of day 9 or day 10 preference tests were found within deprivation condition across days. Therefore, these data were pooled for subsequent analyses, and presented in the following section accordingly.
Conditioned sucrose vs. water preference in OLETF and LETO rats
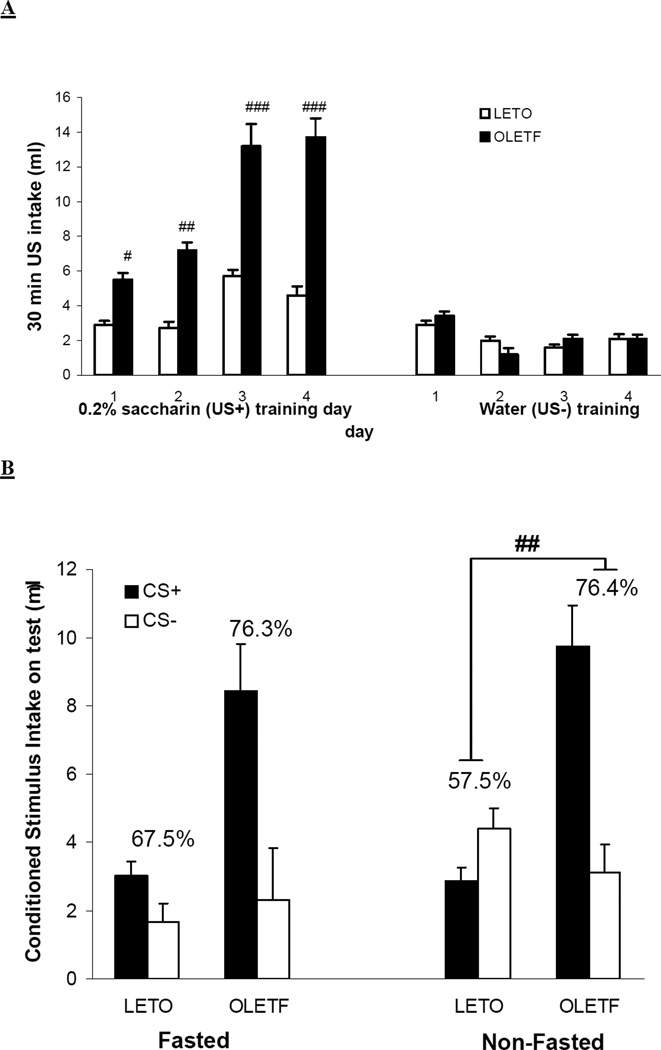
Figure 5A illustrates intake of US solutions during the 8 day training period. OLETF rats consumed more sucrose (US+) than LETO rats during the 30 min access period [F(4,18)=33.78; P<0.05]. Post-hoc analyses showed this effect across all four days of presentation (largest P<0.05). Water (US−) intake was not significantly different between strains. Figure 5B shows results of conditioned preference testing after all rats were given a 30 min two-bottle choice test between CS+ and CS− flavored solutions under either food-deprived or non-deprived conditions. OLETF rats preferred the CS+ flavor to a greater extent than LETO rats under both deprived [80.8 ± 4.9% and 70.8 ± 5.4% for OLETF and LETO rats, respectively: F(1,18)= 13.08.; P<0.05] and non-deprived conditions [82.1 ± 5.3 % and 53.6 ± 7.5% for OLETF and LETO rats, respectively: F(1,18)= 39.81; P<0.01]. When examining the effect of motivational state on conditioned sucrose preference, we observed that LETO rats exhibited a significantly increased CS+ preference when food deprived on test [F(1,18)=19.08; P<0.01], in contrast to OLETF rats that did not show altered preference for CS+ after overnight food deprivation (P=0.726). A similar effect was observed when OLETF rats’ sucrose intake during conditioning was clamped to the amount of LETO rats for either non-deprived or food deprived condition (data not shown).
Fig. 5.
Raw 0.3M sucrose (US+) and water (US−) intakes during one bottle conditioning phase (A) and percent preference for CS+ during two-bottle CS+ vs. CS− choice tests in food-deprived or non-deprived OLETF and LETO rats (B). After consuming a fixed 3 ml volume of flavored saccharin (CS), OLETF rats consumed more sucrose (US+) than LETO rats during a 30 min access period across all four days of presentation. Water (US−) intake was not significantly different between strains (A) (#P<0.05,##P<0.01,###P<0.001; indicates between strain differences in US intake on conditioning days). When given a 30 min two-bottle choice test between both CS+ and CS− solutions, OLETF rats preferred the CS+ flavor to a greater extent than LETO rats regardless of motivational state on test. In contrast, OLETF rats did not show altered preference for CS+ after overnight food deprivation, whereas LETO rats exhibited a significantly increased CS+ preference when food deprived on test (B) CS+ preference percentages are illustrated above SEM bars for raw CS (+/−) intakes within each group. (*P<0.05; indicates within strain preference differences between fasting and non-fasting tests: #P<0.05, ##P<0.01; indicates between strain preference differences within deprivation condition on test).
Conditioned saccharin vs. water preference in OLETF and LETO rats
Figure 6A shows intake of US solutions across the 8-day training period. OLETF rats consumed more of the non-caloric sweet saccharin (US+) than LETO rats during the 30-min access period directly following CS+ consumption [F(4,18)=26.78; P<0.05]. Post-hoc analyses revealed intake to be persistently higher in OLETF rats compared to LETO rats each day of presentation (largest P<0.05). Water (US−) intake was again not significantly different between strains.
Fig. 6.
Intakes of 0.2% saccharin (US+) and water (US−) during one bottle conditioning phase (A) and preference percentages for CS+ during two-bottle CS+ vs. CS− choice tests in non-deprived or food-deprived OLETF and LETO rats (B). After consuming a fixed 3 ml volume of flavored saccharin (CS), OLETF rats consumed more saccharin (US+) than LETO rats during a 30 min access period across all four days of presentation. Water (US−) intake was not significantly different between strains except on the first day of training (A) (#P<0.05, ##P<0.01,###P<0.001; indicates between strain differences in US intake on conditioning days). When given a 30 min two-bottle choice test between both CS+ and CS− solutions, OLETF rats preferred the CS+ flavor to a greater extent than LETO rats in the non-fasted condition. When food deprived on test, no strain differences were noted in CS+ preference. Both LETO and OLETF rats did not show altered preference for CS+ within strain after overnight food deprivation (B) CS+ preference percentages are given above SEM bars for raw CS (+/−) intakes within each experimental group. (##P<0.01; indicates between strain preference differences within deprivation condition on test).
Results of conditioned saccharin preference testing are presented in Figure 6B. Under conditions identical to those imposed in the previous experiment, OLETF rats preferred the CS+ flavor to a greater extent than LETO rats under only non-deprived conditions [76.4 ± 4.3% and 57.5 ± 5.9% for OLETF and LETO rats, respectively: F(1,18)= 28.66; P<0.01], while no significant difference was noted between strains under deprived conditions (P=0.894). In addition, LETO rats also exhibited no significant increase in CS+ preference when food deprived on test (P=0.087) relative to non-deprived preference. Similarly, OLETF rats showed comparable preference for CS+ regardless of motivational state on test (P=0.219).
Conditioned sucrose vs. saccharin preference in OLETF and LETO rats
Results of raw intakes during sucrose (US+) and saccharin (US−) conditioning periods are shown in Figure 7A. ANOVA results showed that OLETF rats consumed significantly greater amounts of both sucrose (US+) [F(4,18)=24.96; P<0.05] and saccharin (US−) [F(4,18)=17.45; P<0.05] solutions during training than LETO rats during the 30 min access period directly following CS(+/−) consumption. Post-hoc analyses revealed intake to be persistently higher in OLETF rats compared to LETO rats each day of presentation for both US+ and US− solutions (largest P<0.05 for both US+ and US−). Conditioned preference testing revealed the sucrose (US+) associated flavor (CS) to be the most preferred. Thus, the results of conditioned preference testing are presented in terms of CS+ preference. Figure 7B depicts results showing OLETF rats preferred the CS+ flavor to a greater extent than LETO rats under only non-deprived conditions [87.6 ± 4.4% and 57.9 ± 4.5% for OLETF and LETO rats, respectively: F(1,18)= 31.19; P<0.01], while no significant difference was noted between strains under deprived conditions (P=0.919). In addition, LETO rats exhibited a significantly increased CS+ preference when food deprived on test [F(1,18)=25.39; P<0.01] relative to non deprived preference, whereas OLETF rats showed similar preference for CS+ regardless of motivational state on test (P=0.622).
Fig. 7.
Intakes of 0.3M sucrose (US+) and 0.2% saccharin (US−) solutions during one bottle conditioning phase (A) and preference percentages for CS+ during two-bottle CS+ vs. CS− choice tests in non-deprived or food-deprived OLETF and LETO rats (B). OLETF rats consumed more sucrose (US+) and saccharin (US−) than LETO rats during a 30 min access period across all four days of presentation after consuming a fixed volume of a flavored saccharin (CS), (A) (#P<0.05, ##P<0.01; indicates between strain differences in US intake on conditioning days). When given a 30 min two-bottle choice test between both CS+ and CS− solutions, OLETF rats preferred the CS+ flavor to a greater extent than LETO rats when non-food deprived on test. No strain differences were noted in CS+ preference following food restriction. In contrast, OLETF rats did not show altered preference for CS+ within strain after overnight food deprivation, whereas LETO rats exhibited a significantly increased CS+ preference when food deprived on test (B) CS+ preference percentages are given above SEM bars for raw CS (+/−) intakes within each experimental group. (**P<0.01; indicates within strain preference differences between fasting and non-fasting tests: ##P<0.01; indicates between strain preference differences within deprivation condition on test).
DISCUSSION
The present results show that OLETF rats exhibit clear deficiencies in modifying sucrose intake after periods of acute food deprivation compared with LETO controls. For both a relatively dilute and concentrated sucrose concentration, OLETF rats show no significant alterations in either sucrose intake or preference, while LETO rats show both increased sucrose intake and preference under food deprivation conditions. Similarly, OLETF rats exhibit a higher conditioned flavor preference for sucrose relative to LETO, regardless of deprivation state. When OLETF and LETO rats are conditioned to prefer a saccharin solution, OLETF rats show a higher preference for the saccharin associated flavor relative to LETO rats when non-deprived, however neither strain shows differential flavor preference for saccharin according to deprivation state on test. These results suggest that orosensory effects of palatable solutions impart a greater role in governing food associations in OLETF rats relative to control, non-mutant LETO rats.
The size of a meal is partly dictated by the manifestation of post-ingestive inhibitory feedback signals. Disruptions of these signals can lead to overconsumption and weight gain. Indeed, we as well as others have shown that OLETF rats suppress intake less than LETO controls in response to a variety of intestinal nutrient infusions (12), as well as gastric fat preloads (38). Also, OLETF rats reduce sucrose sham feeding less than LETO rats following gastric balloon distention (16) suggesting that mechano-detection may be also impaired in these animals. While there is no doubt that aberrations in these signaling mechanisms play a major role in leading to excessive meal size (34), the amount consumed within a meal can also be modulated by the feed-forward effects resultant from orosensory stimulation (43). In this regard, we have recently reported ingestive abnormalities in the OLETF rats characteristic of both enhanced taste sensitivity and orosensory controls of sucrose intake which are manifest in the absence of gastric/post-gastric satiation effects (17, 21). In fact, non food-deprived, pre-diabetic, OLETF rats will sham feed more of a concentrated sucrose solution than age-matched LETO rats, thus overconsuming sucrose in the absence of post-absorptive effects (17). Furthermore, recent data from our laboratory using an automated gustometer have shown OLETF rats to exhibit increased licking of multiple concentrations of sucrose, fructose, and saccharin when granted only short 10 sec access to solutions, pointing to a generalized increase in sweet taste preference (21). The current experiments complement these existing studies by allowing the animals to experience and associate sucrose as both an oral and post-ingestive stimulus. By testing rats under food-deprived and free feeding conditions, we are able to assess how these associations may be weakened or strengthened, without modifying exposure to the stimulus itself. In fact, in the conditioning studies, the rats are tested solely on conditioned associations, as no sucrose is presented during testing.
The possibility that central taste functions are impaired in the OLETF rat, including an increased sensitivity to the incentive motivational effect (i.e. palatability) of ingestive stimuli is further supported by the results reported here. The observation that the magnitude of sucrose intake is similar in both non-deprived and 24 hr food-deprived OLETF rats suggests that an increase in orosensory sensitivity that is already present in ad libitum-fed conditions could be a major contributor to enhanced sucrose intake in addition to known satiation deficits. Specifically, free feeding OLETF rats overconsume rat chow by 30% relative to age-matched LETO rats (34). However, when given access to a palatable sucrose solution, the degree of hyperphagia observed in non food deprived OLETF rats increases by almost 60% for 0.3M sucrose and 146% for 1.0M sucrose relative to freely fed LETO controls. Barring differences between liquid and solid foods on this effect, the augmented magnitude of sucrose hyperphagia relative to chow consumption suggests that other factors than caloric properties of the sucrose, such as palatability, exacerbate the hyperphagic behavior of the OLETF rat. It could be argued that the failure of OLETF rats to increase sucrose intake when deprived may have been due to a ceiling effect as non-deprived intake was large. Similarly, the failure to observe an increase in 1.0M sucrose preference in OLETF rats might be due to their high preference when non-deprived. However, inspection of data from Figure 4 shows that this was not the case. Specifically, under the “high” restriction condition, OLETFs consumed ~17 ml of 1.0 M sucrose, while in the non-deprived condition (“none”), they consumed ~24 ml of 1.0 M sucrose. In fact, OLETFs showed a moderately decreased intake of the 1.0 M sucrose despite an extra “7-ml room” that could have allowed them to increase intake further, at least to the levels of controls. However, they did not. This rejects the argument that the ceiling effect might have contributed to their failure to increase intake when deprived. Although intake is not limited by mere volume, and is concentration-dependent, our data demonstrate that OLETF rats are capable of drinking larger quantities. For instance, in Figure 2, OLETF rats drank ≥35 ml of 0.3 M sucrose. Furthermore, in an earlier report, we showed that OLETF rats drank ≥26 ml of 0.7 M glucose in half the time (30 min) compared to the current study (12). Nevertheless, one condition where OLETF might have encountered a ceiling effect is when tested for preference under the “low” deprivation. In this condition, total intake was approaching, but not reaching, the intake during non-deprived condition. Therefore, we can cautiously assume that in our study during the 60-min recording period, OLETF rats would have been able to increase their intake beyond the amount consumed under food deprivation.
It is well known that the post-ingestive, or nutritive properties of nutrients are able to condition flavor preference (40, 42). Previous work has focused on the effects of food deprivation on conditioned flavor preference in normal (i.e. non-mutant) rats using similar degrees of food deprivation employed in the current design (7, 9, 10). However, several of these studies have examined specifically how flavor preferences are modulated by food deprivation using food exposures during conditioning phases that have been previously associated with a specific caloric need state before preference testing, i.e. low or high food deprivation conditions. In all three of our flavor conditioning tests, CS solutions and the associated tastants (US) were presented when the animal was under ad libitum fed conditions prior to testing. Thus, our design aimed at the effects of differential caloric motivational state specifically on the expression of conditioned flavor preferences and not the acquisition of these preferences (i.e., ad libitum chow availability throughout the conditioning phase).
In our first flavor conditioning test, we showed that food-deprived OLETF rats exhibit no difference in conditioned flavor preference for sucrose when either ad libitum fed or food-deprived on test, while LETO rats showed significantly increased flavor preference for sucrose when food-deprived on test. These results support our hypothesis that while in LETO rats a calorie-association controls preference for sucrose over taste-association under deprived conditions, in OLETF rats the performance on test was not enhanced by increasing caloric drive. We also showed that when comparing between strains, OLETF rats exhibit a higher flavor preference for sucrose relative to LETO rats regardless of deprivation state. Such results may be indicative of a generalized stronger degree of sucrose preference formation in OLETF relative to LETO rats. One possible explanation for this effect is that, among OLETF rats, sucrose possesses an inherently greater reinforcing effect as unconditioned stimulus (37, 41). Such an effect may be related to differences in gustatory functions or the sensitivity of the reward systems, both of which aspects are currently under investigation in our laboratory. While beyond the scope of the present design, investigation of the reinforcing effects of sucrose through operant conditioning procedures may support this notion more directly. Nonetheless, we are still able to provide clear evidence that when tested for conditioned preference, increased caloric need due to food deprivation appears to play a minimal role in the expression of conditioned sucrose preference in the OLETF rat. In other words, orosensory effects of sucrose in hyperphagic OLETF rats are stronger to form preferences than in LETO rats, irrespective of deprivation state.
Our results of enhanced conditioned saccharin preference in non-deprived OLETF rats compared to LETO controls further support this hypothesis. As saccharin is a non-caloric sweet tastant, nutrient satiation deficiencies in the OLETF rat could not account for increased saccharin intake or preference in these animals. We have recently published evidence of enhanced licking of saccharin solutions in the OLETF rat in brief 10 s access testing (21). Thus, one possible explanation for increased saccharin intake during our conditioning phase is an enhanced gustatory sensitivity for the sweet taste of saccharin. Finally, neither strain showed significant alterations in conditioned preference for saccharin in the deprivation state on test consistent with previous reports of conditioned saccharin preference (19). This finding lends support to the notion that an increased potential of sucrose to form conditioned preferences is based on its caloric predictive value or metabolic effects. Such a possibility can be addressed by examining sham feeding of sucrose during conditioning.
Our final study showed that when replacing water (US−) with a more palatable non-caloric sweet saccharin solution, we obtained similar results of conditioned sucrose (US+) preference as that of our earlier experiment. Specifically, OLETF rats showed a higher conditioned preference under non-deprived conditions relative to LETO rats. Secondly, OLETF rats did not show intake differences by motivational state on test, while LETO rats showed a significant increase in conditioned sucrose preference in the food deprivation test. This study suggests that while control LETO rats increase their conditioned preference for a caloric sweet taste over one that is non-caloric when food–deprived, OLETF rats do not appear to display such effects.
In conditioning experiments where US intakes were not clamped during training, we readily observed increased sucrose intake in OLETF rats relative to LETO rats. One can argue that greater reinforcement might lead to stronger preferences, and therefore OLETF rats’ tests results cannot be attributed solely to strain differences in learning about sucrose associates. The existing literature on the influence of the strength of the reinforcement stimulus on forming subsequent preferences is inconclusive. Some suggest that the magnitude of conditioned preference increases after additional training (42), while others show no such correlations (32). In our tests, when OLETF sucrose intakes were clamped to the levels of LETO during conditioning training, OLETF rats did not increase CS+ when deprived compared to non-deprived. Thus, the increased sucrose intake during training seems to have no effect on our results. However, even this method has its limitations (see 50). In particular, OLETF rats drank significantly less CS+/sucrose solution in the clamped study than they normally did when intakes were unlimited. This may have resulted in a “frustration” effect that could reduce their attraction to the CS+ flavor during preference tests. Furthermore, OLETF rats over-consume in part due to reduced satiation signaling, suggesting that increased US intake under ad libitum access conditions is not directly due to increased US exposure effects, but rather may be an artifact of reduced negative feedback signals normally limiting intake.
Overall, the present findings are likely due to deficiencies in the integration of post-ingestive and orosensory signals determining meal size. Recent work from our laboratory has demonstrated that neurons expressing c-fos immunoreactivity in response to CCK are virtually absent in the OLETF rat (13). These data confirm that CCK-related satiation deficits in the OLETF extend to regions of the hindbrain known to relay satiation signals from the gut to the brain via CCK-1 receptor activation. A separate body of work has also shown that the absence of CCK-1 receptors in the dorsomedial hypothalamus may be responsible for altered NPY expression in the obese OLETF rat (3, 35). Of particular interest to the current results are our recent reports that OLETF rats exhibit enhanced intake of sucrose when post-ingestive effects of the solution are eliminated or largely minimized (17, 21). Furthermore, it must also be mentioned that the observed deficits in OLETF rats may be due to the absence of CCK-1 receptor mediated effects on specific taste receptors encoding for sweet stimuli. Recent evidence has supported a possible role for CCK, acting through CCK-1 receptor activation, in taste receptor cells (26). Specifically, it has been shown that many CCK-responsive taste cells are also sensitive to the two bitter stimuli quinine and caffeine (26, 31). The possible impact of CCK-sensitive cells and sweet stimuli sensitivity is unknown, however such studies may help to determine why the CCK-1 receptor deficient OLETF rat may exhibit altered orosensory effects of sweet solutions. In addition, deficient responding to the gustatory relays from taste buds to the NTS and/or projections to the gustatory centers of the parabrachial nucleus (PBN) could also be implicated in these results.
It is also possible that deficits in processing food reward could help explain enhanced orosensory control of sweet preference. For example, dopamine activity, particularly within the mesolimbic pathway, increases after ingestion of palatable sucrose solutions and when orosensory components of these tastants were isolated (22, 46). With relevance to the current model, CCK stimulates DA release by acting on CCK-1 receptors (14). Central CCK-1 receptors are not ubiquitous, in fact only specific populations are known to exist, within limited regions such as the dorsomedial hypothalamus (36, 47), striatum (20), and the caudo-medial shell of the NAcc (25, 30). In the caudal NAcc, DA and CCK are co-released in vivo after administration of drugs that increase DA neuronal firing rate (14, 29). A similar co-release of DA and CCK in the ventral tegmental area (VTA) has been shown to affect DA cell firing rate (23). Moreover, we have shown an altered D2 dopamine receptor function related to sucrose intake in addition to lower D2 receptor binding in the striatum of pre-diabetic OLETF rats of similar age (33). These neural alterations could function to enhance the effects of the hyperphagia in the OLETF rat through not only independent mechanisms, but also collectively, resulting in an overall augmentation of meal size.
Taken together, the present studies indicate that among OLETF rats, both intake of and preference for sucrose, as well as a flavor conditioned sucrose preference, has a relatively stronger orosensory component compared to control LETO animals. Furthermore, food deprivation conditions, which lead to increased consumption of sucrose or sucrose associated flavors in LETO rats, appear to have little impact on consumption of these same fluids in OLETF rats. Thus, it appears that OLETF rats form preferences for sucrose based largely on orosensory and hedonic properties of the solution, rather than post-ingestive associations.
ACKNOWLEDGMENTS
The authors wish to thank Otsuka Pharmaceutical Co. (Tokushima, Japan) for the generous donation of the OLETF and LETO animals used to perform this research. We also wish to thank Dr. Anthony V. Azzara for his critical reading of an earlier version of the manuscript.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-065709.
References
- 1.Azzara AV, Sclafani A. Flavor preferences conditioned by intragastric sugar infusions in rats: maltose is more reinforcing than sucrose. Physiol Behav. 1998;64:535–541. doi: 10.1016/s0031-9384(98)00113-9. [DOI] [PubMed] [Google Scholar]
- 2.Berridge KC. Modulation of taste affect by hunger, caloric satiety, and sensory-specific satiety in the rat. Appetite. 1991;16:103–120. doi: 10.1016/0195-6663(91)90036-r. [DOI] [PubMed] [Google Scholar]
- 3.Bi S, Ladenheim EE, Schwartz GJ, Moran TH. A role for NPY overexpression in the dorsomedial hypothalamus in hyperphagia and obesity of OLETF rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R254–R260. doi: 10.1152/ajpregu.2001.281.1.R254. [DOI] [PubMed] [Google Scholar]
- 4.Bi S, Moran TH. Response to acute food deprivation in OLETF rats lacking CCK-A receptors. Physiol Behav. 2003;79:655–661. doi: 10.1016/s0031-9384(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 5.Blumberg S, Haba D, Schroeder M, Smith GP, Weller A. Independent ingestion and microstructure of feeding patterns in infant rats lacking CCK-1 receptors. Am J Physiol Regul Integr Comp Physiol. 2005 doi: 10.1152/ajpregu.00379.2005. [DOI] [PubMed] [Google Scholar]
- 6.Campbell BA. Absolute and relative sucrose preference thresholds for hungry and satiated rats. J Comp Physiol Psychol. 1958;51:795–800. doi: 10.1037/h0039001. [DOI] [PubMed] [Google Scholar]
- 7.Capaldi ED, Campbell DH, Sheffer JD, Bradford JP. Conditioned flavor preferences based on delayed caloric consequences. J Exp Psychol Anim Behav Process. 1987;13:150–155. [PubMed] [Google Scholar]
- 8.Capaldi ED, Hunter MJ. Taste and odor in conditioned flavor preference learning. Animal Learning & Behavior. 1994;22:355–365. [Google Scholar]
- 9.Capaldi ED, Myers DE. Taste preference as a function of food deprivation during original taste exposure. Animal Learning & Behavior. 1982;10:211–219. [Google Scholar]
- 10.Capaldi ED, Myers DE, Campbell DH, Sheffer JD. Conditioned flavor preferences based on hunger level during original flavor exposure. Animal Learning & Behavior. 1983;11:107–115. [Google Scholar]
- 11.Collier G, Bolles R. Hunger, thirst, and their interaction as determinants of sucrose consumption. J Comp Physiol Psychol. 1968;66:633–641. doi: 10.1037/h0026538. [DOI] [PubMed] [Google Scholar]
- 12.Covasa M, Ritter RC. Attenuated satiation response to intestinal nutrients in rats that do not express CCK-A receptors. Peptides. 2001;22:1339–1348. doi: 10.1016/s0196-9781(01)00461-2. [DOI] [PubMed] [Google Scholar]
- 13.Covasa M, Ritter RC. Reduced CCK-induced Fos expression in the hindbrain, nodose ganglia, and enteric neurons of rats lacking CCK-1 receptors. Brain Res. 2005;1051:155–163. doi: 10.1016/j.brainres.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Crawley JN. Cholecystokinin-dopamine interactions. Trends Pharmacol Sci. 1991;12:232–236. doi: 10.1016/0165-6147(91)90558-a. [DOI] [PubMed] [Google Scholar]
- 15.De Jonghe BC, Di Martino C, Hajnal A, Covasa M. Brief intermittent access to sucrose differentially modulates prepulse inhibition and acoustic startle response in obese CCK-1 receptor deficient rats. Brain Res. 2005;1052:22–27. doi: 10.1016/j.brainres.2005.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Jonghe BC, Hajnal A, Covasa M. Decreased gastric mechanodetection, but preserved gastric emptying, in CCK-1 receptor deficient OLETF rats. Am J Physiol Gastrointest Liver Physiol. 2006 doi: 10.1152/ajpgi.00109.2006. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Jonghe BC, Hajnal A, Covasa M. Increased oral and decreased intestinal sensitivity to sucrose in obese, prediabetic CCK-A receptor-deficient OLETF rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R292–R300. doi: 10.1152/ajpregu.00481.2004. [DOI] [PubMed] [Google Scholar]
- 18.Elizalde G, Sclafani A. Flavor preferences conditioned by intragastric polycose infusions: a detailed analysis using an electronic esophagus preparation. Physiol Behav. 1990;47:63–77. doi: 10.1016/0031-9384(90)90043-4. [DOI] [PubMed] [Google Scholar]
- 19.Fedorchak PM, Bolles RC. Hunger enhances the expression of calorie- but not taste-mediated conditioned flavor preferences. J Exp Psychol Anim Behav Process. 1987;13:73–79. [PubMed] [Google Scholar]
- 20.Graham WC, Hill DR, Woodruff GN, Sambrook MA, Crossman AR. Reduction of [125I]Bolton Hunter CCK8 and [3H]MK-329 (devazepide) binding to CCK receptors in the substantia nigra/VTA complex and its forebrain projection areas following MPTP-induced hemi-parkinsonism in the monkey. Neurosci Lett. 1991;131:129–134. doi: 10.1016/0304-3940(91)90353-u. [DOI] [PubMed] [Google Scholar]
- 21.Hajnal A, Covasa M, Bello NT. Altered taste sensitivity in obese, pre-diabetic OLETF rats lacking CCK-1 receptors. Am J Physiol Regul Integr Comp Physiol. 2005 doi: 10.1152/ajpregu.00412.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2004;286:R31–R37. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton ME, Freeman AS. Effects of administration of cholecystokinin into the VTA on DA overflow in nucleus accumbens and amygdala of freely moving rats. Brain Res. 1995;688:134–142. doi: 10.1016/0006-8993(95)00518-u. [DOI] [PubMed] [Google Scholar]
- 24.Harris JA, Gorissen MC, Bailey GK, Westbrook RF. Motivational state regulates the content of learned flavor preferences. J Exp Psychol Anim Behav Process. 2000;26:15–30. doi: 10.1037//0097-7403.26.1.15. [DOI] [PubMed] [Google Scholar]
- 25.Heidbreder C, Gewiss M, De Potter W, De Witte P. Regional amines levels in the rat brain following intra-accumbens cholecystokinin and intraperitoneal amphetamine pretreatment. Arch Int Physiol Biochim Biophys. 1992;100:267–273. doi: 10.3109/13813459208998113. [DOI] [PubMed] [Google Scholar]
- 26.Herness S, Zhao FL, Lu SG, Kaya N, Shen T. Expression and physiological actions of cholecystokinin in rat taste receptor cells. J Neurosci. 2002;22:10018–10029. doi: 10.1523/JNEUROSCI.22-22-10018.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holder MD. Conditioned preferences for the taste and odor components of flavors: blocking but not overshadowing. Appetite. 1991;17:29–45. doi: 10.1016/0195-6663(91)90082-4. [DOI] [PubMed] [Google Scholar]
- 28.Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992;41:1422–1428. doi: 10.2337/diab.41.11.1422. [DOI] [PubMed] [Google Scholar]
- 29.Ladurelle N, Durieux C, Roques BP, Dauge V. Different modifications of the dopamine metabolism in the core and shell parts of the nucleus accumbens following CCK-A receptor stimulation in the shell region. Neurosci Lett. 1994;178:5–10. doi: 10.1016/0304-3940(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 30.Lanca AJ, De Cabo C, Arifuzzaman AI, Vaccarino FJ. Cholecystokinergic innervation of nucleus accumbens subregions. Peptides. 1998;19:859–868. doi: 10.1016/s0196-9781(98)00032-1. [DOI] [PubMed] [Google Scholar]
- 31.Lu SG, Zhao FL, Herness S. Physiological phenotyping of cholecystokinin-responsive rat taste receptor cells. Neurosci Lett. 2003;351:157–160. doi: 10.1016/j.neulet.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Lucas F, Azzara AV, Sclafani A. Flavor preferences conditioned by intragastric polycose in rats: more concentrated polycose is not always more reinforcing. Physiol Behav. 1997;63:7–14. doi: 10.1016/s0031-9384(97)00364-8. [DOI] [PubMed] [Google Scholar]
- 33.Margas WM, Acharya NK, Covasa M, Hajnal A. Society for the Study of Ingestive Behavior: Annual Meeting. Pittsburgh, PA: Appetite; 2005. Obese OLETF rats show augmented ingestive and motor responses to dopamine D2 receptor stimulation. in press. [Google Scholar]
- 34.Moran TH, Katz LF, Plata-Salaman CR, Schwartz GJ. Disordered food intake and obesity in rats lacking cholecystokinin A receptors. Am J Physiol. 1998;274:R618–R625. doi: 10.1152/ajpregu.1998.274.3.R618. [DOI] [PubMed] [Google Scholar]
- 35.Moran TH, Lee P, Ladenheim EE, Schwartz GJ. Responsivity to NPY and melanocortins in obese OLETF rats lacking CCK-A receptors. Physiol Behav. 2002;75:397–402. doi: 10.1016/s0031-9384(01)00667-9. [DOI] [PubMed] [Google Scholar]
- 36.Moran TH, Robinson PH, Goldrich MS, McHugh PR. Two brain cholecystokinin receptors: implications for behavioral actions. Brain Res. 1986;362:175–179. doi: 10.1016/0006-8993(86)91413-7. [DOI] [PubMed] [Google Scholar]
- 37.Reilly S. Reinforcement value of gustatory stimuli determined by progressive ratio performance. Pharmacol Biochem Behav. 1999;63:301–311. doi: 10.1016/s0091-3057(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz GJ, Whitney A, Skoglund C, Castonguay TW, Moran TH. Decreased responsiveness to dietary fat in Otsuka Long-Evans Tokushima fatty rats lacking CCK-A receptors. Am J Physiol. 1999;277:R1144–R1151. doi: 10.1152/ajpregu.1999.277.4.R1144. [DOI] [PubMed] [Google Scholar]
- 39.Sclafani A. Learned controls of ingestive behaviour. Appetite. 1997;29:153–158. doi: 10.1006/appe.1997.0120. [DOI] [PubMed] [Google Scholar]
- 40.Sclafani A. Oral and postoral determinants of food reward. Physiol Behav. 2004;81:773–779. doi: 10.1016/j.physbeh.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 41.Sclafani A, Ackroff K. Reinforcement value of sucrose measured by progressive ratio operant licking in the rat. Physiol Behav. 2003;79:663–670. doi: 10.1016/s0031-9384(03)00143-4. [DOI] [PubMed] [Google Scholar]
- 42.Sclafani A, Ackroff K. The relationship between food reward and satiation revisited. Physiol Behav. 2004;82:89–95. doi: 10.1016/j.physbeh.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 43.Smith GP. The direct and indirect controls of meal size. Neurosci Biobehav Rev. 1996;20:41–46. doi: 10.1016/0149-7634(95)00038-g. [DOI] [PubMed] [Google Scholar]
- 44.Smith M, Duffy M. Consumption of sucrose and saccharine by hungry and satiated rats. J Comp Physiol Psychol. 1957;50:65–69. doi: 10.1037/h0041824. [DOI] [PubMed] [Google Scholar]
- 45.Takiguchi S, Takata Y, Funakoshi A, Miyasaka K, Kataoka K, Fujimura Y, Goto T, Kono A. Disrupted cholecystokinin type-A receptor (CCKAR) gene in OLETF rats. Gene. 1997;197:169–175. doi: 10.1016/s0378-1119(97)00259-x. [DOI] [PubMed] [Google Scholar]
- 46.Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- 47.Woodruff GN, Hill DR, Boden P, Pinnock R, Singh L, Hughes J. Functional role of brain CCK receptors. Neuropeptides. 1991;19(Suppl):45, 56. doi: 10.1016/0143-4179(91)90082-t. [DOI] [PubMed] [Google Scholar]
- 48.Yiin YM, Ackroff K, Sclafani A. Flavor preferences conditioned by intragastric nutrient infusions in food restricted and free-feeding rats. Physiol Behav. 2005;84:217–231. doi: 10.1016/j.physbeh.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 49.Yiin YM, Ackroff K, Sclafani A. Food deprivation enhances the expression but not acquisition of flavor acceptance conditioning in rats. Appetite. 2005;45:152–160. doi: 10.1016/j.appet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 50.Yu WZ, Sclafani A, Delamater AR, Bodnar RJ. Pharmacology of flavor preference conditioning in sham-feeding rats: effects of naltrexone. Pharmacol Biochem Behav. 1999;64:573–584. doi: 10.1016/s0091-3057(99)00124-0. [DOI] [PubMed] [Google Scholar]
- 51.Zverev YP. Effects of caloric deprivation and satiety on sensitivity of the gustatory system. BMC Neurosci. 2004;5:5. doi: 10.1186/1471-2202-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]