SUMMARY
This study determined the genetic background of virulence and resistance genes of MRSA ST398 in Austria. From 2004 up to 2008 a total of 41 human isolates of MRSA ST398 were investigated for virulence and resistance gene patterns using DNA microarray chip analysis. Highly similar virulence gene profiles were found in 29 (70·7%) of the isolates but genes encoding Panton–Valentine leukocidin, enterotoxins, or toxic shock syndrome toxin were not detected. Genes conferring resistance to tetracycline and erythromycin-lincosamide were common as all but one of the isolates exhibited tetM and/or tetK, which are involved in tetracycline resistance, and 12 (29·9%) were positive for ermC, conferring resistance to erythromycin/lincosamide. SplitsTree analysis showed that 40 isolates were closely related. Changes in virulence and resistance gene patterns were minimal over the observed time period.
Key words: Antibiotic resistance, Austria, DNA microarray, MRSA ST398, spa typing, Staphylococcus aureus
In recent years the emergence of methicillin-resistant Staphylococcus aureus (MRSA) sequence type 398 (ST398), known as live-stock-associated MRSA (LA-MRSA) has attracted particular attention. This is due to its association with pigs and pig farming and its ability to colonize or infect people, such as farmers, who work in close contact with these animals [1, 2]. Less frequently MRSA ST398 has been found in a variety of other farm animals, such as horses, poultry, or cattle and from foods of animal origin. Although inter-human transmission has been described, the incidence of MRSA ST398, especially in hospitals, appears to be much lower than for other MRSA genotypes [1].
MRSA ST398 usually lacks the virulence determinants that are typically found in hospital-acquired MRSA or community-acquired MRSA. The majority of analysed MRSA ST398 isolates carried haemolysin-encoding genes, only a small number of isolates carried the bicomponent leukotoxin Panton–Valentine (lukPV) genes or staphylococcal enterotoxins (SE; se genes). Genes for other toxins, i.e. exfoliative toxins (ET), leukocidins or toxic shock syndrome toxins (TSST-1) have not as yet been reported in MRSA ST398 isolates [3, 4].
Phenotypically, MRSA ST398 is resistant to oxacillin and all β-lactam antibiotics. Resistance to tetracycline is also very common due to its frequent use in pig and cattle farming [5]. Resistance to other antibiotics, especially erythromycin, clindamycin, aminoglycosides or trimethoprim/sulfamethoxazole has been reported; however, multi-resistance to ⩾3 antimicrobial groups is uncommon [1, 3].
In Austria intensive pig farming with about 1·2 million pigs is concentrated in two regions (north and south-east) which are served by two microbiological laboratories and these account for practically all human MRSA ST398 isolates reported in Austria [2, 6].
The aim of this study was to determine whether the virulence and antimicrobial resistance gene profiles of MRSA ST398 in human isolates in Austria differ between the two regions or whether animal trading throughout the country resulted in the spread of identical MRSA ST398 clones.
From January 2004 to December 2008, 41 MRSA ST398 isolates were identified from a total of about 1900 MRSA human primary isolates, from routine samples at two microbiological laboratories in Austria (27 from Linz, 14 from Graz). As published previously, contact with animals (predominantly pigs and/or cows, and horses or hens only) was documented in 32 patients. Clinical manifestations ranged from solely colonization to purulent infections including a case of complicated osteomyelitis. The number of MRSA ST398 detected per year increased steadily from one isolate in 2004 to 16 in 2008 [2, 6].
All MRSA ST398 isolates were retested for susceptibility to the following antimicrobial agents: oxacillin, tetracycline, erythromycin, clindamycin, gentamicin, trimethoprim/sulfamethoxazole, quinupristin/dalfopristin, fusidic acid, daptomycin, rifampicin, linezolid, vancomycin, and chloramphenicol by E-tests (bioMérieux, France) according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) [7]. Isolates were also further characterized by spa typing, multilocus sequence typing (MLST) and SCCmec typing as described previously [6].
Identibac MRSA bacterial genotyping system (Identibac, UK; StaphyType, Alere Technologies GmbH, Germany) was used to characterize the isolates, according to the manufacturer's instructions and recently published recommendations [8]. This DNA microarray comprises 334 target sequences, which correspond to 171 distinct genes and their allelic variants, including S. aureus specific genes, accessory gene regulator (agr) alleles and genes coding for virulence factors (toxins, enterotoxins, haemolysins, proteases, biofilm formation molecules). Hybridization patterns were analysed using Splits Tree4 software (www.splitstree.org), designed to compute unrooted phylogenetic networks from molecular sequence data. Gene profiles were converted to ‘sequence-like’ strings of information, defining present genes as ‘A’ (positive), absent genes as ‘T’ (negative) and spots with ambiguous signal intensities as missing [9].
Spa typing of the 41 MRSA ST398 isolates assigned 36 to spa type t011, two each to t034 and t2346, and one to t1451. Types t011 and t034 were found in both the north and south-east of Austria whereas t2346 was confined to the north; the t1451 isolate originated in the south-east. All, but one, of the isolates were of SCCmec type V and the outlier (t034) was of SCCmec type IV. All isolates were of agr type I.
As expected resistance to oxacillin was universal but 13 (31·7%) isolates exhibited a multidrug resistance phenotype with resistance to ⩾3 classes of antimicrobial agents. All but one isolate were resistant to tetracycline and 13 expressed constitutive erythromycin-clindamycin resistance (cMLSB); three isolates were resistant to gentamicin and one to chloramphenicol. The single tetracycline-susceptible isolate was of type t034 and originated from northern Austria.
None of the isolates exhibited resistance to trimethoprim/sulfamethoxazole, quinupristin/dalfopristin, fusidic acid, daptomycin, rifampicin, linezolid, and vancomycin.
Determination of the resistance gene profiles using the DNA microarray demonstrated concordant results with susceptibility data. All isolates were positive for the mecA gene and 40 harboured the ampicillin-penicillin resistance gene blaZ and the tetracycline resistance gene tetM; 28 isolates carried the tetK gene. Regarding genes involved in resistance to eryth romycin-clindamycin (MLSB), ermC was detected in 13 isolates, which was in agreement with phenotypic resistance patterns but ermA and ermB were not identified in any of the isolates and none harboured other macrolide resistance genes msrA, mefA, mpbBM and the clindamycin resistance gene linA. Three isolates carried the streptogramin resistance gene vga, two isolates the aminoglycoside resistance genes aacA-aphD and one was positive for flexA (conferring resistance to chloramphenicol). A single isolate carried the mecA gene only and no further resistance genes.
All 41 isolates were negative for genes encoding PVL (lukS-PVL, lukF-PVL), enterotoxins (ent genes), and the toxic shock syndrome toxin (tst-1). By contrast all isolates were positive for genes encoding leukocidins (lukF and lukS as part of the hlg locus), haemolysins (hl, hld, hlIII), aureolysin (aur), glutamyl endopeptidase/V8-protease (sspA) and staphopain A (sspP). The frequency of other genes was as follows: leukocidin genes lukX in 40 and lukY in 36 (87·8%) isolates, the haemolysin genes hlgA in 39 (95·1%), and hla in 32 (78·0%) isolates, and the staphopain B gene sspB in 39 (95·1%) isolates. The sak gene encoding staphylokinase in combination with scn encoding a staphylococcal complement inhibitor was detected in four (9·8%) isolates, chp (CHIPS) gene coding for chemotaxis inhibitory protein in three (7·3%) and the arcD-SCC gene coding for arginine/ornithine antiporter in only a single (2·4%) isolate. Twenty-nine (70·7%) isolates demonstrated highly similar virulence gene profiles including the leukocidin genes (lukF, lukS, lukX, lukY), haemolysin genes (hlgA, hl, hla, hld, hlIII), aureolysin gene (aur), glutamyl endopeptidase/V8-protease gene (sspA), staphopain B gene (sspB), and staphopain A gene (sspP). Other gene profiles were represented by one or two isolates.
SplitsTree analysis was used for calculation of an unrooted phylogenetic network depicting strain relatedness, using the data on presence/absence of genes generated by DNA microarray. Except for one strain of spa type t034, all isolates showed highly similar gene patterns suggesting they were closely related (see Fig. 1). Besides several minor clusters, one major cluster comprised eight of the 14 MRSA ST398 isolates from the south-east and two from the north of Austria. Four isolates exhibited identical gene profiles indicative of the same strain (Fig. 1). The analysis did not result in a clear separation of isolates from the two geographical regions but some time-dependent genetic changes were evident as 9/10 isolates recovered from 2004 to 2006 clustered very closely together, while those from 2007 and 2008 showed greater genetic variations, depicted in increased distance in SplitsTree.
Fig. 1.
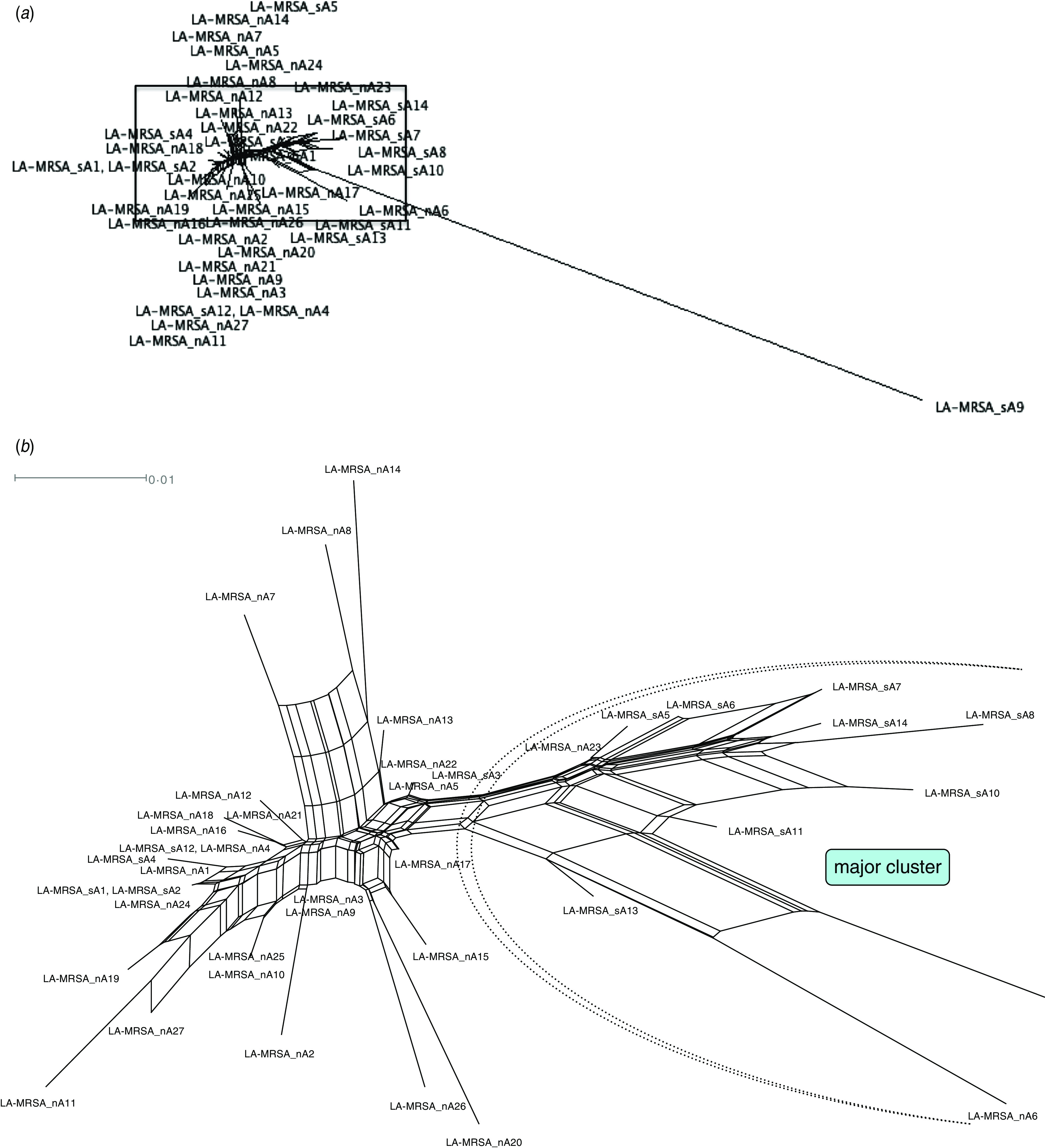
[colour online]. SplitsTree visualizing the similarity among gene profiles obtained by DNA microarray analysis of 41 human isolates of MRSA ST398 in Austria (2004–2008). (a) Overview over the full SplitsTree depicting all 41 isolates. (b) Detail zooming in on the region depicting the 40 highly similar MRSA ST398 isolates, while omitting atypical isolate LA-MRSA_sA9. Among minor clusters, one major cluster containing almost exclusively strains isolated from the south of Austria was found.
In accordance with previous studies, resistance to tetracycline was almost universal among the isolates and related to the presence of the tetM gene [3], although two-thirds of the isolates also carried the tetK gene. The finding of more than one tet gene might be explained by the high antibiotic pressure exerted by the widespread use of tetracycline in animal farming [5]. The absence of ermA or ermB encoding MLSB resistance in the isolates is in contrast to the predominance of ermA in oxacillin-susceptible S. aureus. It has been also suggested that ermB is more common in staphylococci from animal sources compared to human sources, but this could not be confirmed by our study [10]. The finding of a single chloramphenicol-resistant isolate in this series corroborates the reported rarity of the fexA gene in MRSA ST398 [3].
Multi-resistance was found in 31·7% of isolates and this rate may be considered low compared to reports from other countries [3, 11]. However, the absence of resistance to ciprofloxacin or trimethoprim/sulfamethoxazole among the isolates contrasts with the reported resistance rates from Germany of 8% for ciprofloxacin and 4% for trimethoprim/sulfamethoxazole [1, 3]. Overall, resistance gene patterns were more homogeneous in isolates from south-eastern Austria than those from northern Austria, as some resistance genes (vga, aacA-aphA, fexA) were only detected in strains from northern Austria.
As described previously, the tested MRSA ST398 isolates lacked genes encoding several clinically important S. aureus-associated virulence factors such as PVL, toxic shock syndrome toxin, exfoliative toxins, or enterotoxins [3, 8]. This is consistent with the fact that clinical diseases in humans due to this genotype are rarely observed [1]. While all isolates were positive for lukS and lukF, no other leukocidin genes, such as lukM, lukD, and lukE were present. Nevertheless, the first isolates of MRSA ST398 harbouring the PVL gene have been described recently in the literature [12] and there is therefore a need for continuous surveillance as acquisition of this virulence trait could have a significant impact on the disease potential of the genotype.
Notably the two MRSA ST398 isolates of spa type t034 differed particularly from others in the series as one of these exhibited the highest number of virulence genes and was susceptible to tetracycline, which is rather uncommon in MRSA [3]. By contrast, the second t034 isolate had fewer virulence genes than all other isolates and also lacked lukX and lukY. While the lack of lukX in S. aureus, including MRSA, has been described before [11], the absence of lukY has not, to our knowledge, been reported. The second isolate was also the only representative from south-eastern Austria to demonstrate resistance to tetracycline and erythromycin-clindamycin. The Splits Tree analysis showed that this strain clustered separately from the rest and epidemiological investigation of animal contact and clinical background of the patient did not provide an explanation for the orign of this strain.
There are two main limitations of this study. First, comparison of MRSA ST398 obtained from human and animal sources within the same time frame was not possible, as screening of animals in Austria did not begin before 2008 [5]; second, the number of included human samples is limited, due to the rarity of this genotype in humans in Austria [2, 6].
To summarize, virulence and resistance gene profiles in Austrian MRSA ST398 appear at the moment to be largely uniform, with only few variations beginning in 2007. Our results support the hypothesis that the MRSA ST398 isolates in Austria may have originated from a single clone that was spread throughout the country by animal trading [13]. Up to now the input of new MRSA ST398 clones appears to be a rare event. The data obtained facilitate worldwide comparison of the virulence gene and antimicrobial resistance properties of this genotype; due to the already described occurrence of the PVL gene in MRSA ST398 continuous surveillance is needed to monitor a possible shift to a more virulent or more resistant form.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Graveland H, et al. Livestock-associated methicillin-resistant Staphylococcus aureus in animals and humans. International Journal of Medical Microbiology 2011; 301: 630–634. [DOI] [PubMed] [Google Scholar]
- 2.Grisold AJ, et al. Occurrence and genotyping using automated repetitive-sequence-based PCR of methicillin-resistant Staphylococcus aureus ST398 in Southeast Austria. Diagnostic Microbiology and Infectious Disease 2010; 66: 217–221. [DOI] [PubMed] [Google Scholar]
- 3.Argudin MA, et al. Virulence and resistance determinants in German Staphylococcus aureus ST398 isolates from non-human origin. Applied and Environmental Microbiology 2011; 77: 3052–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monecke S, et al. Microarray based study on virulence-associated genes and resistance determinants of Staphylococcus aureus isolates from cattle. Veterinary Microbiology 2007; 125: 128–140. [DOI] [PubMed] [Google Scholar]
- 5.Springer B, et al. Methicillin-resistant Staphylococcus aureus: a new zoonotic agent? Wiener Klinische Wochenschrift 2009; 121: 86–90. [DOI] [PubMed] [Google Scholar]
- 6.Krziwanek K, Metz-Gercek S, Mittermayer H. Methicillin-resistant Staphylococcus aureus ST398 from human patients, Upper Austria. Emerging Infectious Diseases 2009; 15: 766–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 18th informational supplement. CLSI document M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA, 2008. [Google Scholar]
- 8.Monecke S, et al. Microarray-based genotyping of Staphylococcus aureus isolates from camels. Veterinary Microbiology 2011; 150: 309–314. [DOI] [PubMed] [Google Scholar]
- 9.Wattinger L, et al. Comparison of Staphylococcus aureus isolates associated with food intoxication with isolates from human nasal carriers and human infections. European Journal of Clinical Microbiology and Infectious Diseases 2012; 31: 455–464. [DOI] [PubMed] [Google Scholar]
- 10.Gherardi G, et al. Macrolide resistance genotypes and phenotypes among erythromycin-resistant clinical isolates of Staphylococcus aureus and coagulase-negative staphylococci, Italy. FEMS Immunology and Medical Microbiology 2009; 55: 62–67. [DOI] [PubMed] [Google Scholar]
- 11.Huber H, et al. Genotypes, antibiotic resistance profiles and microarray-based characterization of methicillin-resistant Staphylococcus aureus strains isolated from livestock and veterinarians in Switzerland. Zoonoses Public Health 2011; 58: 343–349. [DOI] [PubMed] [Google Scholar]
- 12.Yao D, et al. Molecular characterization of Staphylococcus aureus isolates causing skin and soft tissue infections (SSTIs). BMC Infectious Diseases 2010; 10: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Loo IH, et al. Methicillin-resistant Staphylococcus aureus in meat products, the Netherlands. Emerging Infectious Diseases 2007; 13: 1753–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]


