Abstract
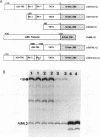
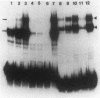
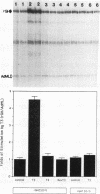
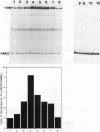
The expression of the rat growth hormone (rGH) gene in the anterior pituitary gland is modulated by Pit-1/GHF-1, a pituitary-specific transcription factor, and by other more widely distributed factors, such as the thyroid hormone receptors (TRs), Sp1, and the glucocorticoid receptor. Thyroid hormone (T3)-mediated transcriptional stimulation of rGH gene expression has been extensively studied in vivo and in vitro including the measurements of (i) rGH mRNA by blot hybridization, (ii) transcriptional rate of rGH gene by nuclear run-on, and (iii) reporter gene expression in which a chimeric plasmid containing 5'-flanking sequences of the rGH gene linked to a reporter gene has been transfected either stably or transiently into pituitary and/or nonpituitary cells. From these studies, it has been suggested that the Pit-1/GHF-1 binding site is necessary for full T3 action. We developed a cell-free in vitro transcription system to examine further the roles of the TRs and Pit-1/GHF-1 in rGH gene activation. Using GH3 nuclear extract as a source of TRs and Pit-1/GHF-1, this in vitro transcription assay showed that T3 stimulation of rGH promoter activity is dependent on the addition of T3 to the GH3 nuclear extract. This transcriptional stimulation was augmented with increasing concentrations of ligand and was T3, but not T4 or reverse T3, specific. T3-mediated stimulation of rGH promoter activity was completely abolished by preincubation of the nuclear extract with rGH-thyroid hormone response element (-200 to -160) but not with Pit-1/GHF-1 (-137 to -65) oligonucleotides. Further, neither deletion of both Pit-1/GHF-1 binding sites nor mutation of the proximal Pit-1/GHF-1 binding site from the rGH promoter abrogated the T3 effect. These results provide evidence that T3-stimulated rGH promoter activity is independent of Pit-1/GHF-1 and raise the possibility that the stimulation of rGH gene expression by T3 might involve direct interaction of TRs with the general transcriptional apparatus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan G. F., Ing N. H., Tsai S. Y., Srinivasan G., Weigel N. L., Thompson E. B., Tsai M. J., O'Malley B. W. Synergism between steroid response and promoter elements during cell-free transcription. J Biol Chem. 1991 Mar 25;266(9):5905–5910. [PubMed] [Google Scholar]
- Bagchi M. K., Tsai S. Y., Tsai M. J., O'Malley B. W. Identification of a functional intermediate in receptor activation in progesterone-dependent cell-free transcription. Nature. 1990 Jun 7;345(6275):547–550. doi: 10.1038/345547a0. [DOI] [PubMed] [Google Scholar]
- Beebe J. S., Darling D. S., Chin W. W. 3,5,3'-triiodothyronine receptor auxiliary protein (TRAP) enhances receptor binding by interactions within the thyroid hormone response element. Mol Endocrinol. 1991 Jan;5(1):85–93. doi: 10.1210/mend-5-1-85. [DOI] [PubMed] [Google Scholar]
- Berkenstam A., Vivanco Ruiz M. M., Barettino D., Horikoshi M., Stunnenberg H. G. Cooperativity in transactivation between retinoic acid receptor and TFIID requires an activity analogous to E1A. Cell. 1992 May 1;69(3):401–412. doi: 10.1016/0092-8674(92)90443-g. [DOI] [PubMed] [Google Scholar]
- Bisson R., Schiavo G. Two different forms of cytochrome c oxidase can be purified from the slime mold Dictyostelium discoideum. J Biol Chem. 1986 Apr 5;261(10):4373–4376. [PubMed] [Google Scholar]
- Bodner M., Castrillo J. L., Theill L. E., Deerinck T., Ellisman M., Karin M. The pituitary-specific transcription factor GHF-1 is a homeobox-containing protein. Cell. 1988 Nov 4;55(3):505–518. doi: 10.1016/0092-8674(88)90037-2. [DOI] [PubMed] [Google Scholar]
- Bodner M., Karin M. A pituitary-specific trans-acting factor can stimulate transcription from the growth hormone promoter in extracts of nonexpressing cells. Cell. 1987 Jul 17;50(2):267–275. doi: 10.1016/0092-8674(87)90222-4. [DOI] [PubMed] [Google Scholar]
- Brent G. A., Harney J. W., Chen Y., Warne R. L., Moore D. D., Larsen P. R. Mutations of the rat growth hormone promoter which increase and decrease response to thyroid hormone define a consensus thyroid hormone response element. Mol Endocrinol. 1989 Dec;3(12):1996–2004. doi: 10.1210/mend-3-12-1996. [DOI] [PubMed] [Google Scholar]
- Brent G. A., Larsen P. R., Harney J. W., Koenig R. J., Moore D. D. Functional characterization of the rat growth hormone promoter elements required for induction by thyroid hormone with and without a co-transfected beta type thyroid hormone receptor. J Biol Chem. 1989 Jan 5;264(1):178–182. [PubMed] [Google Scholar]
- Burnside J., Darling D. S., Carr F. E., Chin W. W. Thyroid hormone regulation of the rat glycoprotein hormone alpha-subunit gene promoter activity. J Biol Chem. 1989 Apr 25;264(12):6886–6891. [PubMed] [Google Scholar]
- Burnside J., Darling D. S., Chin W. W. A nuclear factor that enhances binding of thyroid hormone receptors to thyroid hormone response elements. J Biol Chem. 1990 Feb 15;265(5):2500–2504. [PubMed] [Google Scholar]
- Castrillo J. L., Bodner M., Karin M. Purification of growth hormone-specific transcription factor GHF-1 containing homeobox. Science. 1989 Feb 10;243(4892):814–817. doi: 10.1126/science.2563596. [DOI] [PubMed] [Google Scholar]
- Corthésy B., Hipskind R., Theulaz I., Wahli W. Estrogen-dependent in vitro transcription from the vitellogenin promoter in liver nuclear extracts. Science. 1988 Mar 4;239(4844):1137–1139. doi: 10.1126/science.2830672. [DOI] [PubMed] [Google Scholar]
- Darling D. S., Burnside J., Chin W. W. Binding of thyroid hormone receptors to the rat thyrotropin-beta gene. Mol Endocrinol. 1989 Sep;3(9):1359–1368. doi: 10.1210/mend-3-9-1359. [DOI] [PubMed] [Google Scholar]
- Desvergne B., Petty K. J., Nikodem V. M. Functional characterization and receptor binding studies of the malic enzyme thyroid hormone response element. J Biol Chem. 1991 Jan 15;266(2):1008–1013. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobner P. R., Kawasaki E. S., Yu L. Y., Bancroft F. C. Thyroid or glucocorticoid hormone induces pre-growth-hormone mRNA and its probable nuclear precursor in rat pituitary cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2230–2234. doi: 10.1073/pnas.78.4.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flug F., Copp R. P., Casanova J., Horowitz Z. D., Janocko L., Plotnick M., Samuels H. H. cis-acting elements of the rat growth hormone gene which mediate basal and regulated expression by thyroid hormone. J Biol Chem. 1987 May 5;262(13):6373–6382. [PubMed] [Google Scholar]
- Freedman L. P., Yoshinaga S. K., Vanderbilt J. N., Yamamoto K. R. In vitro transcription enhancement by purified derivatives of the glucocorticoid receptor. Science. 1989 Jul 21;245(4915):298–301. doi: 10.1126/science.2473529. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Franco R., Weinberger C., Albert V. R., Evans R. M., Rosenfeld M. G. A c-erb-A binding site in rat growth hormone gene mediates trans-activation by thyroid hormone. Nature. 1987 Oct 22;329(6141):738–741. doi: 10.1038/329738a0. [DOI] [PubMed] [Google Scholar]
- Ing N. H., Beekman J. M., Tsai S. Y., Tsai M. J., O'Malley B. W. Members of the steroid hormone receptor superfamily interact with TFIIB (S300-II). J Biol Chem. 1992 Sep 5;267(25):17617–17623. [PubMed] [Google Scholar]
- Ingraham H. A., Chen R. P., Mangalam H. J., Elsholtz H. P., Flynn S. E., Lin C. R., Simmons D. M., Swanson L., Rosenfeld M. G. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell. 1988 Nov 4;55(3):519–529. doi: 10.1016/0092-8674(88)90038-4. [DOI] [PubMed] [Google Scholar]
- Izumo S., Mahdavi V. Thyroid hormone receptor alpha isoforms generated by alternative splicing differentially activate myosin HC gene transcription. Nature. 1988 Aug 11;334(6182):539–542. doi: 10.1038/334539a0. [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L., Tsai S. Y., Weigel N. L., Allan G. F., Riley D., Rodriguez R., Schrader W. T., Tsai M. J., O'Malley B. W. The progesterone receptor stimulates cell-free transcription by enhancing the formation of a stable preinitiation complex. Cell. 1990 Jan 26;60(2):247–257. doi: 10.1016/0092-8674(90)90740-6. [DOI] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Mangelsdorf D. J., Evans R. M. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992 Jan 30;355(6359):446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig R. J., Brent G. A., Warne R. L., Larsen P. R., Moore D. D. Thyroid hormone receptor binds to a site in the rat growth hormone promoter required for induction by thyroid hormone. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5670–5674. doi: 10.1073/pnas.84.16.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar M. A., Hodin R. A., Darling D. S., Chin W. W. Identification of a rat c-erbA alpha-related protein which binds deoxyribonucleic acid but does not bind thyroid hormone. Mol Endocrinol. 1988 Oct;2(10):893–901. doi: 10.1210/mend-2-10-893. [DOI] [PubMed] [Google Scholar]
- Lefevre C., Imagawa M., Dana S., Grindlay J., Bodner M., Karin M. Tissue-specific expression of the human growth hormone gene is conferred in part by the binding of a specific trans-acting factor. EMBO J. 1987 Apr;6(4):971–981. doi: 10.1002/j.1460-2075.1987.tb04847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. S., Green M. R. Mechanism of action of an acidic transcriptional activator in vitro. Cell. 1991 Mar 8;64(5):971–981. doi: 10.1016/0092-8674(91)90321-o. [DOI] [PubMed] [Google Scholar]
- Martin K. J. The interactions of transcription factors and their adaptors, coactivators and accessory proteins. Bioessays. 1991 Oct;13(10):499–503. doi: 10.1002/bies.950131003. [DOI] [PubMed] [Google Scholar]
- Murray M. B., Towle H. C. Identification of nuclear factors that enhance binding of the thyroid hormone receptor to a thyroid hormone response element. Mol Endocrinol. 1989 Sep;3(9):1434–1442. doi: 10.1210/mend-3-9-1434. [DOI] [PubMed] [Google Scholar]
- Nelson C., Albert V. R., Elsholtz H. P., Lu L. I., Rosenfeld M. G. Activation of cell-specific expression of rat growth hormone and prolactin genes by a common transcription factor. Science. 1988 Mar 18;239(4846):1400–1405. doi: 10.1126/science.2831625. [DOI] [PubMed] [Google Scholar]
- Petty K. J., Morioka H., Mitsuhashi T., Nikodem V. M. Thyroid hormone regulation of transcription factors involved in malic enzyme gene expression. J Biol Chem. 1989 Jul 5;264(19):11483–11490. [PubMed] [Google Scholar]
- Raaka B. M., Samuels H. H. Regulation of thyroid hormone nuclear receptor levels in GH1 cells by 3,5,3'-triiodo-L-thyronine. Use of dense amino acid labeling to determine the influence of hormone on the receptor half-life and the rate of appearance of newly synthesized receptor. J Biol Chem. 1981 Jul 10;256(13):6883–6889. [PubMed] [Google Scholar]
- Samuels H. H., Shapiro L. E. Thyroid hormone stimulates de novo growth hormone synthesis in cultured GH1 cells: evidence for the accumulation of a rate limiting RNA species in the induction process. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3369–3373. doi: 10.1073/pnas.73.10.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Stanley F., Shapiro L. E. Dose-dependent depletion of nuclear receptors by L-triiodothyronine: evidence for a role in induction of growth hormone synthesis in cultured GH1 cells. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3877–3881. doi: 10.1073/pnas.73.11.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S., Casanova J., Stanley F. Thyroid hormone action: in vitro characterization of solubilized nuclear receptors from rat liver and cultured GH1 cells. J Clin Invest. 1974 Oct;54(4):853–865. doi: 10.1172/JCI107825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S. Thyroid hormone action in cell culture: domonstration of nuclear receptors in intact cells and isolated nuclei. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3488–3492. doi: 10.1073/pnas.70.12.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Schaufele F., West B. L., Baxter J. D. Synergistic activation of the rat growth hormone promoter by Pit-1 and the thyroid hormone receptor. Mol Endocrinol. 1992 Apr;6(4):656–665. doi: 10.1210/mend.6.4.1584227. [DOI] [PubMed] [Google Scholar]
- Shupnik M. A., Ridgway E. C. Thyroid hormone control of thyrotropin gene expression in rat anterior pituitary cells. Endocrinology. 1987 Aug;121(2):619–624. doi: 10.1210/endo-121-2-619. [DOI] [PubMed] [Google Scholar]
- Stringer K. F., Ingles C. J., Greenblatt J. Direct and selective binding of an acidic transcriptional activation domain to the TATA-box factor TFIID. Nature. 1990 Jun 28;345(6278):783–786. doi: 10.1038/345783a0. [DOI] [PubMed] [Google Scholar]
- Weinberger C., Thompson C. C., Ong E. S., Lebo R., Gruol D. J., Evans R. M. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986 Dec 18;324(6098):641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- Wood W. M., Kao M. Y., Gordon D. F., Ridgway E. C. Thyroid hormone regulates the mouse thyrotropin beta-subunit gene promoter in transfected primary thyrotropes. J Biol Chem. 1989 Sep 5;264(25):14840–14847. [PubMed] [Google Scholar]
- Yaffe B. M., Samuels H. H. Hormonal regulation of the growth hormone gene. Relationship of the rate of transcription to the level of nuclear thyroid hormone-receptor complexes. J Biol Chem. 1984 May 25;259(10):6284–6291. [PubMed] [Google Scholar]
- Ye Z. S., Forman B. M., Aranda A., Pascual A., Park H. Y., Casanova J., Samuels H. H. Rat growth hormone gene expression. Both cell-specific and thyroid hormone response elements are required for thyroid hormone regulation. J Biol Chem. 1988 Jun 5;263(16):7821–7829. [PubMed] [Google Scholar]
- Yen P. M., Darling D. S., Carter R. L., Forgione M., Umeda P. K., Chin W. W. Triiodothyronine (T3) decreases binding to DNA by T3-receptor homodimers but not receptor-auxiliary protein heterodimers. J Biol Chem. 1992 Feb 25;267(6):3565–3568. [PubMed] [Google Scholar]
- Yu V. C., Delsert C., Andersen B., Holloway J. M., Devary O. V., När A. M., Kim S. Y., Boutin J. M., Glass C. K., Rosenfeld M. G. RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell. 1991 Dec 20;67(6):1251–1266. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Hoffmann B., Tran P. B., Graupner G., Pfahl M. Retinoid X receptor is an auxiliary protein for thyroid hormone and retinoic acid receptors. Nature. 1992 Jan 30;355(6359):441–446. doi: 10.1038/355441a0. [DOI] [PubMed] [Google Scholar]