Abstract
Background
Twin and family studies have demonstrated that adolescent alcohol use and behavior problems are influenced by a combination of genetic and environmental factors. More recently, studies have begun to investigate how genetic and environmental influences may interact, with efforts underway to identify specific environmental variables that moderate the expression of genetic predispositions. Previously, we have reported that community-level factors, including urban/rural residency, migration rates, and prevalence of young adults, moderate the importance of genetic effects on alcohol use in late adolescence (ages 16 to 18). Here, we extend these findings to test for moderating effects of these socioregional factors on alcohol use and behavior problems assessed in a younger sample of adolescent Finnish twins.
Methods
Using data from the population-based Finnish twin study, FinnTwin12, biometric twin models were fit to data on >1,400 twin pairs to examine the significance of each of the socioregional moderating variables on alcohol use measured at age 14, and behavior problems, measured at age 12.
Results
We find no evidence of a moderating role of these socioregional variables on alcohol use; however, there was significant moderation of genetic influences on behavior problems, with effects limited to girls. Genetic influences assumed greater importance in urban settings, communities with greater migration, and communities with a higher percentage of slightly older adolescents.
Conclusions
The moderation effects observed on behavior problems in early adolescence paralleled the effects found on alcohol use late in adolescence in an independent sample, providing further support for the idea that behavior problems may represent an earlier manifestation of the predisposition to subsequent alcohol problems. Our findings also support the growing body of evidence suggesting that females may be more susceptible to a variety of environmental influences than males.
Keywords: Neighborhoods, Community, Genetics, Gene–Environment Interaction, Behavior Problems
Early adolescent alcohol use represents cause for considerable concern. According to the Monitoring the Future (MTF) study, approximately 70% of 12th graders, 60% of 10th graders, and 40% of 8th graders in the United States report having consumed alcohol at some point in their lives (Johnston et al., 2006). Among younger adolescents, nearly one-third of adolescents report using alcohol before age 13 (Grunbaum et al., 2004), and approximately 10% of 9- to 10-year-olds report that they have consumed alcohol (Donovan et al., 2004). Data from the European School Survey Project on Alcohol and Other Drugs (ESPAD), which collected data from 15- to 16-year-old students in 35 European countries, indicate similar patterns of problematic drinking: in Finland, 54% of surveyed adolescents reported drinking during the past 30 days (Hibell et al., 2004). Furthermore, alcohol use among adolescents is often high risk, characterized by drinking to excess (Miller et al., 2007). It has been reported that approximately 90% of the alcohol consumed by 12- to 17-year-old adolescents is consumed in binge-drinking episodes (U.S. Department of Justice, 1999). The MTF and ESPAD reports indicated that 22% of adolescents in the United States and 40% of adolescents in Finland report binge drinking in the past 30 days (Grube, 2001).
Adolescent alcohol use has been found to be associated with a variety of negative outcomes, including poor school performance, accepting rides from drivers who have been drinking, increased sexual activity, smoking, using illicit drugs, attempting suicide, and becoming a victim of dating violence (Miller et al., 2007). In addition to its effect in impairing decision making, adolescent alcohol use has been linked to physical, psychological and neurological problems, including sleep disturbance (Clark et al., 2001) and long-term learning and memory impairments (Zeigler et al., 2005). In addition, numerous studies have documented an association between early onset alcohol use and the subsequent development of alcohol dependence (DeWit et al., 2000; Grant and Dawson, 1997; Gruber et al., 1996; McGue et al., 2001).
Some of these negative consequences and concomitants of alcohol use may be due to a strong association between adolescent alcohol use and other behavior problems (Jessor and Jessor, 1977). Conduct problems are a robust predictor of both concurrent and future alcohol problems, as demonstrated in both school-based and clinically-ascertained samples. In a longitudinal study of >500 teenage boys, conduct problems were the sole form of psychopathology that predicted growth in alcohol use across 6 years of study (White et al., 2001). Several studies of adolescents with alcohol use disorders have concluded that, among all childhood behavioral disorders, conduct problems exhibits the strongest association with alcohol problems (Molina et al., 2002; Moss and Lynch, 2001).
Both genetic and environmental factors are important in patterns of adolescent alcohol use (Hopfer et al., 2003) and behavior problems (Moffitt, 2005; Rhee and Waldman, 2002). However, understanding genetic and environmental influences on these outcomes necessitates a dynamic, developmental perspective. While the initiation of alcohol use appears to be largely impacted by environmental factors (Hopfer et al., 2003; Pagan et al., 2006), frequency of alcohol use shows dramatic shifts in the relative importance of genetic and environmental effects across adolescence. In an older Finnish twin cohort, we found that genetic influences accounted for approximately one-third of the variation in drinking patterns at age 16, and increased to half of the variation by age 18 (Rose et al., 2001b). Environmental influences shared by the twins (called common environmental effects), on the other hand, accounted for the largest portion of variance in drinking at age 16, but dropped to only approximately 15% of the variance by age 18. Similarly, in a Dutch twin study, Koopmans and Boomsma (1996) found that genetic and environmental effects differed significantly between twins aged 15 to 16 and twins aged 17 and older, with genetic influences increasing in older ages.
Behavior problems also show considerable heritability, with meta-analytic estimates on the order of 40 to 50% (Moffitt, 2005; Rhee and Waldman, 2002). Of particular relevance to this project is the robust finding that the overlap between childhood conduct problems and later alcohol problems is due largely to shared genetic factors. This has been demonstrated across multiple twin samples (Kendler et al., 2003; Krueger, 1999; Krueger et al., 2002; Slutske et al., 1998; Young et al., 2000). In addition, an offspring of twins study found elevated rates of conduct disorder in alcohol-dependent fathers, with transmission patterns supporting the common genes hypothesis (Haber et al., 2005). Specific genes that have been associated with adult alcohol dependence have been associated with conduct problems in younger children/adolescents (rather than early adolescent alcohol dependence) (Dick et al., 2006), again suggesting that childhood behavior problems may be an early manifestation of an underlying predisposition to subsequent alcohol problems. The association with behavior problems may emerge earlier in development because genetic factors are apparent in behavior problems (showing up early in childhood) prior to their impact on patterns of alcohol use (where genetic influences assume greater importance later in adolescence).
Parallel to the literature suggesting multi-faceted developmental changes in the etiological factors impacting substance use and behavior problems, there is also an emerging literature documenting how specific environmental factors moderate the importance of genetic effects. A growing number of variables have been shown to moderate the relative importance of genetic effects on substance use and dependence and antisocial behavior. One of the earliest illustrations of gene–environment interaction in the area of substance use research demonstrated that genetic influences on alcohol use were greater among unmarried women, whereas having a marriage-like relationship reduced the impact of genetic influences on drinking (Heath et al., 1989). Religiosity has also been shown to moderate genetic influences on alcohol use among females, with genetic factors playing a larger role among individuals without a religious upbringing (Koopmans et al., 1999). Social contact (Kaprio et al., 1990; Rose et al., 1990) and co-twin dependency (Penninkilampi-Kerola et al., 2005) have also been shown to moderate twin similarity, with reduced genetic effects and enhanced environmental influences among more co-dependent pairs. Genetic influences on adolescent substance use are also enhanced in environments with lower parental monitoring (Dick et al., 2007c) and in the presence of substance-using friends (Dick et al., 2007b). Similar effects have been demonstrated more recently for antisocial behavior: genetic influences on antisocial behavior were higher in the presence of delinquent peers (Button et al., 2007) and in environments characterized by high parental negativity (Feinberg et al., 2007), low parental warmth (Feinberg et al., 2007), and high paternal punitive discipline (Button et al., 2008). However, the moderating role of the family environment on antisocial behavior and substance use appears to be complex, as one study found that the heritability of antisocial behavior was higher in families with lower levels of family dysfunction (Button et al., 2005), and the same study that reported elevated genetic variance in the presence of high paternal punitive discipline also found elevated genetic effects associated with low maternal punitive disciplines, which the authors hypothesized may reflect differential response to parenting by mothers and fathers.
It is likely that a variety of mechanistic processes underlie these gene–environment interaction effects. One theoretical paper suggested 4 processes by which social context may moderate the relative importance of genetic effects (Shanahan and Hofer, 2005). The environment may (1) trigger or (2) compensate for a genetic predisposition, (3) control the expression of genetic predisposition, or (4) enhance a genetic predisposition (referring to the accentuation of “positive” genetic predispositions). These processes are not mutually exclusive and can represent different ends of a continuum. It is likely that this is the case for several environments that have been shown to moderate substance use and antisocial behavior. For example, family environment and peer substance use/delinquency likely constitute a spectrum of risk or protection, and family/friend environments that are at the “poor” extreme may trigger genetic predispositions toward substance use and antisocial behavior, whereas positive family and friend relationships may compensate for genetic predispositions toward substance use and antisocial behavior. Social control also appears to be a particularly relevant process in substance use, as it is likely that being in a marriage-like relationship and/or being raised with a religious upbringing exert social norms that constrain behavior and thereby reduce genetic predispositions toward substance use.
We have previously demonstrated an important moderating role for socioregional influences on alcohol use in late adolescence in data from a Finnish twin study of older adolescents (FinnTwin16). Specifically, we found that genetic influences were higher in urban environments, whereas common environmental influences played a much larger role in rural environments (Rose et al., 2001b). It is likely that the urban/rural moderation effects reflect a composite of different processes at work. Accordingly, we subsequently expanded these analyses to incorporate more specific information about neighborhood environments, using government collected information about the specific municipalities in which the twins resided (Dick et al., 2001). We found that genetic influences were stronger in environments characterized by higher rates of migration in and out of the municipality; conversely, shared environmental influences predominated in local communities characterized by little migration. We hypothesized that migration rates provide some indication of neighborhood stability, and that neighborhoods characterized by higher stability engender more community monitoring, leading to reduced opportunity to express genetic predispositions. In the context of the aforementioned theoretical perspective, this presumably reflects social control processes. We also found that genetic predispositions were stronger in communities comprised of a higher percentage of young adults slightly older than our age 18 Finnish twins, and we hypothesized that this reflected greater opportunity to express individual differences as settings with more slightly older young adults presumably offer a larger number of role models, access to alcohol, and niche-picking opportunities.
Here, we report a series of analyses that follow-up that initial report. We examined the moderating role of urban/rural residency, migration rates, and percentage of slightly older adolescents in an independent, younger sample of Finnish twins, in which we have reports of behavior problems at age 12 and alcohol use at age 14. We tested whether these variables that showed significant moderation of genetic effects on alcohol use in late adolescence (age 18), would also moderate influences on alcohol use earlier in adolescence (age 14), addressing the question of potential developmental change in the importance of etiological factors impacting alcohol use. We also tested whether there were moderating effects on the related phenotype of behavior problems, based on the literature suggesting shared etiological factors influencing behavior problems and alcohol use and that behavior problems may represent an earlier manifestation of the genetic predisposition that later impacts alcohol problems.
METHODS
Participants and Procedures
FinnTwin12 (FT12) is a population-based, developmental twin study of health-related behaviors and correlated risk factors (Kaprio et al., 2002). It consists of 5 consecutive birth cohorts (1983–1987) of twins identified in Finland’s Population Registry Center (PRC), permitting exhaustive and unbiased ascertainment of all twins living and resident in the country. Questionnaires were mailed to all eligible families, of which 87% (n = 2,724 families) completed the initial family questionnaire. Noncompliance at these initial stages was associated neither with family structure, area of residence within Finland, parental age, nor sex or zygosity of the adolescent twin pairs. Immediately on receipt of the completed family questionnaire, individual questionnaires were mailed to both co-twins and both their parents (including parents not residing with either twin child). The twins’ self-report questionnaires were mailed in the late autumn of the year in which the consecutive birth cohorts reached age 11, and most twins (89.4%) returned their questionnaires before the end of that year (mean age at response 11.4 years, SD = 0.3). We obtained teacher ratings during winter/spring of the year each cohort turned age 12, by requesting the classroom teacher for all twins enrolled into FT12 to complete a teacher version of the Multidimensional Peer Nomination Inventory (MPNI) (Pulkkinen et al., 1999). Ratings were completed by 93% of teachers. In Finnish culture, twin children are seldom placed in separated classrooms; in nearly 90% of the twin pairs in this study, both co-twins were in the same class and, therefore, were rated by the same teachers (Happonen et al., 2002). Finnish children do not yet change classes for different school subjects at this age; accordingly, the classroom teacher would teach the majority of the students’ classes. Additionally, it is common for classroom teachers to transition with their class of students across the elementary school years; accordingly, teachers generally know their students quite well. All twins were sent a follow-up questionnaire within 3 months of the date they reached age 14 (mean age at response 14.1 years, SD = 0.1). Of the 2,567 twin pairs who completed questionnaires at age 12, both co-twins in 2,355 of these pairs (92%) later completed the age 14 follow-up.
Zygosity was determined using a well-validated questionnaire completed by both co-twins at the baseline, containing items regarding similarity and confusability (Kaprio et al., 2002). This was supplemented by parental information and comparisons of school photographs for twins whose zygosity could not be determined definitively from information in the questionnaires.
Measures
Alcohol Use
Adolescent alcohol use was measured at age 14 using an item that asked subjects to report how frequently they drink alcohol, with 4 response options (once a week or more; about 1 to 2 times a month; less often than once a month; never, I don’t drink alcohol). A total of 1,432 same-sex twin pairs (373 female MZ, 340 male MZ, 341 female DZ, and 378 male DZ) responded to the item and were used in analyses. For alcohol use frequency, 66% of the sample reported that they had never used alcohol, 19% reported drinking less often than once a month, 11% reported using alcohol about 1 or 2 times per month, and 3% reported using alcohol once per week or more. After adjusting for the familial clustering in the data, girls are slightly more likely to report alcohol use than boys at this early age [F(2.77, 3965.03) = 3.39, p = 0.02], as has been discussed previously in this sample (Rose et al., 2001a).
Behavior Problems
Behavior problem data were collected from 1,411 same-sex twin pairs (357 female MZ, 343 male MZ, 337 female DZ, and 374 male DZ) at age 12 using the MPNI (Pulkkinen et al., 1999). The MPNI was developed during the course of the Finnish twin studies for use by teachers, parents, and peers as assessors of behavioral and emotional problems of the twins. This study used teacher reports of behavior problems. The behavior problems measure is the composite of 17 items comprising 3 subscales: aggression (6 items), hyperactivity–impulsivity (7 items), and inattention (4 items). Items were presented in the format of statements (e.g. “hurts other kids when they’re angry…”), and the teacher was asked to rate each item on a 4-point scale where 0 = does not apply, 1 = applies sometimes, but not consistently, 2 = certainly applies, but not in a pronounced way, and 3 = applies in a pronounced way. The scores on the 3 subscales are averaged to yield a composite behavior problems score. Scores ranged from 0 to 3 with a mean (M) of 0.88 and a standard deviation (SD) of 0.66 for males and ranged from 0 to 2.73 (M = 0.48, SD = 0.47) for females. The mean score for males was significantly higher than the mean score for females [F(1, 2821) = 346.93, p < 0.001].
Neighborhood Variables
Neighborhood variables were obtained from SuomiCD, which contains a collection of Finnish government statistics compiled for each of the country’s 452 municipalities, based on data from 1998. We analyzed urban/rural residency, migration rate inside the municipality, and percentage of older adolescent residents (aged 15 to 19). Seventy-two percent of the sample resided in urban municipalities and 28% resided in rural municipalities. Migration inside the municipality ranged from 2% to 16% with a mean of 9.48% and standard deviation of 3.46. The percentage of residents aged 15 to 19 ranged from 4% to 11% (M = 6.58, SD = 0.93). These variables showed varying degrees of correlation. Urban/rural residency correlated 0.67 (p < 0.01) with migration rates and −0.11 (p < 0.01) with percentage of older adolescents. Urban municipalities had higher rates of migration and slightly fewer older adolescent residents (age 15 to 19). Migration rates and older adolescents were correlated −0.31 (p < 0.01).
Statistical Analyses
Comparisons of the similarity of MZ and DZ twin pairs yield information about the degree of influence that can be attributed to genetic and environmental factors for a particular outcome (Plomin et al., 2001). The basic genetically informative twin model partitions variance in a behavior into additive genetic influences (A), common environmental influences (C), and unique environmental influences (E). Genetic influences correlate 1.0 between monozygotic (MZ) twins, who share all of their genes identical-by-descent, and 0.5 between dizygotic (DZ) twins, who share, on average, 50% of their segregating genes, as do ordinary siblings. Common environmental effects, as defined in biometrical twin modeling, refer to all environmental influences that make siblings more similar to one another. By definition, these influences correlate 1.0 between both MZ and DZ twins. Unique environmental influences are uncorrelated between co-twins and have the effect of decreasing the covariance between siblings.
We first conducted univariate modeling of each of the outcomes of interest (behavior problems and alcohol use), and tested whether the variance components differed significantly for girls and boys. Models constraining estimates of genetic and environmental variance to be equal across the sexes fit poorly for both alcohol use frequency and behavior problems; accordingly, tests of moderation were conducted separately for males and females.
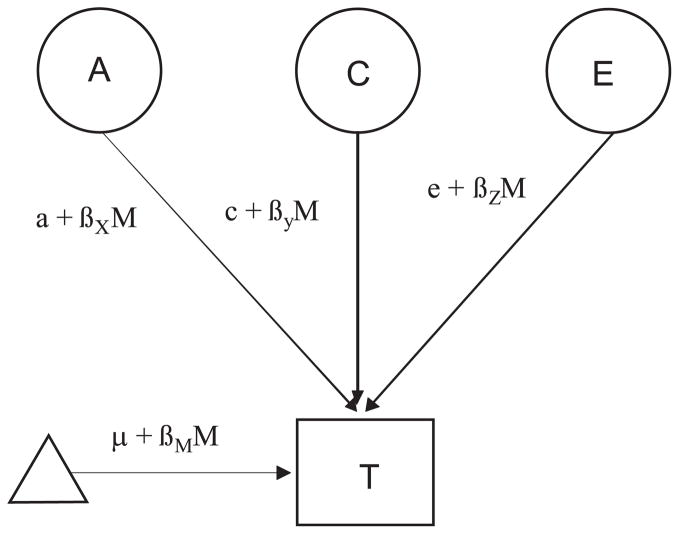
Moderation models were fit to test whether the variance components differed as a function of each of the neighborhood variables. Figure 1 shows a classic twin model (for only 1 twin in the pair) that has been modified to include a moderation component (Purcell, 2002). The standard paths a, c, and e, indicating the magnitude of effect of additive genetic influences, common environmental influences, and unique environmental influences, now each include a β term, which indicates the significance of a potential moderator variable M on each of these genetic and environmental influences. The value of M changes from subject to subject, taking on the value of the measured variable for that subject (i.e., the socioregional variables in our models). In the moderation model, the additive genetic value is a linear function of the moderator M, represented by the equation a + βXM, where βX is an unknown parameter to be estimated from the data, representing the magnitude of the moderating effect. If βX is significantly different from zero, there is evidence for a moderating effect. A similar logic follows for the βY and βZ pathways, which represent the extent to which a specific moderator variable alters the importance of common and unique environmental influences, respectively. In other words, the moderation model allows us to test whether the importance of additive genetic effects (a), common environmental effects (c), and unique environmental effects (e) are changing as a function of the measured variable. The pathway μ + βMM models main effects of the moderator variable on the outcome. Also included in this pathway are any gene–environment correlation effects between the moderator variable and outcome. Thus, any covariance between the moderator and the outcome is incorporated into the means model.
Fig. 1.
Moderation model. The latent variable A, represented in a circle, indicates additive genetic influences on the trait (T) of interest. C represents common (shared) environmental influences on a trait, and latent E represents unique environmental influences, which are uncorrelated between the twins. The triangle indicates the mean/thresholds for T and is necessary when modeling raw data. The standard paths a, c, and e, indicating the magnitude of effect of each latent variable on the trait, each include a β term, which indicates the significance of a measured moderator variable M on each of these genetic and environmental influences.
All modeling was conducted using the raw data option in Mx (Neale, 2000). Mx is a structural equation modeling program developed specifically for the use of twin and family data. When the outcome is ordinal (such as our measure of alcohol use), the model involves the use of thresholds, rather than means. Both quasi-continuous moderating variables were standardized for analyses. The first application of the moderation model using quasi-continuous environmental moderators was to the study of socioregional factors on alcohol use among young adults using an older Finnish twin sample (Dick et al., 2001). These models have subsequently been detailed and expanded (Purcell, 2002). The significance of each of the parameters in the model can be tested by dropping a parameter and evaluating the change in −2 log likelihood between the initial model and the nested submodel. This difference is evaluated using a chi-square distribution. A significant change in fit between the models (p < 0.05) for the difference in degrees of freedom indicates that dropping the parameter caused a significant decrease in fit of the model, indicating that pathway significantly contributes to the outcome trait and should be retained in the model. Model-fitting proceeded in a series of steps. We first tested the significance of the main effect of the moderator (i.e., the neighborhood variable). We then tested the significance of moderation effects by dropping all moderation (3 df test, βX, βY, and βZ dropped). When this test was significant, we conducted further testing to determine what specific variance components showed significant moderation by sequentially dropping and testing the significance of each of the moderating effects one by one.
RESULTS
Standardized estimates (variance component divided by total variance) of additive genetic, common environmental, and unique environmental influences on age 14 alcohol use frequency and age 12 behavior problems are presented in Table 1. Constraining the variance components equal between males and females caused a significant decrease in fit for both alcohol use frequency (χ2Δ = 19.18, 3 df, p < 0.01) and behavior problems (χ2Δ = 8.47, 3 df, p = 0.04). For alcohol use frequency, the A and C parameters could be constrained equal (for A: χ2Δ <0.01, 1 df, p = 0.98; for C: χ2Δ = 1.36, 1 df, p = 0.24); however, constraining the E parameter caused a significant decrease in fit (χ2Δ = 15.38, 1 df, p < 0.01). For behavior problems, constraining both the A and C pathways equal caused a significant decrease in fit (χ2Δ = 8.47, 2 df, p = 0.01). Dropping the paths separately suggested that the significance was more strongly related to constraining the A pathway (dropping A: χ2Δ = 3.13, 1 df, p = 0.08; dropping C: χ2Δ = 1.76, 1 df, p = 0.18). Constraining the E pathways equal also caused a significant decrease in fit (χ2Δ = 6.62, 1 df, p = 0.01).
Table 1.
Standardized Estimates (With 95% Confidence Intervals) of Additive Genetic (A), Common Environmental (C), and Unique Environmental (E) Influences on Alcohol Use Frequency and Behavior Problems for Males and Females
| Variance component | Male | Female |
|---|---|---|
| Alcohol use frequency | ||
| A | 0.27 (0.05–0.50) | 0.27 (0.14–0.43) |
| C | 0.49 (0.28–0.67) | 0.63 (0.47–0.75) |
| E | 0.24 (0.18–0.33) | 0.10 (0.07–0.14) |
| Behavior problems | ||
| A | 0.50 (0.38–0.63) | 0.67 (0.54–0.81) |
| C | 0.34 (0.21–0.45) | 0.22 (0.07–0.34) |
| E | 0.16 (0.14–0.19) | 0.12 (0.10–0.14) |
Alcohol Use Frequency
Urban/Rural Analyses
There was no main effect of urban/rural residency on alcohol use frequency in males; constraining the distributions of use to be equal across urban and rural communities did not cause a significant reduction in model fit (χ2Δ = 2.93, 3 df1, p = 0.40). However, there was a significant main effect of urban/rural residency on female alcohol use (χ2Δ = 8.24, 3 df, p = 0.04). Females living in rural communities were more likely to endorse abstinence (rural: 68.0%; urban: 64.4%) or light drinking [drinking less often than once a month (rural: 20.3%; urban: 18.3%)]; urban females were more likely to report heavier drinking [drinking about 1 or 2 times a month (urban: 13.5%; rural: 10.9%) or once a week or more (urban: 3.7%; rural: 0.8%)].
The univariate model constraining genetic, common environmental and unique environmental influences on age 14 alcohol use frequency to be equal across urban and rural settings did not provide a significant decrease in goodness of fit for males (χ2Δ = 2.81, 3 df, p = 0.42) or females (χ2Δ = 0.22, 3 df, p = 0.97). Thus, the magnitude of importance of genetic and environmental influences on adolescent alcohol use did not significantly differ between urban and rural municipalities for either sex.
Migration
There was no main effect (χ2Δ = 1.20, 1 df, p = 0.27) or moderating effect of migration (moderation of ACE dropped: χ2Δ = 0.50, 3 df, p = 0.92) on alcohol use frequency among males. For female alcohol use, dropping the main effect caused a significant decrease in goodness of fit (χ2Δ = 4.79, 1 df, p = 0.03). Alcohol use was more frequent in municipalities with higher rates of migration. Like in males, there were no significant moderating effects of migration on genetic or environmental influences on alcohol use frequency in females (moderation of ACE dropped: χ2Δ = 3.22, 3 df, p = 0.36).
Older Adolescents
There was no main effect (χ2Δ = 2.69, 1 df, p = 0.10) or moderating effect of percentage of older adolescents (moderation of ACE dropped: χ2Δ = 1.74, 3 df, p = 0.63) on alcohol use frequency among males. Among females, dropping the main effect caused a significant decrease in goodness of fit (χ2Δ = 6.89, 1 df, p = 0.01). There was also a marginally significant moderation effect (moderation of ACE dropped: χ2Δ = 7.85, 3 df, p = 0.05). Dropping the moderation of the individual variance components indicated that this effect was due to moderation of the E component (moderation of A dropped: χ2Δ = 0.60, 1 df, p = 0.44; moderation of C dropped: χ2Δ = 0.86, 1 df, p = 0.35; moderation of E dropped: χ2Δ = 5.88, 1 df, p = 0.02). As the percentage of residents age 15 to 19 increased, the degree of unique environmental influence decreased, from 23% to 3% across the distribution of older individuals.
Behavior Problems
Urban/Rural Analyses
For both males and females, the model constraining the mean scores for behavior problems to be equal across urban and rural environments did not produce a significant decrease in goodness of fit (males: χ2Δ = 1.68, 1 df, p = 0.20; females: χ2Δ = 0, 1 df, p = 0.99). In males, constraining genetic, common environmental and unique environmental influences on behavior problems to be equal across urban and rural settings was not significant (χ2Δ = 7.21, 3 df, p = 0.07). However, for females, there was significant evidence of moderation between urban and rural settings (moderation of ACE: χ2Δ = 27.69, 3 df, p < 0.01). Moderation of each of the variance components was significant (moderation of A: χ2Δ = 3.92, 1 df, p = 0.05; moderation of C: χ2Δ = 4.97, 1 df, p = 0.03; moderation of E: χ2Δ = 18.41, 1 df, p < 0.01). Standardized estimates (with confidence intervals in parentheses) of genetic and environmental influences on behavior problems in urban and rural environments are presented in Table 2. Genetic influences were lower and common environmental influences higher, in rural settings.
Table 2.
Standardized Estimates (With 95% Confidence Intervals) of Additive Genetic (A), Common Environmental (C), and Unique Environmental (E) Influences on Behavior Problems for Females in Urban and Rural Neighborhoods
| Urban | Rural | |
|---|---|---|
| A | 0.74 (0.58–0.88) | 0.47 (0.32–0.69) |
| C | 0.12 (0.00–0.28) | 0.46 (0.24–0.62) |
| E | 0.14 (0.11–0.17) | 0.06 (0.05–0.09) |
Migration
There was no main effect of migration on behavior problems in either males (χ2Δ = 0.92, 1 df, p = 0.34) or females (χ2Δ = 0.27, 1 df, p = 0.61). In males, there were no moderation effects (moderation of ACE dropped: χ2Δ = 2.08, 3 df, p = 0.56). In females, dropping the moderating effect of migration was significant (moderation of ACE dropped: χ2Δ = 37.19, 3 df, p < 0.01). Dropping the moderation of the individual variance components indicated that this effect was due to moderation of the E component (moderation of A dropped: χ2Δ = 0.20, 1 df, p = 0.66; moderation of C dropped: χ2Δ = 1.14, 1 df, p = 0.29; moderation of E dropped: χ2Δ = 33.71, 1 df, p = <0.01). As the level of migration increased, the degree of unique environmental influence on female behavior problems increased. Unique environmental influences accounted for about 3% of the variance in female behavior problems in neighborhoods with the lowest rates of migration and around 22% of the variance in neighborhoods with the highest rates of migration.
Older Adolescents
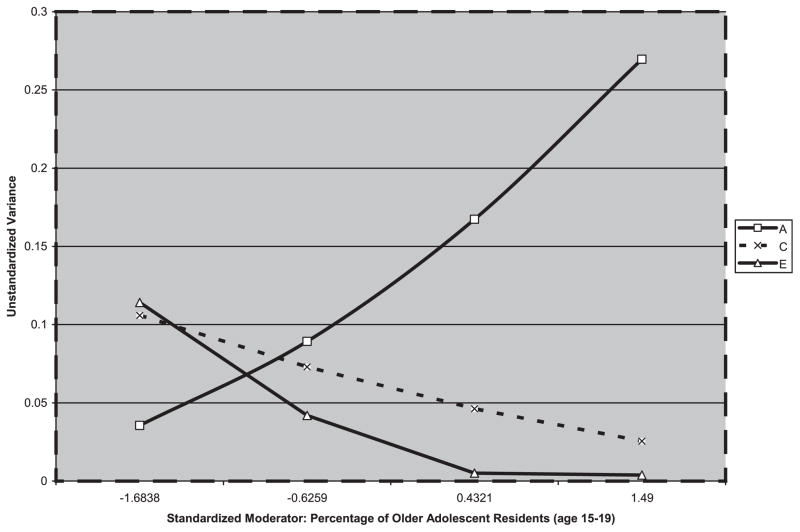
There was no main effect of the percentage of slightly older adolescents on behavior problems among males (χ2Δ = 0.10, 1 df, p = 0.76) or females (χ2Δ = 0.59, 1 df, p = 0.44). For males, dropping the moderation effect was not significant (χ2Δ = 7.22, 3 df, p = 0.07). For females, dropping the moderation did cause a significant decrease in goodness of fit (χ2Δ = 105.52, 3 df, p < 0.01). Dropping the moderation of the A pathway (χ2Δ = 11.21, 1 df, p < 0.01) and dropping the moderation of the E pathway (χ2Δ = 101.85, 1 df, p < 0.01) both caused significant decreases in goodness of fit. However, dropping moderation of the C pathway alone was not significant (χ2Δ = 1.60, 1 df, p = 0.21). Figure 2 shows the changing genetic and environmental influences on female behavior problems at varying levels of older adolescent residents, presented such that the reader can evaluate the results from the full model before any moderation effects were dropped as a result of significance testing. As the percentage of residents age 15 to 19 increased, genetic influences on female behavior problems increased significantly and unique environmental influences on female behavior problems decreased significantly.
Fig. 2.
Changing variance in additive genetic effects, common environmental effects, and unique environmental effects on age 12 behavior problems in girls across increasing levels of percentage of older adolescents in the neighborhood.
DISCUSSION
This study grows out of previous analyses of an older Finnish twin sample in which we found that urban/rural residency, migration rates, and percentage of slightly older individuals significantly moderated genetic and environmental influences on alcohol use at age 18 (Dick et al., 2001; Rose et al., 2001b). Here, we extend these analyses to a younger sample of Finnish twins and test for moderating effects of these variables on alcohol use at age 14 and behavior problems at age 12. For alcohol use, we find main effects of each of the neighborhood variables on alcohol use, but only in females. We find no evidence of moderation of genetic effects on alcohol use in either males or females. For behavior problems, we find no main effects of these neighborhood variables, but we find significant moderation effects, again limited to females. Genetic effects were higher in urban settings and in neighborhoods characterized by more slightly older adolescents, whereas, common environmental influences played a larger role in rural settings.
These findings add to the growing literature underscoring the importance of studying how environmental factors interact with genetic predispositions to understand pathways of risk that contribute to deleterious outcomes, rather than simply testing for main effects of genes and environments. Interestingly, we find no interactive effects of neighborhood influences and genetic susceptibility with respect to alcohol use at age 14. At age 14, the majority of individuals in our sample (64%) had not yet initiated alcohol use. Of those who had reported initiation, 21% reported using alcohol “rarely,” another approximately 13% reported drinking 1 to 2 times monthly, and approximately 3% weekly (Rose et al., 2001b). At this early stage of the initiation and establishment of alcohol use, we find only modest evidence of genetic influences (approximately 27%), in line with other studies finding that the initial stage of alcohol use are largely influenced by common environmental factors (Hopfer et al., 2003). We know that dramatic changes occur in the relative importance of genetic and environmental influences on alcohol use across adolescence, as individuals move from initial experimentation to more established, regular patterns of use (Koopmans and Boomsma, 1996; Rose et al., 2001b). Genetic influences assume increasing influence (Pagan et al., 2006), as illustrated by the fact that they account for but 27% of the variance at age 14, but nearly 50% by age 18, based on data from older adolescent Finnish twins (Rose et al., 2001b). The considerable and dynamic changes that occur from early to late adolescence on influences on alcohol use are further echoed here, where neighborhood factors that play a significant role in moderating genetic susceptibility to alcohol use later in adolescence, do not show similar effects on alcohol use at age 14.
Interestingly, the moderating effects that we found on behavior problems in early adolescence largely parallel the effects we reported previously with alcohol use in older adolescents. Genetic influences on behavior problems at age 12 were more important in urban settings, whereas common environmental influences assumed greater importance in rural settings. These findings are exactly what have previously been reported for alcohol use in later adolescence in a slightly older Finnish twin sample (Rose et al., 2001b). Interestingly, a replication of the urban/rural interaction in the Minnesota twin sample expanded the phenotype to incorporate both substance use and rule-breaking behavior (symptoms of conduct, oppositional defiant, and antisocial personality disorders) at age 17 (Legrand et al., 2007). Additionally, we find that genetic influences assume greater importance on behavior problems at age 12 in neighborhoods with a larger percentage of slightly older adolescents (ages 15 to 19), parallel to the effect reported for alcohol use at age 18 with slightly older young adults (ages 20 to 24; grouping predetermined by SuomiCD statistics). The fact that we find parallel moderation effects associated with neighborhoods on behavior problems in early adolescence, but not on alcohol use, is also interesting in light of the literature suggesting that early behavior problems share a genetic predisposition with adult alcohol problems (Kendler et al., 2003; Krueger et al., 2002; Slutske et al., 1998), and may represent an earlier manifestation of a predisposition to adult alcohol dependence that manifests as behavior problems even prior to involvement in and establishment of alcohol use patterns (Dick et al., 2006). Our finding that neighborhood factors that moderate influences on alcohol use in late adolescence show parallel effects on behavior problems at age 12, but no effects yet on alcohol use at age 14, may further support this idea. We also note that behavior problems as measured at age 12 already show a substantial heritability (50 to 60%), compared to alcohol use at age 14, which is still largely influenced by common environment.
It is striking that all of the significant effects that we found were limited to girls. Similar sex differences were reported previously in a study of gene–environment interaction involving socioeconomic status and adolescent antisocial behavior, in which neighborhood conditions had a greater moderating effect on genetic and environmental influences on female antisocial behavior than male antisocial behavior at ages 16 to 17 (Tuvblad et al., 2006). But in addition to the moderation effects found only in females, we also found that main effects of each of the neighborhood variables on alcohol use at age 14 were limited to girls. Interestingly, in previous analyses of age 14 drinking in this sample, we found evidence that common environmental influences on alcohol use at this age showed some sex-specific effects (Rose et al., 2001a). Girls are also somewhat, albeit not significantly so, more likely to report drinking than are boys at this age, perhaps reflecting the earlier developmental maturation of girls, compared to age-matched boys, and its accompanying association with older peers and boyfriends. There is a growing body of evidence suggesting that females may be more susceptible to a variety of environmental influences than males (Kokkevi et al., 2007; Simons-Morton et al., 2001; Wang et al., 1995; Yeh et al., 2006). In this sample, we have previously reported that girls are more sensitive to the effects of reduced parental monitoring (Rose et al., 2001a) and substance use among friends (Dick et al., 2007a). Here, we find that neighborhood factors may also play a more significant role in both mean levels of alcohol use in girls, and in moderating the importance of genetic susceptibilities on the manifestation of behavior problems.
Although we found that urban/rural residency and composition of slightly older adolescents showed moderating effects on behavior problems that paralleled those reported previously for alcohol use in later adolescence, we did not find significant moderation effects associated with migration for genetic or common environmental influences. The only significant moderating effect of migration was on the unique environmental component, which played a larger role in neighborhoods with higher levels of migration. This is consistent with what we have previously reported (Dick et al., 2007b; Rose et al., 2001a), in which most of the moderation effects we observed with unique environmental influences were such that unique environmental influences assumed greater importance in neighborhoods characterized by more opportunity and/or less social control. This is true here as well for both urban/rural residency and migration rates. However, we also observed significant moderation of the E component with percentage of older adolescents for both behavior problems and alcohol use, with unique environmental influences decreasing in importance as there were more older adolescents in the neighborhood, contrary to expected. It is not immediately obvious why this would be so; the interpretation of the E component is complicated by the fact that it reflects not only the influence of environmental factors unique to the individual twins, but also the error component. The opposite direction of effect observed for the E component with older adolescents may also reflect the inverse correlation (albeit very low) between percentage of older adolescents and urban/rural residency (which also showed moderation of E). However, it is notable that both urban/rural residency and percentage of older adolescents show moderating effects on genetic influences, despite low correlation between the variables, suggesting that the effect of the composition of older adolescents is not merely a reflection of urban/rural status.
In conclusion, we find that neighborhood variables that moderated genetic and environmental influences on alcohol use in late adolescence do not show parallel moderating effects on alcohol use early in adolescence. Instead, we find analogous moderating effects on behavior problems in early adolescence: genetic influences assumed greater importance in urban settings and in neighborhoods containing more older adolescents. However, these effects were limited to girls. In addition, all 3 neighborhood variables showed main effects on alcohol use, but only in girls. These findings suggest that girls may be particularly sensitive to environmental influences. They also add to the growing literature illustrating the importance of identifying specific environments that modify genetic risk.
Acknowledgments
The Finnish Twin studies have been supported by the National Institute of Alcohol Abuse and Alcoholism (grants AA-12502, AA-00145, and AA-09203 to RJR), the Academy of Finland (grants 100499, 205585, and 118555 to JK), and the Finnish Centre of Excellence Programme (to LP and JK). This study has been supported by a grant from the National Institute of Alcohol Abuse and Alcoholism (AA-15416 to DMD).
Footnotes
Note: 3df tests reflect constraining the 3 thresholds, for the 4 level alcohol use measure, to be equal across urban and rural settings.
References
- Button TM, Corley RP, Rhee SH, Hewitt JK, Young SE, Stallings MC. Delinquent peer affiliation and conduct problems: a twin study. J Abnorm Psychol. 2007;116:554–564. doi: 10.1037/0021-843X.116.3.554. [DOI] [PubMed] [Google Scholar]
- Button TM, Lau JY, Maughan B, Eley TC. Parental punitive discipline, negative life events and gene-environment interplay in the development of externalizing behavior. Psychol Med. 2008;38:29–39. doi: 10.1017/S0033291707001328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button TM, Scourfield J, Martin N, Purcell S, McGuffin P. Family dysfunction interacts with genes in the causation of antisocial symptoms. Behav Genet. 2005;35:115–120. doi: 10.1007/s10519-004-0826-y. [DOI] [PubMed] [Google Scholar]
- Clark DB, Lynch KG, Donovan JE, Block GD. Health problems in adolescents with alcohol use disorders: self-report, liver injury, and physical examination findings and correlates. Alcohol Clin Exp Res. 2001;25:1350–1359. [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Dick DM, Bierut L, Hinrichs AL, Fox L, Bucholz KK, Kramer JR, Kuperman S, Hesselbrock V, Schuckit M, Almasy L, Tischfield JA, Porjesz B, Begleiter H, Nurnberger JI, Jr, Xuei X, Edenberg HJ, Foroud T. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behav Genet. 2006;36:577–590. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- Dick DM, Pagan JL, Holliday C, Viken R, Pulkkinen L, Kaprio J, Rose RJ. Gender differences in friends’ influences on adolescent drinking: a genetic epidemiological study. Alcohol Clin Exp Res. 2007a;31:2012–2019. doi: 10.1111/j.1530-0277.2007.00523.x. [DOI] [PubMed] [Google Scholar]
- Dick DM, Pagan JL, Viken R, Purcell S, Kaprio J, Pulkkinen L, Rose RJ. Changing environmental influences on substance use across development. Twin Res Hum Genet. 2007b;10:315–326. doi: 10.1375/twin.10.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Rose RJ, Viken RJ, Kaprio J, Koskenvuo M. Exploring gene-environment interactions: socioregional moderation of alcohol use. J Abnorm Psychol. 2001;110:625–632. doi: 10.1037//0021-843x.110.4.625. [DOI] [PubMed] [Google Scholar]
- Dick DM, Viken R, Purcell S, Kaprio J, Pulkkinen L, Rose RJ. Parental monitoring moderates the importance of genetic and environmental influences on adolescent smoking. J Abnorm Psychol. 2007c;116:213–218. doi: 10.1037/0021-843X.116.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan JE, Leech SL, Zucker RA, Loveland-Cherry CJ, Jester JM, Fitzgerald HE, Puttler LI, Wong MM, Looman WS. Really underage drinkers: alcohol use among elementary students. Alcohol Clin Exp Res. 2004;28:341–349. doi: 10.1097/01.alc.0000113922.77569.4e. [DOI] [PubMed] [Google Scholar]
- Feinberg ME, Button TM, Neiderhiser JM, Reiss D, Hetherington EM. Parenting and adolescent antisocial behavior and depression: evidence of genotype × parenting environment interaction. Arch Gen Psychiatry. 2007;64:457–465. doi: 10.1001/archpsyc.64.4.457. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiological Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Grube JW. Comparison of Drinking Rates and Problems: European Countries and the United States. Pacific Institute for Research and Evaluation, Office of Juvenile Justice Enforcing the Underage Drinking Laws Program; Calverton, MD: 2001. [Google Scholar]
- Gruber E, DiClemente RJ, Anderson MM, Lodico M. Early drinking onset and its association with alcohol use and problem behavior in late adolescence. Prev Med. 1996;25:293–300. doi: 10.1006/pmed.1996.0059. [DOI] [PubMed] [Google Scholar]
- Grunbaum JA, Kann L, Kinchen S, Ross J, Hawkins J, Lowry R, Harris WA, McManus T, Chyen D, Collins J. Youth risk behavior surveillance–United States, 2003. MMWR Surveill Summ. 2004;53:1–96. [PubMed] [Google Scholar]
- Haber JR, Jacob T, Heath A. Paternal alcoholism and offspring conduct disorder: evidence for the ‘common genes’ hypothesis. Twin Res Hum Genet. 2005;8:120–131. doi: 10.1375/1832427053738782. [DOI] [PubMed] [Google Scholar]
- Happonen M, Pulkkinen L, Kaprio J, Van der Meere J, Viken RJ, Rose RJ. The heritability of depressive symptoms: multiple informants and multiple measures. J Child Psychol Psychiatry. 2002;43:471–479. doi: 10.1111/1469-7610.00038. [DOI] [PubMed] [Google Scholar]
- Heath AC, Jardine R, Martin NG. Interactive effects of genotype and social environment on alcohol consumption in female twins. J Stud Alcohol. 1989;50:38–48. doi: 10.15288/jsa.1989.50.38. [DOI] [PubMed] [Google Scholar]
- Hibell B, Andersson B, Bjarnason T, Ahlstrom S, Balakireva O, Kokkevi A, Morgan M. The ESPAD Report 2003: Alcohol and Other Drug Use Among Students in 35 European Countries. Swedish Council for Information on Alcohol and Other Drugs; Stockholm: 2004. [Google Scholar]
- Hopfer CJ, Crowley TJ, Hewitt JK. Review of twin and adoption studies of adolescent substance use. J Am Acad Child Adolesc Psychiatry. 2003;42:710–719. doi: 10.1097/01.CHI.0000046848.56865.54. [DOI] [PubMed] [Google Scholar]
- Jessor R, Jessor SL. Problem Behavior and Psychosocial Development: A Logitudinal Study of Youth. Academic Press; New York: 1977. [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Teen Drug Use Continues Down in 2006, Particularly Among Older Teens; But Use of Prescription-Type Drugs Remains High. Vol. 2007. University of Michigan News and Information Services; Ann Arbor, MI: 2006. [Google Scholar]
- Kaprio J, Koskenvuo M, Rose RJ. Change in cohabitation and intra-pair similarity of monozygotic (MZ) cotwins for alcohol use, extraversion, and neuroticism. Behav Genet. 1990;20:265–276. doi: 10.1007/BF01067794. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Pulkkinen L, Rose RJ. Genetic and environmental factors in health-related behaviors: studies on Finnish twins and twin families. Twin Res. 2002;5:358–365. doi: 10.1375/136905202320906101. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott C, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kokkevi A, Richardson C, Florescu S, Kuzman M, Stergar E. Psychosocial correlates of substance use in adolescence: a cross-national study in six European countries. Drug Alcohol Depend. 2007;86:67–74. doi: 10.1016/j.drugalcdep.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Koopmans JR, Boomsma DI. Familial resemblances in alcohol use: genetic or cultural transmission? J Stud Alcohol. 1996;57:19–28. doi: 10.15288/jsa.1996.57.19. [DOI] [PubMed] [Google Scholar]
- Koopmans JR, Slutske WS, van Baal GCM, Boomsma DI. The influence of religion on alcohol use initiation: evidence for genotype × environment interaction. Behav Genet. 1999;29:445–453. doi: 10.1023/a:1021679005623. [DOI] [PubMed] [Google Scholar]
- Krueger RF. The structure of common mental disorders. Arch Gen Psychiatry. 1999;56:1088–1096. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. J Abnorm Psychol. 2002;111:411–424. [PubMed] [Google Scholar]
- Legrand LN, Keyes M, McGue M, Iacono WG, Krueger RF. Rural environments reduce the genetic influence on adolescent substance use and rule-breaking behavior. Psychol Med. 2007;00:1–10. doi: 10.1017/S0033291707001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand L, Malone S, Elkins I. Origins and consequences of age at first drink. I. Associations with substance use disorders, disinhibitory behavior and psycholopathology, and P3 amplitude. Alcohol Clin Exp Res. 2001;25:1156–1165. [PubMed] [Google Scholar]
- Miller JW, Naimi TS, Brewer RD, Jones SE. Binge drinking and associated health risk behaviors among high school students. Pediatrics. 2007;119:76–85. doi: 10.1542/peds.2006-1517. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. Genetic and environmental influences on antisocial behaviors: evidence from behavioral-genetic research. Adv Genet. 2005;55:41–104. doi: 10.1016/S0065-2660(05)55003-X. [DOI] [PubMed] [Google Scholar]
- Molina BSG, Bukstein OG, Lynch KG. Attention-deficit/hyperactivity disorder and conduct disorder symptomatology in adolescents with alcohol use disorder. Psychol Addict Behav. 2002;16:161–164. doi: 10.1037//0893-164x.16.2.161. [DOI] [PubMed] [Google Scholar]
- Moss HB, Lynch KG. Comorbid disruptive behavior disorder symptoms and their relationship to adolescent alcohol use disorders. Drug Alcohol Depend. 2001;64:75–83. doi: 10.1016/s0376-8716(00)00233-7. [DOI] [PubMed] [Google Scholar]
- Neale MC. The use of Mx for association and linkage analyses. GeneScreen. 2000;1:107–111. [Google Scholar]
- Pagan JL, Rose RJ, Viken RJ, Pulkkinen L, Kaprio J, Dick DM. Genetic and environmental influences on stages of alcohol use across adolescence and into young adulthood. Behav Genet. 2006;36:483–497. doi: 10.1007/s10519-006-9062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninkilampi-Kerola V, Kaprio J, Moilanen I, Rose RJ. Co-twin dependence modifies heritability of abstinence and alcohol use: a population-based study of Finnish twins. Twin Res Hum Genet. 2005;8:232–244. doi: 10.1375/1832427054253095. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral Genetics. Worth; London: 2001. [Google Scholar]
- Pulkkinen L, Kaprio J, Rose RJ. Peers, teachers and parents as assessors of the behavioural and emotional problems of twins and their adjustment: the Multidimensional Peer Nomination Inventory. Twin Res. 1999;2:274–285. doi: 10.1375/136905299320565762. [DOI] [PubMed] [Google Scholar]
- Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Res. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Waldman I. Genetic and environmental influences on antisocial behavior: a meta-analysis of twin and adoption studies. Psychol Bull. 2002;128:490–529. [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken Rj, Kaprio J. Gene-environment interaction in patterns of adolescent drinking: regional residency moderates longitudinal influences on alcohol use. Alcohol Clin Exp Res. 2001a;25:637–643. [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Drinking or abstaining at age 14? A genetic epidemiological study. Alcohol Clin Exp Res. 2001b;25:1594–1604. [PubMed] [Google Scholar]
- Rose RJ, Kaprio J, Williams CJ, Viken R, Obremski K. Social contact and sibling similarity: facts, issues, and red herrings. Behav Genet. 1990;20:763–778. doi: 10.1007/BF01065919. [DOI] [PubMed] [Google Scholar]
- Shanahan MJ, Hofer SM. Social context in gene-environment interactions: retrospect and prospect. J Gerontol B Psychol Sci Soc Sci. 2005;60(Spec1):65–76. doi: 10.1093/geronb/60.special_issue_1.65. [DOI] [PubMed] [Google Scholar]
- Simons-Morton B, Haynie DL, Crump AD, Eitel P, Saylor KE. Peer and parent influences on smoking and drinking among early adolescents. Health Educ Behav. 2001;28:95–107. doi: 10.1177/109019810102800109. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Dinwiddle SH, Madden PAF, Bucholz KK, Dunne MP, Statham DJ, Martin NG. Common genetic risk factors for conduct disorder and alcohol dependence. J Abnorm Psychol. 1998;107:363–374. doi: 10.1037//0021-843x.107.3.363. [DOI] [PubMed] [Google Scholar]
- Tuvblad C, Grann M, Lichtenstein P. Heritability for adolescent antisocial behavior differs with socioeconomic status: gene-environment interaction. J Child Psychol Psychiatry. 2006;47:734–743. doi: 10.1111/j.1469-7610.2005.01552.x. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Justice. Drinking in America: Myths, Realities, and Prevention Policy. Pacific Institute for Research and Evaluation; Calverton, MD: 1999. [Google Scholar]
- Wang MQ, Fitzhugh EC, Westerfield RC, Eddy JM. Family and peer influences on smoking behavior among American adolescents: an age trend. J Adolesc Health. 1995;16:200–203. doi: 10.1016/1054-139X(94)00097-X. [DOI] [PubMed] [Google Scholar]
- White HR, Zie M, Thompson W, Loeber R, Stouthamer-Loeber M. Psychopathology as a predictor of adolescent drug use trajectories. Psychol Addict Behav. 2001;15:210–218. [PubMed] [Google Scholar]
- Yeh MY, Chiang IC, Huang SY. Gender differences in predictors of drinking behavior in adolescents. Addict Behav. 2006;31:1929–1938. doi: 10.1016/j.addbeh.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. Am J Med Genet. 2000;96:684–695. [PubMed] [Google Scholar]
- Zeigler DW, Wang CC, Yoast RA, Dickinson BD, McCaffree MA, Robinowitz CB, Sterling ML. The neurocognitive effects of alcohol on adolescents and college students. Prev Med. 2005;40:23–32. doi: 10.1016/j.ypmed.2004.04.044. [DOI] [PubMed] [Google Scholar]