Abstract
Tetanus is an acute disease manifested by motor system and autonomic nervous system instability. Maternal and neonatal tetanus occur where deliveries are performed under unsanitary circumstances and unhygienic umbilical cord practices are prevalent. Neonatal tetanus is almost always fatal in the absence of medical care. These deaths can be prevented with changes in traditional obstetrical practices and maternal immunization. This situation led to the development of the Maternal and Neonatal Elimination Initiative by the World Health Organization. Using a three-pronged approach, tetanus can be eliminated via promotion of hygienic practices during delivery, maternal and childhood immunization, and close surveillance.
Key words: Tetanus, Maternal and neonatal mortality, MNT initiative
Tetanus is an acute disease manifested by motor system and autonomic nervous system instability. It is caused by a neurotoxin produced by the anaerobic bacterium Clostridium tetani.1 Although tetanus can affect anyone, women and infants are particularly at risk when deliveries and cord stump care are performed in unsanitary conditions, leading to tissue contamination. Tetanus contributes to a large proportion of maternal and neonatal mortality worldwide, estimated in 2008 to have claimed approximately 180,000 lives per year. In 1988, an estimated 787,000 newborns died of tetanus.2 This led to the development of the Maternal and Neonatal Elimination Initiative by the World Health Organization (WHO). In 2010, 58,000 newborns died of tetanus,3 equivalent to a 93% mortality reduction from the 1980s and highlighting the positive impact of the WHO initiative. However, much remains to be done, as over 30 countries have yet to eliminate maternal and neonatal tetanus (MNT).4
MNT occurs where deliveries are performed under unsanitary circumstances and unhygienic umbilical cord practices are prevalent. Neonatal tetanus (NT) is almost always fatal in the absence of medical care. With medical care—and depending on the availability of intensive care—the mortality rate of NT ranges from 10% to 60%.5 These deaths can be prevented with changes in traditional obstetrical practices and maternal immunization.
In 1988, the global mortality rate of NT was estimated to be 6.7 per 1000 live births, which translates to approximately 787,000 newborn deaths.2 NT was identified as a global health problem by the WHO, and an initiative was established in 1989 to eliminate it by 1995. Because the spores for C tetani, the causative agent of tetanus, are present in soil, it is impossible to eradicate tetanus from our environment; however, NT can be considered eliminated when the goal of < 1 case per 1000 live births is reached.
In 1999, maternal tetanus was added to the NT elimination initiative.6 Since the WHO MNT initiative was established, significant progress has been made toward elimination of MNT, although it remains a public health problem in more than 30 resource-limited countries.4 In 2010, an estimated 58,000 neonates died from NT globally.3
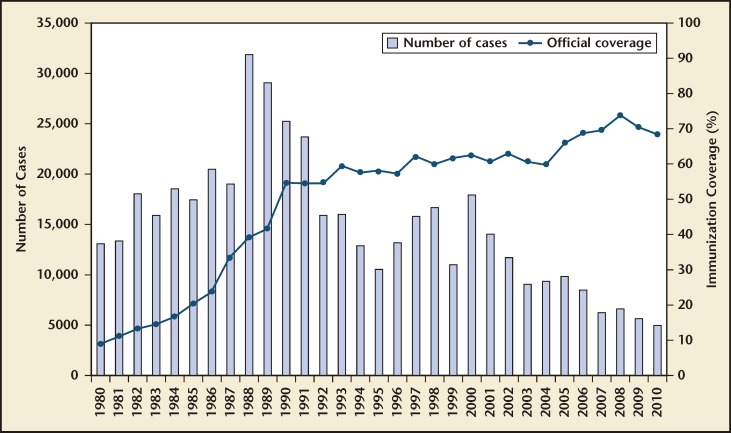
Figure 1 shows the global reported cases of NT from 1980 to 2010. It also shows the percentage of maternal immunization with tetanus toxoid in countries where NT is a public health problem. The accuracy of these numbers may be limited by the incomplete surveillance of NT in most resourcelimited countries.
Figure 1.
Neonatal tetanus global annual reported cases and TT2plus coverage, 1980–2010. TT2plus, second and subsequent doses of tetanus toxoid. Reprinted with permission from the World Health Organization.
Pathophysiology
Tetanus is a preventable disease caused by the neurotoxin produced by the bacterium C tetani, a grampositive obligate anaerobic spore-forming bacillus.7 The organism is widely distributed in soil and in the gastrointestinal tracts of farm animals, dogs, rats, and approximately 10% of the human population. The spores produced by this bacterium can be found on skin surfaces and are particularly resistant to heat and antiseptics. This bacterium produces two exotoxins: tetanolysin (which plays no role in the pathogenesis of the disease), and tetanospasmin (tetanus toxin), which is responsible for the clinical manifestations of the disease.8
Deep, penetrating wounds with the appropriate environment for anaerobic growth are necessary for the development of tetanus. Common points of entry are postpartum or postabortion infections of the uterus, wounds on the lower extremities, nonsterile intramuscular injections, and compound fractures. However, minor trauma can lead to tetanus, and no point of entry is identified in up to 30% of patients.9
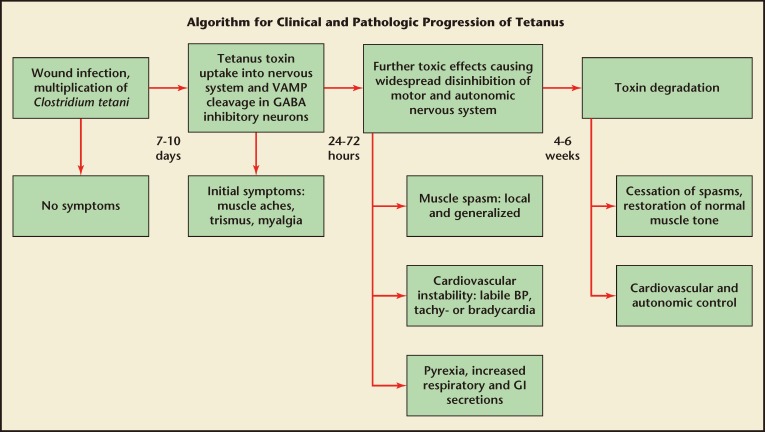
Once produced, the diseasecausing toxin is transported by intra-axonal transport to motor nuclei of the cranial nerves or ventral horns of the spinal cord,1,10 where, in its active form, it blocks the action of inhibitory neurons and leads to sustained excitatory discharge in the motor and autonomic nervous systems (Figure 2). This results in the sustained muscle spasms and hyperadrenergic state that are characteristic of tetanus. The incubation period of tetanus varies from 1 day to several months; however, onset of symptoms usually occurs within 8 days. The period of onset is the time between the first symptom and the start of spasms. Shorter incubation periods and/or periods of onset are associated with worse outcomes.11 In NT, the younger the age of the neonate, the worse the prognosis.1
Figure 2.
Clinical and pathologic progression of tetanus. BP, blood pressure; GABA, gamma-aminobutyric acid; GI, gastrointestinal; VAMP, vesicle-associated membrane protein. Reprinted with permission from Thwaites and Yen.8
Symptoms
Tetanus produces a wide range of clinical features that are often divided into generalized and localized disease. Generalized tetanus is the most common and most severe form and often presents with trismus, although it can also present with cephalic or localized disease. Generalized tetanus is often associated with autonomic overactivity, which is manifested as irritability, restlessness, diaphoresis, and tachycardia. This can progress to cardiac arrhythmias, labile hypertension, or hypotension and fever. Generalized tetanus is classically associated with painful tonic contractions of skeletal muscles and intermittent muscular spasms. Triggers for tetanic spasms include loud noises, touch, or light. Strong contractions of the thoracic and/or pharyngeal muscles can lead to periods of apnea or airway obstruction. Localized tetanus, which involves tonic and spastic muscle contractions in only one extremity or body region, is rare and often progresses to generalized tetanus.6 Both maternal and NT are examples of generalized tetanus.
Neonates with tetanus initially present with irritability and trouble with sucking and feeding within the first few days of life. In the majority of cases, onset of symptoms occurs between postnatal days 3 and 14.12 The affected neonate then proceeds to develop generalized symptoms, including stiffness of the jaw and neck, and generalized spasms and rigidity of the abdominal and back muscles.13 Maternal tetanus is defined as tetanus that occurs during pregnancy or within 6 weeks after delivery, abortion, or spontaneous pregnancy loss. Patients often present with the generalized symptoms described above.14
Diagnosis
The diagnosis of tetanus is based on clinical presentation. There are no microbiologic, chemical, hematologic, or radiographic studies that provide a gold standard for diagnosis. Tissues cultures are positive in < 50% of patients. Symptoms associated with tetanus include trismus, risus sardonicus, muscle rigidity, nuchal rigidity, and opisthotonus. The differential diagnosis for tetanus includes stiff person syndrome, meningitis, neuroleptic malignant syndrome, and rabies. The progression as well as constellation of symptoms helps to distinguish tetanus from other diseases. When meningitis is suspected, a lumbar puncture must be performed to rule it out.15
Treatment
Patients with a presumed diagnosis of tetanus must be managed in an intensive care unit. This includes patients with mild symptoms, given the possibility of progression to generalized disease. Medical management of tetanus includes airway management, sedation, pain control, antibiotic treatment, appropriate wound management, antitoxin therapy, and supportive measures.16–18
Airway management is of the utmost importance in patients with tetanus. Persistent generalized muscle rigidity, despite treatment with benzodiazepines, is an indication for intubation. Patients with respiratory compromise and those at risk of aspiration due to severe dysphagia should also be intubated. Traditionally, tetanic spasms have been managed with neuromuscular blocking agents. However, recent studies suggest effective management of rigidity and autonomic dysfunction with magnesium sulfate.19,20
Surgical debridement of tetanus wounds is associated with improved survival. Gangrenous limbs must be amputated. In women with postpartum tetanus, a hysterectomy is indicated if the uterus is gangrenous, or if there is invasive bacterial sepsis or injury of the uterus.
Tetanus antitoxin (human tetanus immune globulin [HTIG]) should be administered to patients with tetanus. It is usually administered intrathecally in patients with localized rigidity and intramuscularly in patients with generalized rigidity.21–24 HTIG needs to be administered before surgical debridement to prevent seeding of the circulatory system with the tetanus toxin. The administration of antibiotics is also recommended to treat infected wounds and to prevent spread of the toxin. The first line of treatment is often metronidazole. However, other antibiotics such as tetracycline, erythromycin, chloramphenicol, and clindamycin may also be used.25
Prevention and Control
The NT elimination initiative was set up as a partnership among WHO, the United Nations Children’s Fund (UNICEF), and the United Nations Population Fund in 1989 with the goal of eliminating NT by 1995. However, there was limited response from countries in which MNT is a public health problem, and the goal was not attained. As stated above, elimination of maternal tetanus was introduced as part of the target in 1999. Although significant progress has been made, data from surveys conducted in 2012 show that MNT has not yet been eliminated in 34 countries. The MNT initiative through the collaboration of its partners advocates a three-pronged approach for MNT elimination: promotion of clean deliveries, promotion of routine immunization of pregnant women with the tetanus toxoid, and surveillance of NT. In areas where an inadequate proportion of pregnant women is vaccinated against tetanus, supplemental immunization activities (SIAs) may be required.4 These SIAs involve targeted vaccination of women who have no history of prior tetanus vaccinations and live in high-risk areas. These women must receive three properly spaced vaccines during their childbearing years.
Tetanus is preventable through immunization. Vaccination against tetanus is part of most childhood immunization programs. However, booster vaccines are required to provide long-term and possibly lifelong immunity. Protection against tetanus is antibody mediated and can be attained either through active or passive immunization. A prior tetanus infection does not guarantee protection against future infections. The WHO provides recommended tetanus vaccination schedules (Table 1).26
Table 1.
Immunization Schedule for Tetanus
| Immunizations With DTP and Td Vaccines Required to Obtain Long-Term Protectiona Against Tetanus | ||||||
|---|---|---|---|---|---|---|
| Recommended schedule | DTP | DTP | DTP | dT | dT | dT |
| Before age 1 or as early as possible after age 6 weeks with ≥ 4-week intervals | eg, 4–7 years | eg, 12–15 years | Early adulthood | |||
| Adolescents and adults with no previous immunization | dT | dT | dT | dT | dT | |
| As early as possible | At least 4 weeks later | At least 6 months later | At least 1 year later | At least 1 year later | ||
| Pregnant women with no previous immunization (or unreliable immunization information) | dT | dT | dT | dT | dT | |
| As early as possible in first pregnancy | At least 4 weeks later | At least 6 months later, or in next pregnancy | At least 1 year later, or in next pregnancy | At least 1 year later, or in next pregnancy | ||
| Pregnant women with 3 childhood DTP doses | dT | dT | dT | |||
| As early as possible in first pregnancy | At least 4 weeks later | At least 1 year later | ||||
| Pregnant women with 4 childhood DTP doses | dT | dT | ||||
| As early as possible in first pregnancy | At least 1 year later | |||||
| Supplementary immunization activities in high-risk areas (women of childbearing age) | dT | dT | dT | dT | dT | |
| During round 1 During round 2, at least 4 weeks after round 1 | During round 3, at least 6 months after round 2 | At least 1 year later (eg, in next pregnancy) | At least 1 year later, or in next pregnancy | |||
Other tetanus-containing combination vaccines can be used as per national immunization schedules.
dT, low-dose diphtheria toxoid; DTP, diphtheria-tetanus-pertussis; Td, diphtheria toxoid. Reprinted with permission from Wkly Epidemiol Rec.26
All tetanus vaccines are based on the tetanus toxoid, a modified form of the tetanus neurotoxin responsible for the disease’s symptoms. The toxoid stimulates production of an antitoxin antibody. NT is prevented through maternal immunization. The maternal antibodies produced in response to the toxoid cross the placenta to the fetus. Thus, vaccination of pregnant women and all women of childbearing age prevents MNT.4
The tetanus toxoid vaccine is available in several forms: as a single tetanus toxoid vaccine, combined with a diphtheria toxoid, with a low-dose diphtheria toxoid, or with diphtheria and pertussis vaccines.
The WHO MNT Elimination Initiative
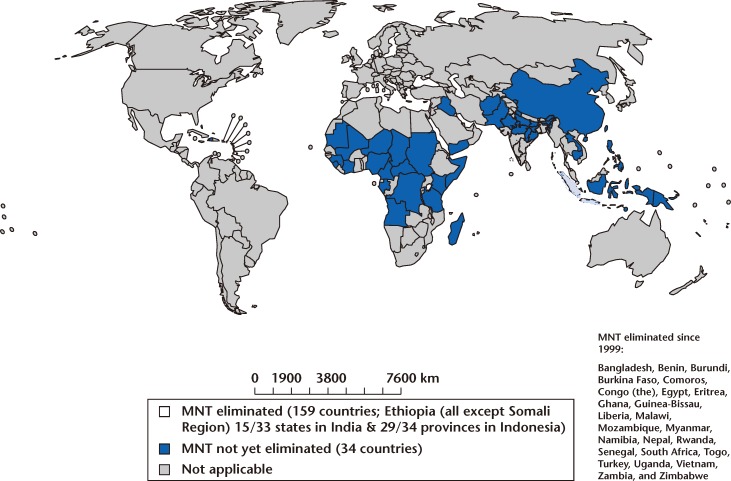
The WHO MNT Elimination Initiative encourages countries in which MNT is prevalent to develop a plan of action for its elimination. This plan is then submitted to UNICEF or other agencies for financial and technical support. Between 2000 and January 2012, 23 countries succeeded in eliminating MNT; however, 34 countries have yet to achieve this goal (Figure 3).4
Figure 3.
Elimination of maternal neonatal tetanus (MNT). Reprinted with permission from the World Health Organization.
Conclusions
MNT contributes significantly to maternal and neonatal mortality in many countries. Using a three-pronged approach, we can eliminate tetanus via promotion of hygienic practices during delivery, maternal and childhood immunization, and close surveillance. Although significant inroads have been made toward eliminating MNT, the goal has yet to be achieved.
Main Points.
Maternal and neonatal tetanus (MNT) occurs where deliveries are performed under unsanitary circumstances and unhygienic umbilical cord practices are prevalent. Neonatal tetanus (NT) is almost always fatal in the absence of medical care.
NT is prevented through maternal immunization. The maternal antibodies produced in response to the toxoid cross the placenta to the fetus; thus, vaccination of pregnant women and all women of childbearing age prevents MNT.
In 1989 a partnership initiative was established with the World Health Organization, the United Nations Children’s Fund, and the United Nations Population Fund to eradicate NT; NT will be considered eliminated when the goal of < 1 case per 1000 live births is reached.
Using a three-pronged approach, tetanus can be eliminated via promotion of hygienic practices during delivery, maternal and childhood immunization, and close surveillance.
References
- 1.Katsarou I, Potamitis N, Papadopoulos K, et al. The father of modern medicine: the first research of the physical factor of tetanus; Paper presented at: 16th Annual European Congress of Clinical Microbiology and Infectious Diseases; April 1–4, 2006; Nice, France. [Google Scholar]
- 2.World Health Organization, authors. Resolution WHA 42.32. Expanded Programme on Immunization. 42nd World Health Assembly. Geneva, Switzerland: World Health Organization; 1989. [Google Scholar]
- 3.Liu L, Johnson HL, Cousens S, et al. Child Health Epidemiology Reference Group of WHO and UNICEF, authors. Global, regional and national causes of child mortality: an updated systemic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization, authors. Maternal and neonatal tetanus (MNT) elimination. [Accessed September 28, 2012].
- 5.Roper MH, Vandelaer JH, Gasse FL, et al. Maternal and neonatal tetanus. Lancet. 2007;370:1947–1959. doi: 10.1016/S0140-6736(07)61261-6. [DOI] [PubMed] [Google Scholar]
- 6.WHO, UNICEF, UNFPA, authors. Maternal and Neonatal Tetanus Elimination by 2005. Strategies for Achieving and Maintaining Elimination. Geneva, Switzerland;: World Health Organization, United Nations Children’s Fund, and United Nations Population Fund; 2000. [Google Scholar]
- 7.Bartlett JG. Tetanus. In: Goldman L, Bennett JC, editors. Cecil Textbook of Medicine. 21st ed. Philadelphia, PA: WB Saunders; 2001. pp. 1675–1677. [Google Scholar]
- 8.Thwaites CL, Yen LM, et al. Tetanus. In: Longo DL, Fauci AS, Kasper DL, et al., editors. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw-Hill; 2012. pp. 1197–1199. [Google Scholar]
- 9.Centers for Disease Control (CDC), authors Tetanus—United States, 1985–1986. MMWR Morb Mortal Wkly Rep. 1987;36:477–481. [PubMed] [Google Scholar]
- 10.Farrar JJ, Yen LM, Cook T, et al. Tetanus. J Neurol Neurosurg Psychiatry. 2000;69:292–301. doi: 10.1136/jnnp.69.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saltoglu N, Tasova Y, Midikli D, et al. Prognostic factors affecting deaths from adult tetanus. Clin Microbiol Infect. 2004;10:229–233. doi: 10.1111/j.1198-743x.2004.00767.x. [DOI] [PubMed] [Google Scholar]
- 12.Blencowe H, Lawn J, Vandelaer J, et al. Tetanus toxoid immunization to reduce mortality from neonatal tetanus. Int J Epidemiol. 2010;39(suppl 1):i102–i109. doi: 10.1093/ije/dyq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogle JW, Anderson MS. Infections: bacterial and spirochetal. In: Hay WW, Levin MJ, Sondheimer JM, Deterding RR, editors. Current Diagnosis & Treatment: Pediatrics. 20th ed. New York, NY: McGraw-Hill; 2011. pp. 1159–1220. [Google Scholar]
- 14.Fauveau V, Mamdani M, Steinglass R, Koblinsky M. Maternal tetanus, magnitude, epidemiology and potential control measures. Int J Gynaecol Obstet. 1993;40:3–12. doi: 10.1016/0020-7292(93)90765-o. [DOI] [PubMed] [Google Scholar]
- 15.Gray P, Roberts D. Tetanus. In: Hall JB, Schmidt GA, Wood LD, editors. Principles of Critical Care. 3rd ed. New York, NY: McGraw-Hill; 2005. pp. 943–948. [Google Scholar]
- 16.Brook I. Current concepts in the management of Clostridium tetani infection. Expert Rev Anti Infect Ther. 2008;6:327–336. doi: 10.1586/14787210.6.3.327. [DOI] [PubMed] [Google Scholar]
- 17.Weinstein L. Tetanus. N Engl J Med. 1973;289:1293–1296. doi: 10.1056/NEJM197312132892408. [DOI] [PubMed] [Google Scholar]
- 18.Sheffield JS, Ramin SM. Tetanus in pregnancy. Am J Perinatol. 2004;21:173–182. doi: 10.1055/s-2004-828605. [DOI] [PubMed] [Google Scholar]
- 19.Mathew PJ, Samra T, Wig J. Magnesium sulphate for treatment of tetanus in adults. Anaesth Intensive Care. 2010;38:185–189. doi: 10.1177/0310057X1003800128. [DOI] [PubMed] [Google Scholar]
- 20.Thwaites CL, Yen LM, Loan HT, et al. Magnesium sulphate for treatment of severe tetanus; a randomized controlled trial. Lancet. 2006;368:1436–1443. doi: 10.1016/S0140-6736(06)69444-0. [DOI] [PubMed] [Google Scholar]
- 21.Cook TM, Protheroe RT, Handel JM. Tetanus: a review of the literature. Br J Anaesth. 2001;87:477–487. doi: 10.1093/bja/87.3.477. [DOI] [PubMed] [Google Scholar]
- 22.Alfrey D, Rauscher LA. Tetanus: a review. Crit Care Med. 1979;7:176–181. doi: 10.1097/00003246-197904000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal M, Thomas K, Peter JV, et al. A randomized double-blind sham-controlled study of intrathecal human anti-tetanus immunoglobulin in the management of tetanus. Natl Med J India. 1998;11:209–212. [PubMed] [Google Scholar]
- 24.Gupta PS, Kapoor R, Goyal S, et al. Intrathecal human tetanus immunoglobulin in early tetanus. Lancet. 1980;2:439–440. doi: 10.1016/s0140-6736(80)91883-8. [DOI] [PubMed] [Google Scholar]
- 25.Ahmadsyah I, Salim A. Treatment of tetanus: an open study to compare the efficacy of procaine penicillin and metronidazole. Br Med J (Clin Res Ed) 1985;291:648–650. doi: 10.1136/bmj.291.6496.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tetanus vaccine. Wkly Epidemiol Rec. 2006;81:198–208. [PubMed] [Google Scholar]