Abstract
Fetal heart rate monitoring is the most common obstetric procedure, and yet it remains a frustrating technology, plagued by false-positive results and miscommunication between providers. A new generation of invasive and noninvasive monitoring technologies is under development and entering the clinic, including the STAN monitor (Neoventa Medical, Mölndal, Sweden), which improves monitoring accuracy by incorporating a proxy of the fetal ST-segment. New noninvasive fetal electrocardiography and uterine contraction monitoring technologies will bring novel metrics and potentially improved safety to obstetrics in coming years.
Key words: Fetal monitoring, Fetal ECG, Uterine EMG, Safety
It might just be the most hated technology we can’t live without; since continuous fetal heart rate (FHR) monitoring became the standard of care in American obstetrics three decades ago, every obstetrician has learned to practice with an imperfect device that is finicky, requires frequent adjustment, accurately predicts fetal health, but does a poor job of predicting injury. At least two National Institutes of Health consensus panels wrestled with fetal monitoring recommendations,1,2 and the American Congress of Obstetricians and Gynecologists revises its guidelines every few years, most recently recommending the use of a three-category system to simplify communication.3
The most fundamental problem with FHR monitoring, aside from technical limitations of the Doppler-based systems, is that nonreassuring FHR patterns rarely signify fetal hypoxia or ischemia, and, as a consequence, physicians and nurses may become complacent regarding nonreassuring heart rate tracings, or tracings demonstrating poor-quality data. Evidence that the use of continuous intrapartum fetal monitoring improves newborn outcomes is conspicuously lacking; what is clear is that the use of this technology leads to more operative vaginal deliveries and cesarean deliveries.3
Because of this, the race is on for better, safer, more reliable fetal monitoring technology that reliably reports the FHR, and indicates hypoxic conditions when they exist (and not when they do not). Obstetricians can look forward to significant advances in monitoring technology in the coming years; this review previews some of these advances.
Traditional Fetal Monitoring Technology
The most frequently used fetal monitoring technology uses a Doppler ultrasound device to “listen” to fetal cardiac activity, either the fetal heart itself or arterial flow through a major fetal vessel. An algorithm in the device calculates the time interval between the loudest points in the cardiac cycle and displays a heart rate. The original fetal monitoring technology, now used as a backup when the noninvasive Doppler fails, is the fetal scalp electrode, which functions as a single-lead invasive electrocardiogram (ECG), with the monitor’s algorithm calculating the R-R interval and displaying a heart rate. Contractions are monitored using either an external strain gauge or an invasive intrauterine pressure catheter.
New Fetal Heart Monitoring Technologies
STAN Monitor
Most of the new technology, either invasive or noninvasive, exploits physiologic voltage. Several devices monitor the fetal ECG waveform, and others analyze the voltage generated by the contracting uterine muscle measured at the skin surface.
The most established of these technologies is the STAN monitor (Neoventa Medical, Mölndal, Sweden). The STAN monitor uses a fetal scalp electrode to monitor not only FHR but also a proxy for the ST segment of the fetal QRS complex—the quantitative ratio between the amplitude of the fetal T wave and the fetal R wave—and the presence or absence of a biphasic ST segment.
The physiologic basis for this test came from cardiology, where researchers demonstrated that repolarization of the adult myocardium is sensitive to hypoxia, which causes an elevation of the ST segment in patients with coronary artery occlusion. Subsequent research on fetal sheep showed that experimental hypoxia leads to elevation in the ST segment and T waves in the ECG of the fetus.4 The challenge from a monitoring perspective was to develop a measurement that was vector independent, one that did not require a set of carefully located electrodes over the fetal chest. The solution was the ratio of the T-wave voltage relative to the R-wave voltage.
Additional studies demonstrated that the combination of the ST metric with FHR monitoring was better than FHR monitoring alone: large European studies showed use of the STAN monitor led to reduced incidence of hypoxic ischemic encephalopathy, and even reduced incidence of cesarean delivery (reassuring data from the STAN monitor allowed women with nonreassuring FHR patterns to continue laboring when they would have had unnecessary cesarean deliveries for false-positive FHR patterns otherwise).5–8
However, because the STAN monitor requires the use of an invasive fetal scalp electrode, and it costs significantly more than standard Doppler-based monitors, it has not been widely adopted outside of its native Scandinavia. In order to broaden appeal of the device to the American obstetric community, Neoventa Medical and the National Institute of Child Health and Human Development are sponsoring an ongoing multicenter clinical trial with the Maternal-Fetal Medicine Units network. Results of the trial, which are delayed due to low enrollment, are expected within the next several years.
Many developers see two lessons in the clinical success and business failure of the STAN monitor: (1) the way to improve the accuracy of the monitor is to exploit features of the ECG waveform, and (2) for a technology to be widely useful, it has to be noninvasive.
The Monica AN24 Monitor
The first FHR monitor using noninvasive fetal ECG technology to arrive in the American clinic was the Monica AN24 monitor (Monica Healthcare, Nottingham, UK), which obtained US Food and Drug Administration (FDA) premarket approval in February 2011. The Monica AN24 collects fetal ECG data from five electrodes placed on the laboring woman’s abdomen.
The technical challenge for the Monica AN24 and other similar monitors is that, at the point where the electrodes are placed on the maternal abdomen, the fetal ECG waveform is overwhelmed by the maternal ECG, which has a voltage 100 times greater than its tiny fetal counterpart. Ambient electrical noise, generated by maternal movement, other nearby electrical devices, and the lights overhead, also overwhelm the fetal ECG signal. Algorithms to filter out the maternal ECG signal and ambient noise have been developed over the past 30 years, but only recently have microprocessors capable of running these algorithms in real time been affordable. In order to overcome this problem of signal to noise ratio, the Monica AN24 device depends on the patient’s abdomen receiving a thorough skin preparation with a special abrading solution that removes the stratum corneum of the epidermis in order to optimize signal collection.
Although the Monica AN24 has not yet gained a significant foothold in the monitoring market, researchers have published data demonstrating high concordance between FHR data obtained using the Monica AN24 and Doppler technology.9,10 The next major milestone will require a noninvasive technology with the capacity to accomplish fetal ECG waveform analysis of the type demonstrated by the STAN monitor. The Monica AN24 is being used for research that exploits the fetal ECG waveform, but data demonstrating the capacity of this device to extract the ECG waveform have not yet been published.
The MindChild MERIDIAN Monitor
One device under development that has demonstrated the capacity to analyze the fetal ECG waveform noninvasively is the MERIDIAN monitor from MindChild Medical (North Andover, MA). The MERIDIAN monitor has been used extensively in preclinical research, and preliminary data demonstrate a high degree of effectiveness, even in obese patients who are difficult to monitor using traditional Doppler technology.
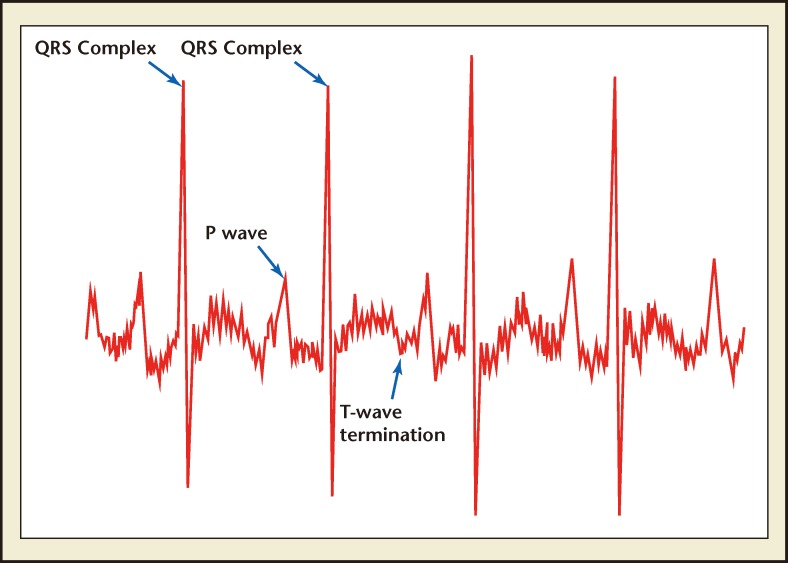
MindChild Medical’s monitor was used in a study that compared the fetal ST segment monitored using noninvasive sensors with the same data collected simultaneously using an invasive fetal scalp electrode. 11 Researchers also are using the MindChild Medical monitor to evaluate fetal ECG waveform patterns that predict in-utero inflammation. In addition to the proven capacity to measure the fetal ECG waveform, the MindChild Medical monitor does not require any skin preparation prior to placement of its abdominal electrodes. Figure 1 demonstrates fetal ECG waveform features extracted using the MindChild Medical MERIDIAN monitor.
Figure 1.
Fetal electrocardiogram waveform features identified using the MindChild Medical MERIDIAN monitor (North Andover, MA).
Other small companies and academic investigators have conducted research on noninvasive fetal ECG technology, but limited information is available about the status of their development efforts, and few published reports exist. The MindChild Medical monitor was approved for clinical use by the FDA is September 2012.
When technology makes the fetal ECG waveform available for antepartum and intrapartum interrogation, the field of fetal ECG analysis will be ripe for investigation in collaborations between pediatric electrophysiologists and obstetric researchers. This type of research, currently conducted only using fetal magnetocardiography, will be significantly easier to accomplish if inexpensive noninvasive fetal ECG equipment can be used. Research questions focused on fetal arrhythmias, prolongations in the fetal PR and QT intervals, and morphologic waveform evaluations generally inaccessible to researchers today will be addressed and answered, and the results will be incorporated into clinical algorithms that will improve the safety of obstetrics.
Newer Nonvoltage Fetal Heart Monitoring Technologies
Although voltage-based technologies are most active in the fetal monitoring space, developers are working on other technologies as well. Lifewave, Inc. (Los Altos, CA) is developing an FHR monitoring system based on radiofrequency technology similar to radar that emits a low-energy electromagnetic signal and analyzes the return waveform that has bounced off moving fetal structures in order to calculate a heart rate. The device has the potential to be very small and portable, but it is not yet approved for use by the FDA, and published data are lacking.
Significant advances also are being made in areas of fetal magnetocardiography and fetal magnetic resonance imaging (MRI), but both technologies require expensive, fixed equipment ill-suited for routine antepartum or intrapartum use. Magnetocardiography utilizes minute shifts in the magnetic fields produced by fetal electrical cardiac activity; in addition to evaluating anatomy, functional MRI sequences can be used to obtain metabolic information about the developing fetus. Although both magnetocardiography and MRI are poised to make major contributions to the understanding of fetal cardiology, anatomic abnormalities, and metabolic disorders in coming years, neither is likely to have a role in intrapartum management or routine antepartum evaluation.
Outside the domain of medical devices, informatics is playing a major role in helping obstetricians do more with the data they have. For example, automated systems that identify abnormalities in traditional FHR tracings and warn clinicians before a high-risk pattern is ignored may reduce the risk of adverse newborn outcomes.12,13
Newer Uterine Contraction Monitoring Technologies
Other exciting technology under development that also exploits physiologic voltage is the uterine contraction monitor based on electromyography (EMG) of the uterine muscle. External tocodynomometers are notoriously inaccurate and subject to movement artifact, particularly in obese patients. Like fetal ECG technology, EMG-based contraction monitors filter out ambient noise and, in this case, voltage from the maternal rectus muscles, and focus on the contraction pattern from the uterine muscle.
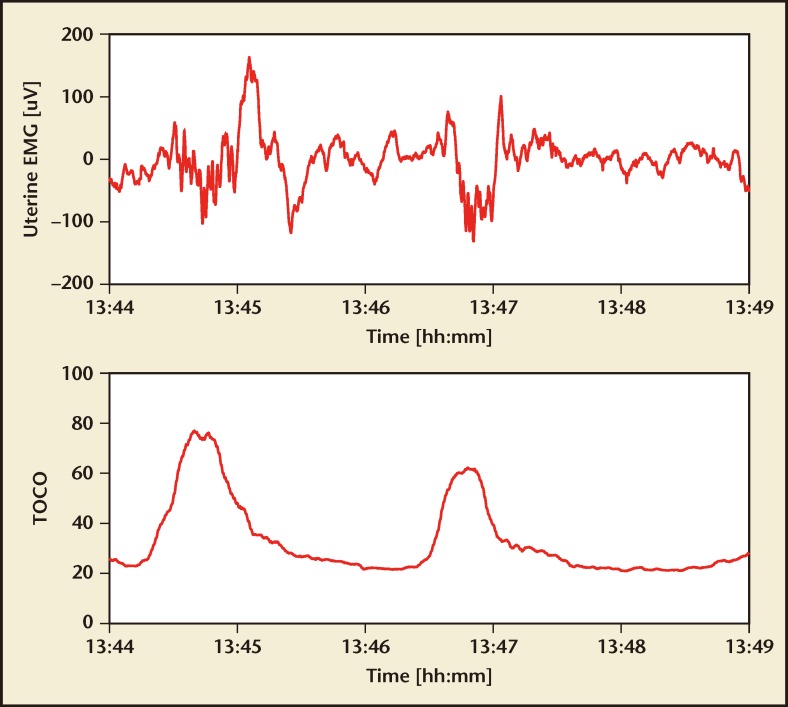
The only EMG-based contraction monitor available for clinical use is part of the Monica AN24 monitor, although other companies, including Nemo Healthcare (Veghel, the Netherlands), Reproductive Research Technologies (Houston, TX), and OB-Tools (Migdal HaEmek, Israel) are developing similar devices. Figure 2, from Nemo Healthcare, demonstrates the uterine EMG signal and the derived contraction monitoring strip.
Figure 2.
Uterine electromyogram (top) and extracted contraction pattern (bottom). EMG, electromyogram; TOCO, tocodynamometer. Image courtesy of Nemo Healthcare (Veghel, the Netherlands).
In addition to bringing more accurate contraction monitoring to obstetrics, there is some evidence that EMG-based contraction monitoring technology can be used to distinguish preterm labor from preterm contractions.14 Although this line of research is extremely preliminary, it has the potential to make an enormous contribution to clinical obstetrics.
Conclusions
FHR monitoring does a reasonably good job of indicating hypoxia and ischemia when these conditions are present. What is needed is technology that does a better job of diagnosing normal: distinguishing the nonreassuring FHR tracings generated by the healthy, oxygenated fetus. If we were better at diagnosing normal, we would pay more attention to abnormal FHR tracings that belie unfolding injury.
The other major challenge for new technology is the diagnosis of nonhypoxic injury, such as inflammatory and metabolic disorders. Technology that could identify fetuses at risk for injury having nothing to do with oxygenation would make a major impact on obstetric safety.
With these goals in mind, the future is likely to see improvements in the informatics and team training systems obstetricians use to evaluate and act on existing FHR tracings, combined with improved metrics leveraging uterine EMG and fetal ECG analysis that increase the diagnostic capacity of fetal monitoring. It may well take an innovative combination of technology, algorithms, and human systems to make obstetrics safer.
MAIN Points.
Fetal heart rate monitoring (FHR) is the most common obstetric procedure, and yet it remains a frustrating technology, plagued by false-positive results and other technical limitations.
Traditional FHR technology uses Doppler ultrasound. Most of the new technology, either invasive or noninvasive, exploits physiologic voltage. Several devices monitor the fetal electrocardiographic (ECG) waveform, and others analyze the voltage generated by the contracting uterine muscle measured at the skin surface.
The technical challenge for noninvasive monitors is that, at the point where the electrodes are placed on the maternal abdomen, the fetal ECG waveform is overwhelmed by the maternal ECG, which has a voltage 100 times greater than its tiny fetal counterpart. Ambient electrical noise, generated by maternal movement, other nearby electrical devices, and the lights overhead, also overwhelm the fetal ECG signal.
Significant advances also are being made in areas of fetal magnetocardiography and fetal magnetic resonance imaging, but both technologies require expensive, fixed equipment ill-suited for routine antepartum or intrapartum use.
Other technology under development that also exploits physiologic voltage is the uterine contraction monitor based on electromyography (EMG) of the uterine muscle. Like fetal ECG technology, EMG-based contraction monitors filter out ambient noise and, in this case, voltage from the maternal rectus muscles, and focus on the contraction pattern from the uterine muscle. In addition to bringing more accurate contraction monitoring to obstetrics, there is some evidence that EMG-based contraction monitoring technology can be used to distinguish preterm labor from preterm contractions.
Footnotes
Dr. Wolfberg is the Chief Medical Officer of MindChild Medical, Inc. (North Andover, MA), a company in which he owns equity.
References
- 1.Electronic fetal heart rate monitoring: research guidelines for interpretation. Am J Obstet Gynecol; National Institute of Child Health and Human Development Research Planning Workshop; 1997. pp. 1385–1390. [PubMed] [Google Scholar]
- 2.Macones GA, Hankins GD, Spong CY, et al. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. Obstet Gynecol. 2008;112:661–666. doi: 10.1097/AOG.0b013e3181841395. [DOI] [PubMed] [Google Scholar]
- 3.American College of Obstetricians and Gynecologists, authors. ACOG Practice Bulletin No. 106: Intrapartum fetal heart rate monitoring: nomenclature, interpretation, and general management principles. Obstet Gynecol. 2009;114:192–202. doi: 10.1097/AOG.0b013e3181aef106. [DOI] [PubMed] [Google Scholar]
- 4.Greene KG. The ECG waveform. In: Whittle M, editor. Baillière’s Clinical Obstetrics and Gynaecology. London, UK: Baillière Tindale; 1987. pp. 131–155. [DOI] [PubMed] [Google Scholar]
- 5.Amer-Wåhlin I, Bördahl P, Eikeland T, et al. ST analysis of the fetal electrocardiogram during labor: Nordic observational multicenter study. J Matern Fetal Neonatal Med. 2002;12:260–266. doi: 10.1080/jmf.12.4.260.266. [DOI] [PubMed] [Google Scholar]
- 6.Amer-Wåhlin I, Hellsten C, Nor’en H, et al. Cardiotocography only versus cardiotocography plus ST analysis of fetal electrocardiogram for intrapartum fetal monitoring: a Swedish randomised controlled trial. Lancet. 2001;358:534–538. doi: 10.1016/s0140-6736(01)05703-8. [DOI] [PubMed] [Google Scholar]
- 7.Luzietti R, Erkkola R, Hasbargen U, et al. European Community Multi-Center Trial “Fetal ECG Analysis During Labor”: ST plus CTG analysis. J Perinat Med. 1999;27:431–440. doi: 10.1515/JPM.1999.058. [DOI] [PubMed] [Google Scholar]
- 8.Norén H, Carlsson A. Reduced prevalence of metabolic acidosis at birth: an analysis of established STAN usage in the total population of deliveries in a Swedish district hospital. Am J Obstet Gynecol. 2010;202:546.e1–546.e7. doi: 10.1016/j.ajog.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 9.Stampalija T, Signaroldi M, Mastroianni C, et al. Fetal and maternal heart rate confusion during intrapartum monitoring: comparison of trans-abdominal fetal electrocardiogram and Doppler telemetry. J Matern Fetal Neonatal. 2012;25:1517–1520. doi: 10.3109/14767058.2011.636090. [DOI] [PubMed] [Google Scholar]
- 10.Reinhard J, Hayes-Gill BR, Schiermeier S, et al. Intrapartum signal quality with external fetal heart rate monitoring: a two way trial of external Doppler CTG ultrasound and the abdominal fetal electrocardiogram [published online ahead of print June 20, 2012] Arch Gynecol Obstet. doi: 10.1007/s00404-012-2413-4. doi:10.1007/s00404-012-2413-4. [DOI] [PubMed] [Google Scholar]
- 11.Clifford G, Sameni R, Ward J, et al. Clinically accurate fetal ECG parameters acquired from maternal abdominal sensors. Am J Obstet Gynecol. 2011;205:47.e1–47.e5. doi: 10.1016/j.ajog.2011.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton EF, Warrick PA. New perspectives in electronic fetal surveillance. J Perinat Med. 2012;0:1–10. doi: 10.1515/jpm-2012-0024. [DOI] [PubMed] [Google Scholar]
- 13.Warrick PA, Hamilton EF, Precup D, Kearney RE. Classification of normal and hypoxic fetuses from systems modeling of intrapartum cardiotocography. IEEE Trans Biomed Eng. 2010;57:771–779. doi: 10.1109/TBME.2009.2035818. [DOI] [PubMed] [Google Scholar]
- 14.Lucovnik M, Maner WL, Chambliss LR, et al. Noninvasive uterine electromyography for prediction of preterm delivery. Am J Obstet Gynecol. 2011;204:228.e1–228.e10. doi: 10.1016/j.ajog.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]