Abstract
Background: Insulin sensitivity (Si) is improved by weight loss and exercise, but the effects of the replacement of saturated fatty acids (SFAs) with monounsaturated fatty acids (MUFAs) or carbohydrates of high glycemic index (HGI) or low glycemic index (LGI) are uncertain.
Objective: We conducted a dietary intervention trial to study these effects in participants at risk of developing metabolic syndrome.
Design: We conducted a 5-center, parallel design, randomized controlled trial [RISCK (Reading, Imperial, Surrey, Cambridge, and Kings)]. The primary and secondary outcomes were changes in Si (measured by using an intravenous glucose tolerance test) and cardiovascular risk factors. Measurements were made after 4 wk of a high-SFA and HGI (HS/HGI) diet and after a 24-wk intervention with HS/HGI (reference), high-MUFA and HGI (HM/HGI), HM and LGI (HM/LGI), low-fat and HGI (LF/HGI), and LF and LGI (LF/LGI) diets.
Results: We analyzed data for 548 of 720 participants who were randomly assigned to treatment. The median Si was 2.7 × 10−4 mL · μU−1 · min−1 (interquartile range: 2.0, 4.2 × 10−4 mL · μU−1 · min−1), and unadjusted mean percentage changes (95% CIs) after 24 wk treatment (P = 0.13) were as follows: for the HS/HGI group, −4% (−12.7%, 5.3%); for the HM/HGI group, 2.1% (−5.8%, 10.7%); for the HM/LGI group, −3.5% (−10.6%, 4.3%); for the LF/HGI group, −8.6% (−15.4%, −1.1%); and for the LF/LGI group, 9.9% (2.4%, 18.0%). Total cholesterol (TC), LDL cholesterol, and apolipoprotein B concentrations decreased with SFA reduction. Decreases in TC and LDL-cholesterol concentrations were greater with LGI. Fat reduction lowered HDL cholesterol and apolipoprotein A1 and B concentrations.
Conclusions: This study did not support the hypothesis that isoenergetic replacement of SFAs with MUFAs or carbohydrates has a favorable effect on Si. Lowering GI enhanced reductions in TC and LDL-cholesterol concentrations in subjects, with tentative evidence of improvements in Si in the LF-treatment group. This trial was registered at clinicaltrials.gov as ISRCTN29111298.
INTRODUCTION
Weight loss and increased physical activity improve insulin sensitivity (Si) and several of the features of metabolic syndrome (1–3), but the evidence with regard to the type and nature of fat and carbohydrate in the diet is less clear.
Dietary guidelines (4, 5) for cardiovascular disease (CVD) prevention advocate a reduction in saturated fatty acids (SFAs) and trans isomeric fatty acids (TFAs) to ≤10% and 1% of energy, respectively. The replacement of SFAs with cis monounsaturated fatty acids (MUFAs) lowers LDL cholesterol concentrations and may improve Si (6–8); replacement with high–glycemic index (HGI) carbohydrates lowers HDL cholesterol concentrations (9) and might impair Si, although lower GI carbohydrates may prevent the fall in HDL cholesterol concentrations, promote weight loss, and improve Si (10, 11).
The RISCK (Reading, Imperial, Surrey, Cambridge, and Kings) study tested the hypothesis that replacing SFAs with MUFAs or carbohydrates and lowering the GI would improve Si and other CVD risk factors in subjects at risk of developing metabolic syndrome.
SUBJECTS AND METHODS
Subjects
Ethical approval for the RISCK study was obtained from the National Research Ethics Service, and written informed consent was given by participants.
Men and women (age range: 30–70 y), who were recruited from the general population, attended a clinic at the participating centers in a fasting state for measurement of height, weight, waist, seated blood pressure (BP), liver function test, glucose and lipid concentrations, and hematology. A score of ≥4 points was required to qualify for entry into the study according to the following point system: a fasting glucose concentration >5.5 mmol/L or insulin concentration >40 pmol/L = 3 points; body mass index (BMI; in kg/m2) >30 or waist >102 cm for men and >88 cm for women = 2 points; BMI of 25–30 or waist >94 cm for men and >80 cm (women) = 1 point; treated hypertension = 2 points; systolic BP >140 mm Hg = 1 point; diastolic BP >90 mm Hg = 1 point; HDL cholesterol concentration <1.0 mmol/L for men and <1.3 mmol/L for women = 2 points; and serum triacylglycerol concentration >1.3 mmol/L = 1 point. A small remuneration was given to subjects for participation in the study. Baseline measures were made from August 2004 to April 2006. Exclusion criteria for this study were as follows: a medical history of ischemic heart disease; a >30% 10-y risk of CVD (5); diabetes mellitus; cancer, pancreatitis, cholestatic liver disease, or renal disease; use of lipid-lowering drugs; systemic corticosteroids, androgens, phenytoin, erythromycin, or drugs for regulating hemostasis (excluding aspirin); exposure to any investigational agent ≤30 d before the study; presence of gastrointestinal disorder or use of a drug that is likely to alter gastrointestinal motility or nutrient absorption; a history of substance misuse or alcoholism; a current pregnancy, planned pregnancy, or given birth in the past 12 mo; an allergy or intolerance to intervention foods; an unwillingness to follow the protocol or to give informed consent; a weight change of >3 kg in the 2 mo before the study; intake of >1 g eicosapentaenoic and docosahexaenoic acids/d; or smoking >20 cigarettes/d.
Study design
A parallel 2 × 2 factorial design compared with a control intervention was used. The primary outcome was a change in Si, and the secondary outcomes were changes in CVD risk factors including lipid profiles and BP associated with metabolic syndrome. Power calculations were based on 113 subjects per group completing the study to give an 80% power to detect a difference in means of 1 (×10−4 mL · μU−1 · min−1) in the index of Si at P = 0.005. A Bonferroni multiplicity adjustment was made in the sample-size calculation for 10 pairwise comparisons. The common SD was assumed to be 2 units. Allowing for dropouts of 15%, the final sample size was 130 subjects per treatment group.
Subjects were randomly assigned to treatments by using a computer-based minimization procedure to balance assignment by age, sex, waist, and HDL cholesterol. The intervention diets were planned to provide similar intakes of dietary energy but to vary in the amount and type of fats and carbohydrates as follows: high-SFA and HGI (HS/HGI) (reference), high-MUFA and HGI (HM/HGI), HM and low-GI (HM/LGI), low-fat and HGI (LF/HGI), and LF and LGI (LF/LGI) diets. The intervention involved the provision of key sources of fat (including spreads, cooking oils, and margarine) and carbohydrates (including bread, pasta, rice, and cereals) in the diet with additional dietary information, tailored to the study group, given to subjects in writing and reinforced at individual study visits (12). The target intake for total fat was 38% of energy in the HS and HM diets and 28% of energy in the LF diets, with carbohydrate intakes of 45% and 55% of energy respectively. The HM and LF diets were designed to reduce dietary SFAs to 10% of energy with a planned MUFA intake of 20% of energy in the HM diets and 12% of energy in the LF and HS/HGI reference diet. The target differential between the HGI and LGI groups was ≈11 and ≈13 GI points respectively. Participants were advised that the dietary advice was designed for weight maintenance. For practical reasons, participants and the nutritionist advising on the dietary changes were not blinded to the treatment allocation. The dietary intervention is described in detail elsewhere (12). In brief, dietary targets were successfully achieved by using a food-exchange model where exchangeable fats and carbohydrates in the habitual diet were replaced by study foods with a specific fatty acid profile and measured GI. Specially formulated fat spreads, cooking and baking fats, and mayonnaises were provided by Unilever Food and Health Research Institute (Unilever R&D, Vlaardingen, Netherlands) and pasta or potatoes, breakfast cereals, breads, rice, and biscuits of appropriate GI were provided. Participants were offered sufficient quantities of study foods for their whole household at fortnightly intervals and given counseling and support to maintain dietary compliance. Unweighed 4-d food diaries (3 weekdays and 1 weekend day) were collected to record the habitual diet (before run-in) of subjects, dietary intakes at baseline, and dietary intakes in the third and the final month of the intervention. Nutrient intakes were estimated by using the food-composition database software DINO (Medical Research Council Human Nutrition Research Unit, Elsie Widdowson Laboratory, Cambridge, United Kingdom), which includes the UK food-composition tables (13) and is supplemented with additional data obtained from manufacturers and from in-house analyses and calculations. The database contained GI values for carbohydrate-containing foods (14). The fatty acid composition of the study fats and oils was based on chemical analyses provided by the suppliers (Unilever Best Food, Unilever R&D).
Participants provided a 24-h urine collection at baseline and follow-up for determination of the urinary microalbumin:creatinine ratio before attending the clinic for the intravenous glucose tolerance test (IVGTT). The day before each clinic visit, advice was given to subjects to avoid fatty foods, refrain from consuming alcohol, and engage in exercise, and participants were provided with a choice of light LF evening meals (2 to 3 MJ; <10 g fat) with the same meal provided on each occasion and were advised to consume the type of carbohydrate to which they had been allocated (HGI or LGI) and with vegetables as required. Subjects were asked to consume the meal before 2200 as described in a previous study (15), after which time they fasted overnight and attended the clinic between 0800 and 1100. Weight (in light clothing) and height (without shoes) and seated BP (16) were measured, and an indwelling venous cannula was inserted into the forearm. Fasting blood samples were collected and a short IVGTT was performed (17). Blood samples were separated at ≤2 h and stored at –80°C pending analysis.
IVGTT
Glucose effectiveness (Sg) and Si were estimated with the MINMOD Millennium program (version 6.02; MINMOD Inc, Pasadena, CA). Two fasting blood samples were taken to measure glucose and insulin. A bolus (0.3 g/kg body weight) of 50% glucose solution (Phoenix Pharma Ltd, Gloucester, United Kingdom) was infused into one cannula over a 1-min period. Insulin (Novo Nordisk Pharmaceuticals Ltd, West Sussex, United Kingdom) was given as a bolus (30 mU/kg body weight) into the same cannula 20 min after administration of the glucose. Blood samples were collected over a 3-h period (2, 4, 8, 19, 22, 30, 40, 50, 70, 100, and 180 min after the start of the glucose infusion). The area under the plasma insulin curve ≤19 min was computed as an indicator of endogenous insulin secretion (AIRG). Each modeled IVGTT was examined to establish the goodness of fit, and if observed glucose and insulin profiles were atypical and/or the fractional SD for Si was >0.2, the result was excluded from the analysis. The Revised Quantitative Insulin Sensitivity Check Index (RQUICKI) index of Si was calculated from fasting glucose, insulin, and nonesterified fatty acid concentrations (18).
Analytic methods
Glucose concentrations were measured with a hexokinase assay (Dimension clinical chemistry system; Dade Behring, Milton Keynes, United Kingdom) (interassay CV: 2.4%), insulin concentrations were measured with an electrochemiluminescence immunoassay (Roche, Indianapolis, IN) on a Roche Elecsys analyzer (Roche) (interassay CVs were 4.5% at 169 pmol/L and 3.6% at 552 pmol/L), and nonesterified fatty acid concentrations were measured with an enzymatic colorimetric assay (Roche Diagnostics, Penzber, Germany). The following assays were determined in the Department of Clinical Chemistry, King's College Hospital (London, United Kingdom). Total cholesterol (TC), HDL cholesterol, and triacylglycerol concentrations were measured by using enzymatic assays on a Bayer Advia Model analyzer (Bayer Diagnostics Europe Ltd, Newbery, United Kingdom) by using reagents supplied by the manufacturer (CVs for TC were 1.1%, 1.5%, and 1.0% at 3.9, 5.2 and 5.7 mmol/L, respectively; CVs for HDL cholesterol were 2.2%, 2.1%, and 2.5% at 0.91, 1.39, and 1.95 mmol/L; CVs for triacylglycerol were 2.5% and 1.5% at 1.32 and 2.36 mmol/L, respectively). LDL cholesterol concentrations were calculated by using the Friedwald formula only if fasting plasma triacylglycerol concentrations were <4.49 mmol/L; intercellular adhesion molecule-1 concentrations were measured by using an enzyme-linked immunosorbent assay (R&D Systems Europe, Abingdon, Oxon) (interassay CV: 6%); C-reactive protein high-sensitivity assay concentrations were measured by using reagents (Rapackiego19, 20–150; PZ Cormay, Lublin, Poland) (interassay CV: 6.97%, 3.34%, and 1.23% at concentrations of 0.047, 0.218, and 0.976 mg/dL, respectively; urinary microalbumin concentrations were measured by using reagents (Bayer Diagnostics Europe Ltd) (CVs were 5.3% at 19 mg/L and 2.8% at 56 mg/L; the minimum detectable concentration of urinary albumin was 6 mg/dL); urinary creatinine was measured by using the kinetic Jaffe method with rate blank correction by using reagents (Bayer Diagnostics Europe Ltd) (CVs were 2.1% at 7.1 mmol/L and 1.4% at 17.7 mmol/L). Plasma apolipoprotein B and A1 were measured by immunoprecipitation assays (Randox Laboratories, Crumlin, United Kingdom) on an ILAB-650 analyzer (Instrumentation Laboratory, Warrington, United Kingdom), and the proportion of small dense LDL (sdLDL) was measured by ultracentrifugation on the LDL fraction on an iodixanol gradient (19) at the University of Surrey. Fibrinogen concentrations were measured by the Clauss method, and factor VII coagulant (FVIIc) was measured by a clotting assay as previously described (15) by using an assay where the deficient plasma was depleted in protein S and protein C (20) in the Hemophilia Centre Laboratories St Thomas's Hospital (London, United Kingdom); interassay CVs were 2% and 3%, respectively. Plasminogen activator inhibitor type 1 (PAI-1) concentrations were measured by using an in-house chromogenic assay (21) at the Institute of Medical Sciences, University of Aberdeen (Aberdeen, United Kingdom) (interassay CV: 8.4%).
Statistical analyses
Data were analyzed by using analysis of covariance (ANCOVA) according to a prespecified analysis plan of regressing follow-up measures against baseline measures with center, ethnicity, baseline waist, baseline age, baseline HDL cholesterol, sex, and diet as covariates. Box Cox regression models were used to select suitable data transformation. Outliers were excluded from the ANCOVA and were defined as points >2.5 times the interquartile range from the median on the transformed scale at baseline, follow-up, or change from baseline. However, inclusion of these points did not materially affect the findings. The unadjusted effect of each diet is expressed as the percentage change from the median value at baseline with 95% CIs. A global test of between-diet differences with 4 df providing a P < 0.05 was prespecified as a condition to be met before further between-diet differences were explored. Additional sensitivity analyses were conducted. The effect of weight change as a covariate was examined post hoc. Correlations are presented as Spearman's r.
RESULTS
Study participation
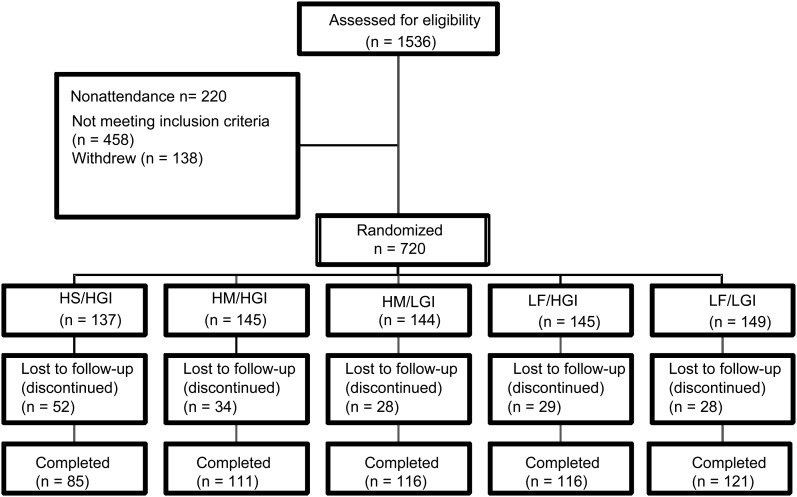
The CONSORT (22) flowchart for recruitment is shown in Figure 1. A total of 549 subjects completed the study, and data from 548 subjects were analyzed (one subject was excluded for statin use in the LF/HGI group). The ethnic mix of subjects was typical of England and predominantly white, with about one-fifth of subjects from ethnic minorities. The details of the participants at screening are shown in Table 1. Few participant were smokers (6.6%); 80% of participants had central obesity; 63% of participants had BP >130/85 mm Hg or were receiving medication for BP (17.5%); 25.5% of participants had low HDL cholesterol concentrations (<1.29 mmol/L in in men and <1.03 mmol/L in women); 27.5% of participants had serum triacylglycerol concentrations >1.7 mmol/L; and 72% of participants had serum TC concentrations >5.0 mmol/L; 47.5% of the subjects had metabolic syndrome according to the criteria of the International Diabetes Federation (23). Their habitual diets provided 35.3%, 13.0%, 11.6%, 1.2%, and 6.2% of energy from total fat, SFAs, MUFAs, TFAs, and polyunsaturated fatty acids.
FIGURE 1.
CONSORT (CONsolidated Standards of Reporting Trials) diagram of subject flow throughout the trial. HS/HGI, high–saturated fatty acid and high–glycemic index diet (reference); HM/HGI, high–monounsaturated fatty acid and HGI diet; HM/LGI, HM and low–glycemic index diet; LF/HGI, low-fat and HGI diet; LF/LGI, LF and LGI diet.
TABLE 1.
Characteristics of participants at screening1
| Characteristic | M (n = 230) | F (n = 318) |
| Age (y) | 52 ± 102 | 51 ± 9 |
| Postmenopause [n (%)] | — | 167 (53.5) |
| Race or ethnic group [n (%)] | ||
| White | 192 (83.5) | 249 (78.3) |
| South Asian | 21 (9.1) | 31 (9.7) |
| Black | 12 (5.2) | 28 (8.8) |
| Other | 5 (2.2) | 10 (3.2) |
| BMI (kg/m2) | 28.3 ± 3.8 | 28.6 ± 5.3 |
| Waist circumference (cm) | 102 ± 10 | 94 ± 12 |
| BMI >30 kg/m2 or waist >94 cm (M) or 84 cm (F) [n (%)] | 186 (80.9) | 254 (79.9) |
| Fasting insulin (pmol/L) | 60 ± 35.7 | 59.5 ± 31.3 |
| >40 pmol/L [n (%)] | 196 (87.5) | 272 (87.5) |
| Fasting glucose (mmol/L) | 5.5 ± 0.7 | 5.3 ± 0.6 |
| >5.6 mmol/L [n (%)] | 97 (42.4) | 107 (33.8) |
| Systolic BP (mm Hg) | 138 ± 16 | 129 ± 17 |
| Diastolic BP (mm Hg) | 84 ± 10 | 80 ± 9.3 |
| BP >130/85 mm Hg or BP medication [n (%)] | 168 (73.0) | 177 (55.7) |
| Total cholesterol (mmol/L) | 5.5 ± 0.9 | 5.5 ± 1.0 |
| >5.0 mmol/L [n (%)] | 164 (71.6) | 229 (72.0) |
| HDL cholesterol (mmol/L) | 1.2 ± 0.3 | 1.5 ± 0.4 |
| <1.03 mmol/L (M) or <1.29 mmol/L (F) [n (%)] | 49 (21.4) | 90 (28.3) |
| TAG (mmol/L) | 1.4 ± 0.8 | 1.2 ± 0.7 |
| >1.7 mmol/L [n (%)] | 75 (32.8) | 75 (23.6) |
| TAG >1.7 mmol or HDL cholesterol <1.03 mmol/L (M) or 1.29 mmol/L (F) [n (%)] | 97 (42.4) | 136 (42.8) |
| Cigarette smokers [n (%)] | 18 (7.8) | 18 (5.6) |
| BP medication [n (%)] | 44 (19.1) | 51 (16.3) |
| Hormone replacement therapy [n (%)] | — | 35 (11.0) |
| Oral contraceptive [n (%)] | — | 12 (3.8) |
| Thyroxine [n (%)] | 2 (0.9) | 23 (7.2) |
BP, blood pressure; TAG, triacylglycerol.
Geometric mean ± SD (all such values).
Body weight was relatively stable but decreased with the LF diets by 0.8 kg (95% CI: 0.4, 1.2 kg) compared with the HM diet (Table 2). This change was correlated with a decrease in reported energy intake (ρ = 0.206; P < 0.0001).
TABLE 2.
Insulin sensitivity (Si), insulin-independent glucose disposal (Sg), insulin response to glucose (AIRg), Revised Quantitative Insulin Sensitivity Check Index (RQUICKI), and weight at baseline [high–saturated fatty acid and high–glycemic index (HS/HGI) diet] and at follow-up after 24 wk of different dietary treatments1
| HS/HGI | HM/HGI | HM/LGI | LF/HGI | LF/LGI | P | |
| Weight (kg)2 | ||||||
| Baseline [median (IQR)] | 79.5 (69.9, 87.8) | 80.5 (70.0, 92.1) | 83.7 (69.6, 93.1) | 80.7 (71.4, 91.4) | 79.4 (70.1, 91.8) | — |
| Follow-up [median (IQR)] | 79.8 (71.0, 88.4) | 78.3 (70.1, 92.4) | 83.9 (70.0, 93.9) | 80.4 (72.1, 90.0) | 78.1 (69.4, 90.1) | — |
| Percentage change [mean (95% CI)] | +0.4 (−0.3, 1.0) | −0.5 (−1.0, 0.0) | +0.2 (−0.2, 0.6) | −1.1 (−1.6, −0.6) | −1.1 (−1.7, −0.5) | 0.0013 |
| Si (×10minus4 mL · μUminus1 · minminus1)4 | ||||||
| Baseline [median (IQR)] | 2.92 (2.00, 4.39) | 2.59 (1.86, 3.50) | 2.71 (1.77, 3.97) | 2.87 (2.00, 4.46) | 2.36 (1.84, 3.79) | — |
| Follow-up [median (IQR)] | 2.69 (2.14, 3.77) | 2.53 (1.80, 3.67) | 2.45 (1.59, 3.81) | 2.56 (1.87, 3.54) | 2.58 (1.97, 4.65) | — |
| Percentage change [mean (95% CI)] | −4.1 (−12.7, 5.3) | +2.1 (−5.8, 10.7) | −3.5 (−10.6, 4.3) | −8.6 (−15.4, −1.1) | +9.9 (2.4, 18.0) | 0.133 and 0.215 |
| Sg (× 10minus3/min)6 | ||||||
| Baseline [median (IQR)] | 16 (12, 19) | 16 (13, 19) | 16 (14, 19) | 17 (13, 19) | 17 (13, 19) | — |
| Follow-up [median (IQR)] | 15 (13, 19) | 16 (13, 19) | 16 (13, 20) | 17 (14, 20) | 17 (13, 20) | — |
| Percentage change [mean (95% CI)] | +2.1 (−5.5, 9.8) | −2.0 (−9.2, 5.2) | +1.9 (−5.3, 9.2) | +4.9 (−2.8, 12.6) | +0.2 (−5.8, 6.3) | 0.833 and 0.815 |
| AIRg (mL · μUminus1 · minminus1)7 | ||||||
| Baseline [median (IQR)] | 387 (228, 632) | 412 (242, 694) | 366 (262, 595) | 362 (216, 692) | 390 (234, 607) | — |
| Follow-up [median (IQR)] | 411 (248, 687) | 482 (286, 708) | 446 (283, 672) | 403 (216, 612) | 376 (221, 644) | — |
| Percentage change [mean (95% CI)] | +7.6 (−2.5, 18.3) | +10.8 (3.4, 18.5) | +2.4 (−5.4, 10.6) | −0.1 (−7.5, 7.7) | +0.9 (−6.2, 8.3) | 0.833 and 0.815 |
| RQUICKI (arbitrary units)8 | ||||||
| Baseline [median (IQR)] | 0.37 (0.35, 0.40) | 0.37 (0.35, 0.39) | 0.37 (0.35, 0.40) | 0.38 (0.36, 0.41) | 0.37 (0.34, 0.41) | — |
| Follow-up [median (IQR)] | 0.37 (0.35, 0.42) | 0.38 (0.35, 0.41) | 0.37 (0.34, 0.41) | 0.38 (0.36, 0.41) | 0.38 (0.35, 0.42) | — |
| Percentage change [mean (95% CI)] | +1.4 (−0.4, 3.3) | +1.1 (−0.5, 2.8) | +1.0 (−0.5, 2.6) | +0.5 (−1.1, 2.1) | +1.8 (0.3, 3.3) | 0.933 and 0.945 |
HM/HGI, high–monounsaturated fatty acid and HGI diet; HM/LGI, HM and low–glycemic index diet; LF/HGI, low-fat and HGI diet; LF/LGI, LF and LGI diet; IQR, interquartile range. Transformations: log(Si), cube root(AIRg), and reciprocal RQUICKI. Outliers were removed. Mean (95% CI) changes were calculated on a transformed scale but expressed as percentage changes from median values at baseline.
n = 85 (HS/HGI), 110 (HM/HG), 115 (HM/LGI), 111 (LF/HGI), and 117 (LF/LGI).
From ANCOVA of transformed follow-up measures on transformed baseline measures adjusted for sex, center, ethnicity, and baseline waist circumference, (log)HDL, and age.
n = 79 (HS/HGI), 103 (HM/HG), 100 (HM/LGI), 108 (LF/HGI), and 108 (LF/LGI).
Adjusted for weight change.
n = 76 (HS/HGI), 99 (HM/HG), 101 (HM/LGI), 104 (LF/HGI), and 105 (LF/LGI).
n = 79 (HS/HGI), 104 (HM/HG), 97 (HM/LGI), 108 (LF/HGI), and 104 (LF/LGI).
n = 79 (HS/HGI), 105 (HM/HG), 102 (HM/LGI), 111 (LF/HGI), and 110 (LF/LGI).
The dietary intake of energy from total fat, SFAs, and MUFAs were close to the target values with the HS/HGI reference diet and LF (LF/HGI and LF/LGI) diets (Table 3). With the HM (HM/HGI and HM/LGI) intervention diets the intake of MUFA was slightly lower than planned, and the intake of polyunsaturated fatty acid was slightly higher than planned. The proportion (mean ± SD) of energy supplied by stearic acid (18:0) with the HS/HGI, HM, and LF diets were 4.0 ± 0.8%, 2.3 ± 0.7%, and 2.1 ± 0.7% of energy, respectively. The intakes (mean ± SD) of TFA with the HS/HGI, HM, and LF diets were 1.0 ± 0.4%, 0.6 ± 0.4%, and 0.6 ± 0.4% of energy, respectively, and were derived primarily from naturally occurring sources (dairy fat, lamb, and beef). The GI was ≈8 GI points lower with the LGI diets than with the HGI diets compared with the target of 11–13 GI points. Further information is provided in Table 3 and elsewhere (12).
TABLE 3.
Changes in energy, source of dietary energy, and carbohydrate quality during the run-in period with a high–saturated fatty acid and a high–glycemic index (HS/HGI) reference diet and at the end of a 24-wk dietary intervention1
| After the 24-wk dietary intervention |
||||||
| 4 wk run-in on HS/HGI (n = 511) | HS/HGI (n = 77) | HM/HGI (n = 98) | HM/LGI (n = 101) | LF/HGI (n = 95) | LF/LGI (n = 110) | |
| Energy (MJ) | 8.59 ± 2.112 | −0.22 (−0.74, 0.30)3,a,b | −0.54 (−1.00, −0.08)a,b | −0.31 (−0.76, 0.15)a | −0.83 (−1.30, −0.37)b,c | −1.31 (−1.75, −0.88)c |
| Protein (g/d) | 80.8 ± 20.7 | 1.2 (−4.8, 7.2) | −2.2 (−7.5, 3.1) | 3.4 (−1.9, 8.6) | −0.3 (−5.7, 5.1) | −2.8 (−7.8, 2.2) |
| Fat (% of energy) | 37.9 ± 5.3 | −0.4 (−2.4, 1.6)a | −2.3 (−4.1, −0.5)a,b | −2.2 (−3.9, −0.4)b | −10.4 (−12.2, −8.6)c | −11.8 (−13.5, −10.1)c |
| SFA (% of energy) | 16.5 ± 3.0 | −0.5 (−1.5, 0.6)a | −7.0 (−7.9, −6.0)b | −6.9 (−7.8, −6.0)b | −7.3 (−8.3, −6.4)b,c | −8.2 (−9.1, −7.3)c |
| MUFA (% of energy) | 11.6 ± 2.1 | −0.1 (−1.2, 1.0)a | 4.6 (3.6, 5.6)b | 4.7 (3.7, 5.6)b | −1.8 (−2.8, −0.8)c | −1.9 (−2.8, −1.0)c |
| PUFA (% of energy) | 5.8 ± 1.7 | 0.0 (−0.7, 0.7)a,c | 0.8 (0.2, 1.4)a,b | 1.1 (0.5, 1.7)b | −0.6 (−1.2, 0.0)c | −0.7 (−1.4, −0.2)c |
| Carbohydrates (% of energy) | 43.0 ± 6.5 | −1.0 (−3.1, 1.0)a | 1.9 (0.1, 3.7)b | 1.6 (−0.2, 3.4)b | 8.1 (6.3, 9.9)c | 8.5 (6.8, 10.2)c |
| Starch (% of energy) | 24.0 ± 5.8 | −0.2 (−2.0, 1.6)a | 1.4 (−0.2, 3.0)a | 1.9 (1.2, 3.2)a,b | 4.2 (2.6, 5.8)c | 5.1 (3.6, 6.6)c |
| Sugar (% of energy) | 18.8 ± 5.4 | −0.8 (−2.3, 0.8)a | 0.5 (−0.9, 1.9)a | −0.5 (−1.9, 0.8)a | 3.8 (2.4, 5.3)b | 3.5 (2.1, 4.8)b |
| NSP (g/d) | 17.6 ± 5.8 | −0.7 (−2.4, −0.9)a | 0.7 (−0.8, 2.1)a,b | 1.7 (0.3, 3.1)bc | 1.0 (−0.5, 2.5)b,c | 0.3 (−1.1, 1.6)a,b |
| Glycemic index | 63.5 ± 3.6 | 0.5 (−0.7, 1.8)a | −0.2 (−1.3, 1.0)a | −8.3 (−9.4, −7.2)b | 0.9 (−0.3, 2.0)a | −7.2 (−8.3, −6.1)b |
| Glycemic load (% of energy) | 27.2 ± 3.9 | −0.5 (−1.8, 0.9)a | 1.1 (−0.1, 2.3)ab | −2.8 (−4.0, −1.6)c | 5.6 (4.4, 6.8)d | 1.6 (0.5, 2.7)b |
HM/HGI, high–monounsaturated fatty acid and HGI diet; HM/LGI, HM and low–glycemic index diet; LF/HGI, low-fat and HGI diet; LF/LGI, LF and LGI diet; SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; NSP, nonstarch polysaccharide. Values in the same row with different superscript letters are significantly different, P < 0.05 (Bonferroni multiple-range test).
Mean ± SD (all such values).
Mean; 95% CI in parentheses (all such values).
Insulin sensitivity
Modeling was successfully achieved for 510 participants out of 544 sets of complete IVGTT data; the number of subjects on whom data were excluded were as follows: 5 subjects in the HS/HGI group, 4 subjects in the HM/HGI group, 12 subjects in the HM/LGI group, 3 subjects in the LF/HGI group, and 10 subjects in the LFLGI group. The correlations between baseline and follow-up measures of Si, Sg, AIRG, and RQUICKI were 0.70, 0.29, 0.82, and 0.73 in the HS/HGI group. The median Si in the subjects indicated that most subjects were moderately insulin resistant (Table 2).
Unadjusted mean percentage changes (95% CIs) after 24 wk treatment were as follows: −4% (−12.7%, 5.3%) in the HS/HGI group, 2.1% (−5.8%, 10.7%) in the HM/HGI group; −3.5% (−10.6%, 4.3%) in the HM/LGI group, −8.6% (−15.4%, −1.1%) in the LF/HGI group, and 9.9% (2.4%, 18.0%) in the LF/LGI group. A global test of between-diet differences revealed no significant effect of the dietary interventions on Si (P = 0.13). Likewise, there were no significant effects of dietary intervention on Sg, AIRG, or the fasting index of Si (RQUICKI) (24). Adjustment for weight change did not alter these statistical conclusions. A post hoc analysis that excluded subjects whose weight changed >3 kg (n = 93) showed an inverse relation between changes in Si with changes in body weight (ρ = −0.112; P = 0.019).
Sensitivity analyses
Inclusion in the analysis plan of a gate-keeping condition (P < 0.05) in the global test of between-diet differences before further between-diet differences can be explored constrained the false-positive rate at 5%. However, it may have concealed further between diet differences. Because the unadjusted 95% CIs of the changes in Si after consumption of the LF/HGI and LF/LGI diets did not overlap, an additional ANCOVA analysis tested for evidence of an interaction between the fat content of the diet and GI (P = 0.017; adjusted for weight change P = 0.027). In the LF condition, a reduction of the GI of the diet increased Si by 12% (P = 0.016; adjusted for weight change P = 0.027) compared with a 5% decrease in Si with a reduction in GI in the HM condition (P = NS). These additional analyses were not adjusted for multiple comparisons. Further additional analyses did not reveal any convincing between-diet differences in any measures of Si, including the type (P = 0.7) or amount (P > 0.18) of fat.
Cardiovascular risk factors
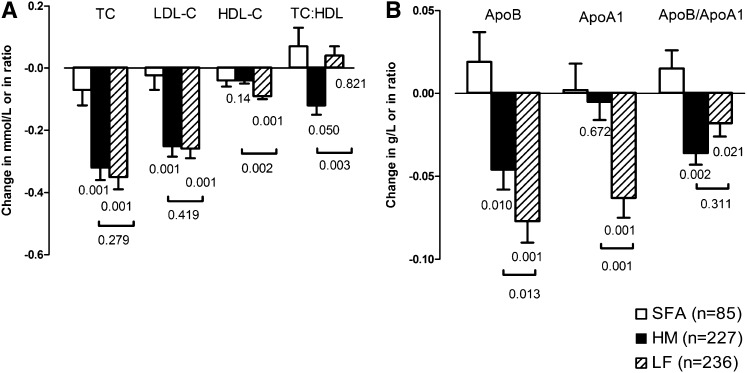
TC and LDL cholesterol concentrations were significantly lower after consumption of all diets with a reduced intake of SFAs than after consumption of the HS/HGI reference diet (P < 0.001 and P < 0.001; Table 4). Reductions in TC (P = 0.01) and LDL cholesterol (P = 0.03) concentrations were greatest in the LGI groups. Apolipoprotein B concentrations differed between treatment groups (P < 0.001) and were lower with the HM diets and lower still with the LF diets than with the HS/HGI reference diet (Figure 2B). HDL cholesterol concentrations were lower with the LF diets than with the HS/HGI reference diet or HM diets (P < 0.001 and P = 0.002, respectively) (Figure 2A). These changes were reflected by similar changes in apolipoprotein A1 concentrations (Figure 2B). There was no difference between the LGI and HGI diets. Only a minority of the subjects showed a preponderance of sdLDL (19% of the men and 4% of the women), which was not affected by the dietary intervention. The TC:HDL cholesterol ratio was significantly lower in the HM group than in the LF group (P = 0.003), but the ratio of apolipoprotein B and A1 was significantly lower in both HM and LF groups (P = 0.002 and P = 0.02, respectively) than it was for subjects who consumed the HS/HGI reference diet. BP fell in all groups from baseline to follow-up, but there were no treatment effects (Table 5). Fibrinogen was positively correlated with BMI (ρ = 0.226; P < 0.001), FVIIc was correlated with TC and triacylglycerol (ρ = 0.129 and ρ = 0.211, respectively; P < 0.001 for both), and PAI-1 activity was correlated with serum triacylglycerol and BMI (ρ = 0.236 and ρ = 0.266, respectively; P < 0.001 for both), as previously reported (25, 26). The urinary microalbumin:creatinine ratio was higher in the LF groups than in the HM groups (P = 0.001). No other significant differences were noted.
TABLE 4.
Serum lipid and lipoprotein concentrations at baseline [high–saturated fatty acid and high–glycemic index (HS/HGI) diet] and at follow-up1
| HS/HGI | HM/HGI | HM/LGI | LF/HGI | LF/LGI | P | |
| Total cholesterol (mmol/L)2 | ||||||
| Baseline [median (IQR)] | 5.6 (4.8, 6.2) | 5.7 (4.9, 6.3) | 5.6 (5.0, 6.4) | 5.5 (5.0, 6.3) | 5.5 (4.8, 6.3) | — |
| Follow-up [median (IQR)] | 5.3 (4.9, 6.1) | 5.5 (4.6, 6.1) | 5.4 (4.6, 6.1) | 5.3 (4.6, 5.9) | 5.2 (4.6, 5.8) | — |
| Percentage change [mean (95% CI)] | −1.2 (−3.1, 0.6) | −3.9 (−5.7, −2.1) | −7.0 (−8.9, −5.0) | −5.7 (−7.4, −4.0) | −6.7 (−8.5, −4.8) | 0.00013 and 0.00064 |
| LDL cholesterol (mmol/L)5 | ||||||
| Baseline [median (IQR)] | 3.4 (3.0, 4.0) | 3.6 (3.1, 4.1) | 3.6 (3.0, 4.1) | 3.6 (3.1, 4.2) | 3.5 (2.8, 4.1) | — |
| Follow-up [median (IQR)] | 3.4 (3.0, 4.1) | 3.5 (2.9, 4.1) | 3.3 (2.7, 3.9) | 3.4 (2.8, 3.9) | 3.2 (2.7, 3.7) | — |
| Percentage change [mean (95% CI)] | −0.6 (−3.4, 2.1) | −5.2 (−7.8, −2.6) | −7.8 (−10.2, −5.5) | −7.0 (−9.2, −4.8) | −7.0 (−9.5, −4.5) | 0.00053 and 0.00154 |
| Plasma sdLDL (%)6 | ||||||
| Baseline [median (IQR)] | 18.8 (12.9, 30.0) | 20.9 (14.2, 31.1) | 20.1 (14.6, 35.5) | 19.2 (12.9, 26.1) | 20.2 (13.2, 31.3) | — |
| Follow-up [median (IQR)] | 20.6 (13.8, 30.7) | 22 (15.8, 37.9) | 21.8 (14.8, 36.7) | 19.5 (12.9, 30.0) | 19.7 (14.3, 35.2) | — |
| Percentage change [mean (95% CI)] | +4.3 (−2.0, 10.8) | +9.8 (3.8, 16.0) | +1.9 (−3.7, 7.6) | +5.2 (0.0, 10.6) | +2.4 (−3.4, 8.3) | 0.503 and 0.394 |
| HDL cholesterol (mmol/L)7 | ||||||
| Baseline [median (IQR)] | 1.3 (1.2, 1.5) | 1.3 (1.1, 1.5) | 1.3 (1.1, 1.6) | 1.3 (1.1, 1.6) | 1.3 (1.2, 1.6) | — |
| Follow-up [median (IQR)] | 1.3 (1.2, 1.4) | 1.3 (1.1, 1.5) | 1.3 (1.1, 1.5) | 1.3 (1.1, 1.5) | 1.2 (1.1, 1.4) | — |
| Percentage change [mean (95% CI)] | −2.0 (−4.3, 0.3) | −2.7 (−4.6, −0.9) | −4.3 (−6.3, −2.2) | −5.9 (−7.7, −4.0) | −7.2 (−8.9, −5.5) | 0.00093 and 0.00094 |
| Triacyglycerols (mmol/L)8 | ||||||
| Baseline [median (IQR)] | 1.4 (1.0, 1.7) | 1.4 (1.1, 1.8) | 1.5 (1.1, 1.9) | 1.2 (0.9, 1.7) | 1.4 (1.0, 1.7) | — |
| Follow-up [median (IQR)] | 1.3 (1.0, 1.7) | 1.4 (1.1, 1.8) | 1.3 (0.9, 1.9) | 1.2 (0.9, 1.8) | 1.3 (1.0, 1.8) | — |
| Percentage change [mean (95% CI)] | −0.6 (−7.1, 6.3) | +1.5 (−3.7, 7.0) | −4.8 (−9.6, 0.2) | +2.9 (−2.0, 8.0) | +0.3 (−4.4, 5.2) | 0.743 and 0.344 |
| Total:HDL-cholesterol ratio9 | ||||||
| Baseline [median (IQR)] | 4.2 (3.6, 4.8) | 4.4 (3.7, 4.9) | 4.3 (3.5, 5.0) | 4.2 (3.5, 4.8) | 4.2 (3.3, 4.7) | — |
| Follow-up [median (IQR)] | 4.2 (3.5, 4.8) | 4.3 (3.7, 4.8) | 4.0 (3.3, 4.7) | 4.1 (3.6, 4.8) | 4.1 (3.4, 4.8) | — |
| Percentage change [mean (95% CI)] | +0.6 (−1.5, 2.8) | −2.4 (−4.3, −0.5) | −2.8 (−4.7, −0.9) | +0.1 (−1.7, 2.0) | −0.8 (−1.1, 2.8) | 0.0373 and 0.00434 |
HM/HGI, high–monounsaturated fatty acid and HGI diet; HM/LGI, HM and low–glycemic index diet; LF/HGI, low-fat and HGI diet; LF/LGI, LF and LGI diet; IQR, interquartile range; sdLDL, small dense LDL. Transformations: log(HDL cholesterol), square root(cholesterol), log(triacylglycerol), and square root(total:HDL cholesterol ratio). Outliers were removed. Mean (95% CI) changes were calculated on a transformed scale but expressed as the percentage change from median values at baseline. P values refer to the global test of significance between the groups. Relevant post hoc analysis results are referred to in Results or depicted in Figure 2.
n = 83 (HS/HGI), 109 (HM/HGI), 111 (HM/LGI), 112 (LF/HGI), and 120 (LF/LGI).
From ANCOVA of transformed follow-up measures on transformed baseline measures adjusted for sex, center, and ethnicity and baseline waist, (log)HDL, and age.
Adjusted for weight change.
n = 83 (HS/HGI), 108 (HM/HGI), 112 (HM/LGI), 112 (LF/HGI), and 120 (LF/LGI).
n = 81 (HS/HGI), 108 (HM/HGI), 110 (HM/LGI), 109 (LF/HGI), and 114 (LF/LGI).
n = 87 (HS/HGI), 107 (HM/HGI), 112 (HM/LGI), 108 (LF/HGI), and 104 (LF/LGI).
n = 83 (HS/HGI), 110 (HM/HGI), 108 (HM/LGI), 113 (LF/HGI), and 121 (LF/LGI).
n = 82 (HS/HGI), 108 (HM/HGI), 113 (HM/LGI), 112 (LF/HGI), and 121 (LF/LGI).
FIGURE 2.
Mean (±SEM) changes in cholesterol, apolipoprotein B (ApoB), and apolipoprotein A1 (ApoA1) after consumption of diets low in saturated fatty acids (SFA) that were high in monounsaturated fatty acids or low in fat compared with a control saturated fat–rich diet. A: Total cholesterol (TC), LDL cholesterol (LDL-C), HDL cholesterol (HDL-C), and TC:HDL cholesterol ratio after consumption of diets low in SFA that were high in monounsaturated fatty acids (HM; n = 227; filled bars) or low in fat (LF; n = 236; hatched bars) compared with a control SFA-rich diet (n = 85; open bars). Values below the bars indicate statistical significance from the SFA diet, and values below horizon bars indicate statistical significance between HM and LF diets (ANCOVA). B: Plasma ApoB, ApoA1, and the ApoB:ApoA1 ratio (ApoB/ApoA1) after consumption of HM (n = 227; filled bars) or LF (n = 236; hatched bars) diets compared with an SFA diet (n = 85; open bars). Values below the bars indicate statistical significance from the SFA diet, and values below horizon bars indicate statistical significance between HM and LF diets (ANCOVA).
TABLE 5.
Blood pressure (BP) and hemostatic and inflammatory risk factors at baseline [high–saturated fatty acid and high–glycemic index (HS/HGI) diet] and at follow-up1
| HS/HGI | HM/HGI | HM/LGI | LF/HGI | LF/LGI | P | |
| Systolic BP (mm Hg)2 | ||||||
| Baseline [median (IQR)] | 126 (117, 138) | 126 (115, 137) | 130 (120, 140) | 130 (120, 143) | 128 (118, 138) | |
| Follow-up [median (IQR)] | 126 (119, 137) | 124 (114, 134) | 128 (116, 136) | 127 (115, 142) | 124 (116, 137) | |
| Percentage change [mean (95% CI)] | −1.5 (−3.2, 0.3) | −2.0 (−3.6, −0.3) | −2.5 (−4.0, −1.0) | −1.7 (−3.1, −0.4) | −1.5 (−3.0, 0.0) | 0.733 and 0.334 |
| Diastolic BP (mm Hg)5 | ||||||
| Baseline [median (IQR)] | 77.8 (71.8, 86.0) | 78 (73.0, 85.0) | 79 (72.5, 84.5) | 81 (74.0, 87.0) | 79.5 (74.0, 87.0) | |
| Follow-up [median (IQR)] | 79 (71.5, 84.0) | 78.5 (72.0, 84.0) | 78.5 (72.5, 85.0) | 78.5 (71.5, 87.0) | 79.3 (73.5, 85.0) | |
| Percentage change [mean (95% CI)] | −0.5 (−2.6, 1.5) | −1.6 (−3.3, 0.0) | −0.9 (−2.6, 0.8) | −1.5 (−2.9, −0.1) | −1.7 (−3.1, −0.2) | 0.993 and 1.004 |
| Fibrinogen (g/L)6 | ||||||
| Baseline [median (IQR)] | 2.68 (2.28, 3.14) | 2.71 (2.40, 3.10) | 2.55 (2.30, 3.03) | 2.79 (2.28, 3.14) | 2.65 (2.32, 3.11) | |
| Follow-up [median (IQR)] | 2.66 (2.34, 3.17) | 2.76 (2.37, 3.24) | 2.70 (2.27, 3.13) | 2.82 (2.43, 3.15) | 2.76 (2.40, 3.21) | |
| Percentage change [mean (95% CI)] | +0.5 (−3.1, 4.1) | +1.6 (−1.2, 4.5) | +1.6 (−1.0, 4.2) | +2.1 (−0.8, 5.0) | +2.4 (−0.3, 5.0) | 0.853 and 0.984 |
| CRP (mg/L)7 | ||||||
| Baseline [median (IQR)] | 0.7 (0.16, 2.30) | 0.54 (0.20, 1.90) | 0.4 (0.14, 1.10) | 0.5 (0.10, 1.95) | 0.57 (0.16, 1.90) | |
| Follow-up [median (IQR)] | 0.95 (0.30, 1.85) | 0.65 (0.20, 2.30) | 0.7 (0.20, 2.00) | 0.7 (0.20, 2.40) | 0.6 (0.20, 1.70) | |
| Percentage change [mean (95% CI)] | +21.3 (−5.8, 55.2) | +3.8 (−21.4, 35.6) | +36.3 (3.0, 78.2) | +22.4 (−7.6, 60.3) | +8.0 (−13.5, 33.9) | 0.863 and 0.904 |
| ICAM-1 (μg/L)8 | ||||||
| Baseline [median (IQR)] | 242 (206, 288) | 240 (218, 283) | 242.2 (219, 283) | 240 (206, 283) | 247 (207, 284) | |
| Follow-up [median (IQR)] | 241.4 (215, 287) | 247.6 (214, 278) | 242 (210, 274) | 242 (214, 273) | 249 (214, 280) | |
| Percentage change [mean (95% CI)] | +0.3 (−3.0, 3.7) | −0.4 (−3.0, 2.3) | −2.2 (−4.9, 0.6) | −0.6 (−3.4, 2.2) | −0.3 (−3.2, 2.6) | 0.593 and 0.574 |
| PAI-1 (IU/L)9 | ||||||
| Baseline [median (IQR)] | 17.0 (12.0, 23.4) | 18.0 (13.4, 22.4) | 17.8 (13.6, 23.0) | 16.7 (12.3, 21.0) | 17.9 (13.6, 21.9) | |
| Follow-up [median (IQR)] | 19.2 (13.0, 24.1) | 19.4 (15.0, 24.8) | 19.6 (14.5, 24.9) | 18 (12.9, 23.4) | 18.4 (14.0, 22.1) | |
| Percentage change [mean (95% CI)] | +7.5 (−1.1, 16.1) | +11.3 (4.6, 18.0) | +7.0 (0.3, 13.8) | +6.4 (−1.1, 13.8) | +2.2 (−4.8, 9.3) | 0.483 and 0.724 |
| FVIIc (%)10 | ||||||
| Baseline [median (IQR)] | 135 (118, 153) | 138 (116, 153) | 133 (110, 150) | 127 (105, 148) | 135 (116, 156) | |
| Follow-up [median (IQR)] | 127 (108, 150) | 133 (111, 155) | 132 (110, 157) | 127 (101, 143) | 128 (103, 149) | |
| Percentage change [mean (95% CI)] | −0.9 (−5.0, 3.2) | −0.6 (−4.4, 3.3) | +1.3 (−2.4, 5.0) | +2.8 (−1.2, 6.8) | −2.7 (−6.2, 0.7) | 0.853 and 0.984 |
| Microalbumin:creatinine (mg/mmol)11 | ||||||
| Baseline [median (IQR)] | 1.27 (0.93, 1.82) | 1.17 (0.83, 1.58) | 1.22 (0.80, 1.62) | 1.2 (0.78, 1.62) | 1.13 (0.82, 1.76) | |
| Follow-up [median (IQR)] | 1.19 (0.78, 1.50) | 1.08 (0.78, 1.58) | 1.11 (0.72, 1.88) | 1.22 (0.80, 1.88) | 1.3 (0.86, 2.00) | |
| Percentage change [mean (95% CI)] | −12.7 (−21.5, −2.8) | −6.7 (−13.4, 0.7) | −4.3 (−11.8, 3.8) | +7.3 (−0.8, 16.1) | +5.8 (−1.7, 13.8) | 0.00163 and 0.0174 |
HM/HGI, high–monounsaturated fatty acid and HGI diet; HM/LGI, HM and low–glycemic index diet; LF/HGI, low-fat and HGI diet; LF/LGI, LF and LGI diet; IQR, interquartile range; CRP, C-reactive protein; ICAM-1, intercellular adhesion molecule-1; PAI-1, plasminogen activator inhibitor type 1; FVIIc, factor VII coagulant. Transformations: log. Outliers were removed. Mean (95% CI) changes were calculated on a transformed scale but expressed as the percentage change from median values at baseline. P values refer to the global test of significance between the groups. Relevant post hoc analysis results are referred to in Results.
n = 84 (HS/HGI), 109 (HM/HGI), 113 (HM/LGI), 113 (LF/HGI), and 120 (LF/LGI).
From ANCOVA of transformed follow-up measures on transformed baseline measures adjusted for sex, center, ethnicity, and baseline waist circumference, (log)HDL cholesterol, and age.
Adjusted for weight change.
n = 83 (HS/HGI), 110 (HM/HGI), 114 (HM/LGI), 113 (LF/HGI), and 120 (LF/LGI).
n = 81 (HS/HGI), 110 (HM/HGI), 112 (HM/LGI), 113 (LF/HGI), and 113 (LF/LGI).
n = 79 (HS/HGI), 107 (HM/HGI), 108 (HM/LGI), 109 (LF/HGI), and 119 (LF/LGI).
n = 80 (HS/HGI), 106 (HM/HGI), 107 (HM/LGI), 109 (LF/HGI), and 115 (LF/LGI).
n = 85 (HS/HGI), 105 (HM/HGI), 111 (HM/LGI), 114 (LF/HGI), and 119 (LF/LGI).
n = 75 (HS/HGI), 97 (HM/HGI), 100 (HM/LGI), 102 (LF/HGI), and 100 (LF/LGI).
n = 80 (HS/HGI), 98 (HM/HGI), 109 (HM/LGI), 101 (LF/HGI), and 108 (LF/LGI).
Additional analyses using parsimonious models did not provide any further evidence that the dietary manipulations had any additional effects on outcomes beyond those identified in the primary analysis.
DISCUSSION
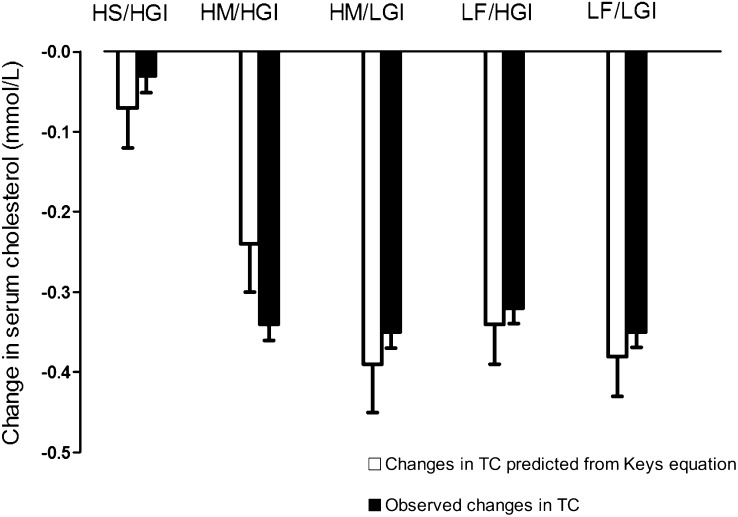
Participants without preexisting CVD or type 2 diabetes, but who were likely to be insulin resistant, were recruited into the RISCK trial. The criteria were purposefully set to identify individuals at an increased risk of developing metabolic syndrome but at a lower level than clinical definitions. The dietary manipulations were smaller than many previous studies but represent achievable public health goals. Measurements of food intake and changes in TC predicted by the Keys equation (Figure 3) indicated good compliance to the changes in dietary fat intake. Although the dietary advice aimed to maintain body weight, there was a small decrease (≈1 kg) after the LF than after the HM diets. This is consistent with the measured reduction in energy intake on the LF diet and reflects incomplete compensation for the reduction in food energy from fat. In contrast, there was no evidence of any effect of GI on body weight.
FIGURE 3.
Mean (±SEM) changes in total cholesterol (TC) compared with predicted changes from the Keys equation (27). Overall test of significance, P < 0.001 (ANCOVA). HS/HGI, high–saturated fatty acid and high–glycemic index diet; HM/HGI, high–monounsaturated fatty acid and HGI diet; HM/LGI, HM and low-GI diet; LF/HGI, low-fat and HGI diet; LF/LGI, LF and LGI diet.
Insulin sensitivity
Two smaller studies that used a glucose suppression test reported favorable effects of MUFA compared with SFA on Si (28, 29). However, these prescribed specific meals were consumed by individuals in a metabolic ward–type regimen. The current study used the IVGTT method, which focuses on the peripheral actions of insulin and is largely unconfounded by the hepatic and gastrointestinal contributions observed in measures such as the oral glucose tolerance test. Our results do not confirm the findings of the KANWU study (7), which also used an IVGTT and a similar dietary prescription to replace SFAs with MUFAs for 3 mo. The KANWU study reported a 5.2% difference in TC between diets and noted that Si fell from 4.13 to 3.71 (×10−4 mL · μU−1 · min−1) on the SFA diet, and on the MUFA diet it increased from 4.76 to 4.86 (×10−4 mL · μU−1 · min−1); the difference between treatments was of borderline statistical significance (P = 0.053). Further analysis suggested a significant difference (P = 0.03) in subjects who consumed <37% of energy as fat during the study period, but the justification of conducting this post hoc comparison is questionable (30) because there was no evidence that the treatment effects differed in the different subgroups (P = 0.26; Lars Berglund, personal communication, 2008). A recent small (n = 60) intervention trial that compared an HS diet to an HM or Mediterranean diet, showed no effect on Si measured by homeostatic model assessment of IR or by the euglycemic clamp method in a subset of (30) subjects after 8 wk intervention (31). Findings from LIPGENE, a large (n = 417) pan-European study, concur with that of RISCK. LIPGENE examined the effect of a similar dietary intervention on Si, as measured by IVGTT, in the metabolic syndrome, and showed no significant change in Si after SFA-rich and MUFA-rich diets (32). Taken together, these findings do not provide convincing evidence that the isoenergetic replacement of SFA with MUFA improves Si.
Reports of the effect of GI on Si are inconsistent, and intervention studies have varied widely in their duration, sample size, subject characteristics and health status, intervention diet, and methods for assessing Si (33, 34). We achieved a reduction in GI similar to the range reported in prospective observational studies (35). To achieve a greater reduction in GI, it would have been necessary to replace a substantial amount of added sugar with starch foods with a LGI that would have resulted in other, poorly controlled, dietary changes. In the current study, there was no overall effect between diets on Si as determined by the prespecified statistical analyses. However, additional sensitivity analyses provided tentative evidence to suggest an improvement on the LF/LGI diet than on the LF/HGI diet, but further research is needed to test if this observation is robust.
Cardiovascular risk factors
This study confirmed the effect of SFA reduction on TC, LDL cholesterol, and apolipoprotein B concentrations and an effect of fat reduction on lowering HDL cholesterol and apolipoprotein A1 concentrations. The current study did not observe the change in serum triacylglycerol concentrations reported in a previous meta-analysis (36). This was probably because participants were provided with a light LF meal on the day that preceded measurement to exclude the acute effects of variations in fat intake. We previously showed that fasting triacylglycerol concentrations do not differ between an HM diet and a high-carbohydrate diet when dietary antecedent intake is standardized (37) and also showed, by using the same model, that changes in fasting and postprandial triacylglycerol (15) and small dense LDL concentrations occur after an increased intake of long-chain n−3 fatty acids (38). The dietary interventions did not alter the proportion of small dense LDLs, which are believed to be the key proatherogenic lipoprotein particle associated with the dyslipidemia of metabolic syndrome, and this finding is consistent with previous reports (39, 40). In contrast to the conclusions of a meta-analysis (33), the current study showed an additional reduction in TC and LDL cholesterol concentrations with lowering GI. However, lowering GI did not prevent the fall in HDL cholesterol or apolipoprotein A1 concentrations. On the basis of a meta-analysis of prospective cohort studies (41), it was argued that the ratio of TC:HDL cholesterol is twice as informative at predicting CVD risk than is TC. In this context, the LF diets did not influence the ratio, but the ratio was lower on the HM diet than on the HS/HGI reference diet.
Elevated CRP, fibrinogen, and PAI-1 activity independently predict the risk of acute coronary syndromes. In line with previous reports (25, 26), a relation between these risk factors and measures of adiposity was shown. However, the current intervention study was unable to show any effects of the type of fat or GI on hemostatic and inflammatory markers of CVD risk, which suggests that diet composition has little effect on these risk factors. This is consistent with the review of the effects of fat conducted for the WHO/FAO, which could find no probable or convincing evidence to indicate that exchanging SFAs for MUFAs, or carbohydrates for SFAs, influenced CRP concentrations (40). An elevated FVIIc concentration is associated with an increased risk of fatal CHD (42, 43) and associated with fasting triacylglycerol and cholesterol concentrations. The Women's Health Initiative (44) reported a 4% fall in FVIIc in women consuming an LF diet. However, FVIIc changes rapidly in response to meals high in fat (≤3 h and persisting for ≤24 h) the findings of this RISCK study, where the measurements were preceded by a light LF meal, indicate that there is no chronic change in dietary FVIIc caused by variations in total and monounsaturated fat intake in agreement with a shorter-term study (37).
Conclusions
This study did not support the hypothesis that isoenergetic replacement of SFAs with MUFAs or carbohydrates improves Si but confirms the favorable effect of reductions in SFA on TC:HDL ratio. Lowering GI enhanced the reduction in TC and LDL cholesterol concentrations, with preliminary evidence from additional analyses of an improvement in Si in the LF treatment.
Acknowledgments
We acknowledge the contributions of the additional RISCK Study Group members—University of Reading: Hannah Farrant (local coordinator); Claire Lawrence, Edel Magee, and Kit Tsoi (research assistants); Darren Cole (database manager); Steve Austin, Hanneke Mfuni, and Kate Guberg (sample analyses of glucose, insulin, and nonesterified fatty acid); Anna Gent, Celia Greenberg, and Caroline Stokes (coding and analyses of dietary data); Mario Siervo and Rosemary Hall (clinicians); Celia Walker (supplementary analyses, Medical Research Council Human Nutrition Research); Imperial College London: Louise Goff (local coordinator); Claire Howard, Namrata Dhopatkar, and Bushra Siddiqui (research assistants); Anne Dornhurst (clinician); Kings College London: Fiona Lewis (local coordinator) Samantha Bowen, L Chen, and Robert Gray (research assistants); Nuala Booth (sample analyses of PAI-1); Gary Moore (sample analyses of FVIIc and fibrinogen); Roy Sherwood (sample analyses of clinical biochemistry); Anthony Leeds, A Shah, G Saran, J Niehuser-Saran, and JA Cockburn (clinicians); University of Reading: Rachel Gitau (local coordinator); Katie Newens and Sean Lovegrove (research assistants); Ana Rodriguez-Mateos (sample analyses of plasma fatty acids); University of Reading and University of Surrey: John Wright (clinician); University of Surrey: Margaret Griffin (local coordinator) and Nicola Harman (lead for lipid subclasses).
The authors' responsibilities were as follows—SAJ: chief investigator and is the guarantor for this article and had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analyses; JAL: principal investigator; CMW: co-investigator for the University of Reading; BAG: principal investigator for University of Surrey; GSF: principal investigator for the Imperial College London; CSM: scientific coordinator; MDC: statistician; LJB: responsible for insulin sensitivity modelling and analysis; and TABS: principal investigator for the Kings College London. The authors and their research groups have a number of links with the food industry. In a personal capacity, GSF is a consultant to Coca-Cola, Premier Foods, and Unilever and TABS has acted as a consultant to Seven Seas and is a member of the Scientific Advisory Committee for the Global Dairy Platform and external scientific review committee of the Malaysian Palm Oil Board, and chairs Cadbury's Global Nutrition Advisory Panel. TABS, BAG, JAL, CMW, SAJ, and GSF have received ad hoc honoraria for lectures or writing articles. In a nonpersonal capacity, BAG was formerly a member of an expert group known as the Fat Panel, which was supported by Dairy Crest, Kerry Gold, and Unilever; SAJ is a member of Scientific Advisory Boards for Coca-Cola, Heinz, PepsiCo, Nestlé and Kellogg's; CMW is a member of PepsiCo's Health and Wellbeing External Advisory Board. CMW and SAJ sit on government advisory boards that also include food industry members. All research groups received products from a range of food companies gratis for research purposes, including Archer Daniel Mills, Croda, Matthews Foods, Nestle, PepsiCo, Jordan, GSK, and Unilever. CSM, MDC, and LJS reported no conflicts of interest.
REFERENCES
- 1.Ilanne-Parikka P, Eriksson JG, Lindstrom J, et al. Effect of lifestyle intervention on the occurrence of metabolic syndrome and its components in the Finnish Diabetes Prevention Study. Diabetes Care 2008;31:805–7 [DOI] [PubMed] [Google Scholar]
- 2.Orchard TJ, Temprosa M, Goldberg R, et al. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med 2005;142:611–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costacou T, Mayer-Davis EJ. Nutrition and prevention of type 2 diabetes. Annu Rev Nutr 2003;23:147–70 [DOI] [PubMed] [Google Scholar]
- 4.Graham I, Atar D, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Eur Heart J 2007;28:2375–414 [DOI] [PubMed] [Google Scholar]
- 5.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97 [DOI] [PubMed] [Google Scholar]
- 6.Garg A. High-monounsaturated-fat diets for patients with diabetes mellitus: a meta-analysis. Am J Clin Nutr 1998;67:577S–82S [DOI] [PubMed] [Google Scholar]
- 7.Vessby B, Unsitupa M, Hermansen K, et al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU Study. Diabetologia 2001;44:312–9 [DOI] [PubMed] [Google Scholar]
- 8.Due A, Larsen TM, Mu H, Hermansen K, Stender S, Astrup A. Comparison of 3 ad libitum diets for weight-loss maintenance, risk of cardiovascular disease, and diabetes: a 6-mo randomized, controlled trial. Am J Clin Nutr 2008;88:1232–41 [DOI] [PubMed] [Google Scholar]
- 9.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr 2003;77:1146–55 [DOI] [PubMed] [Google Scholar]
- 10.Leeds AR. Glycemic index and heart disease. Am J Clin Nutr 2002;76:286S–9S [DOI] [PubMed] [Google Scholar]
- 11.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 2002;287:2414–23 [DOI] [PubMed] [Google Scholar]
- 12.Moore C, Gitau R, Goff L, et al. Successful manipulation of the quality and quantity of fat and carbohydrate consumed by free-living individuals using a food exchange model. J Nutr 2009;139:1534–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCance and Widdowson's the composition of foods. 6th ed Cambridge, United Kingdom: Royal Society of Chemistry and the Food Standards Agency, 2002 [Google Scholar]
- 14.Aston LM, Jackson D, Monsheimer S, et al. Developing a methodology for assigning glycaemic index values to foods consumed across Europe. Obes Rev 2010;11:92–100 [DOI] [PubMed] [Google Scholar]
- 15.Sanders TA, Lewis F, Slaughter S, et al. Effect of varying the ratio of n−6 to n−3 fatty acids by increasing the dietary intake of alpha-linolenic acid, eicosapentaenoic and docosahexaenoic acid, or both on fibrinogen and clotting factors VII and XII in persons aged 45-70 y: the OPTILIP study. Am J Clin Nutr 2006;84:513–22 [DOI] [PubMed] [Google Scholar]
- 16.Williams B, Poulter NR, Brown MJ, et al. British Hypertension Society guidelines for hypertension management 2004 (BHS-IV): summary. BMJ 2004;328:634–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steil GM, Murray J, Bergman RN, Buchanan TA. Repeatability of insulin sensitivity and glucose effectiveness from the minimal model. Implications for study design. Diabetes 1994;43:1365–71 [DOI] [PubMed] [Google Scholar]
- 18.Perseghin G, Caumo A, Caloni M, Testolin G, Luzi L. Incorporation of the fasting plasma FFA concentration into QUICKI improves its association with insulin sensitivity in nonobese individuals. J Clin Endocrinol Metab 2001;86:4776–81 [DOI] [PubMed] [Google Scholar]
- 19.Davies IG, Graham JM, Griffin BA. Rapid separation of LDL subclasses by iodixanol gradient ultracentrifugation. Clin Chem 2003;49:1865–72 [DOI] [PubMed] [Google Scholar]
- 20.Miller GJ, Stirling Y, Esnouf MP, et al. Factor VII-deficient substrate plasmas depleted of protein C raise the sensitivity of the factor VII bio-assay to activated factor VII: an international study. Thromb Haemost 1994;71:38–48 [PubMed] [Google Scholar]
- 21.Verheijen JH, Mullaart E, Chang GT, Kluft C, Wijngaards G. A simple, sensitive spectrophotometric assay for extrinsic (tissue-type) plasminogen activator applicable to measurements in plasma. Thromb Haemost 1982;48:266–9 [PubMed] [Google Scholar]
- 22.Moher D, Schulz KF, Altman DG. for the Consort Group. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA 2001;285:1987–91 [DOI] [PubMed] [Google Scholar]
- 23.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 2006;23:469–80 [DOI] [PubMed] [Google Scholar]
- 24.Rabasa-Lhoret R, Bastard JP, Jan V, et al. Modified quantitative insulin sensitivity check index is better correlated to hyperinsulinemic glucose clamp than other fasting-based index of insulin sensitivity in different insulin-resistant states. J Clin Endocrinol Metab 2003;88:4917–23 [DOI] [PubMed] [Google Scholar]
- 25.Mutch NJ, Wilson HM, Booth NA. Plasminogen activator inhibitor-1 and haemostasis in obesity. Proc Nutr Soc 2001;60:341–7 [DOI] [PubMed] [Google Scholar]
- 26.Meade TW, Chakrabarti R, Haines AP, North WR, Stirling Y. Characteristics affecting fibrinolytic activity and plasma fibrinogen concentrations. BMJ 1979;1:153–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keys A, Parlin R. Serum cholesterol response to changes in dietary lipids. Am J Clin Nutr 1966;19:175–81 [DOI] [PubMed] [Google Scholar]
- 28.Paniagua JA, Gallego de la Sacristana A, Romero I, et al. Monounsaturated fat-rich diet prevents central body fat distribution and decreases postprandial diponectin expression induced by a carbohydrate-rich diet in insulin-resistant subjects. Diabetes Care 2007;30:1717–23 [DOI] [PubMed] [Google Scholar]
- 29.Pérez-Jiménez F, Lopez-Miranda J, Pinillos MD, et al. A Mediterranean and a high-carbohydrate diet improve glucose metabolism in healthy young persons. Diabetologia 2001;44:2038–43 [DOI] [PubMed] [Google Scholar]
- 30.Matthews JN, Altman DG. Statistics notes. Interaction 2: Compare effect sizes not P values. BMJ 1996;313:808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bos MB, de Vries JH, Freskens EJ, et al. Effect of a high monounsaturated fatty acids diet and a Mediterranean diet on serum lipids and insulin sensitivity in adults with mild abdominal obesity. Nutr Metab Cardiovasc Dis (Epub ahead of print 17 August 2009) [DOI] [PubMed] [Google Scholar]
- 32.Tierney AC, McMonagle J, Shaw DI, et al. Effects of dietary fat modification on insulin sensitivity and other risk factors of the metabolic syndrome—LIPGENE: an European randomized dietary intervention study. Int J Obes (in press) [DOI] [PubMed] [Google Scholar]
- 33.Kelly S, Frost G, Whittaker V, Summerbell C. Low glycaemic index diets for coronary heart disease. Cochrane Database Syst Rev 2004;CD004467. [DOI] [PubMed] [Google Scholar]
- 34.Wolever TM, Mehling C, Chiasson JL, et al. Low glycaemic index diet and disposition index in type 2 diabetes (the Canadian trial of carbohydrates in diabetes): a randomised controlled trial. Diabetologia 2008;51:1607–15 [DOI] [PubMed] [Google Scholar]
- 35.Jakobsen MU, Dethlefsen C, Joensen AM, et al. Intake of carbohydrates compared with intake of saturated fatty acids and risk of myocardial infarction: importance of the glycemic index. Am J Clin Nutr 2010;91:1764–8 [DOI] [PubMed] [Google Scholar]
- 36.Mensink RP, Katan MB. Effect of dietary fatty acids on serum lipids and lipoproteins: a meta-analysis of 27 trials. Arterioscler Thromb 1992;12:911–9 [DOI] [PubMed] [Google Scholar]
- 37.Sanders TA, Oakley FR, Crook D, Cooper JA, Miller GJ. High intakes of trans monounsaturated fatty acids taken for 2 weeks do not influence procoagulant and fibrinolytic risk markers for CHD in young healthy men. Br J Nutr 2003;89:767–76 [DOI] [PubMed] [Google Scholar]
- 38.Griffin MD, Sanders TA, Davies IG, et al. Effects of altering the ratio of dietary n−6 to n−3 fatty acids on insulin sensitivity, lipoprotein size, and postprandial lipemia in men and postmenopausal women aged 45−70 y: the OPTILIP Study. Am J Clin Nutr 2006;84:1290–8 [DOI] [PubMed] [Google Scholar]
- 39.Rivellese AA, Maffettone A, Vessby B, et al. Effects of dietary saturated, monounsaturated and n−3 fatty acids on fasting lipoproteins, LDL size and post-prandial lipid metabolism in healthy subjects. Atherosclerosis 2003;167:149–58 [DOI] [PubMed] [Google Scholar]
- 40.Sanders TA. Fat and fatty acid intake and metabolic effects in the human body. Ann Nutr Metab 2009;55:162–72 [DOI] [PubMed] [Google Scholar]
- 41.Lewington S, Whitlock G, Clarke R, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007;370:1829–39 [DOI] [PubMed] [Google Scholar]
- 42.Meade TW, Ruddock V, Stirling Y, Chakrabarti R, Miller GJ. Fibrinolytic activity, clotting factors, and long-term incidence of ischaemic heart disease in the Northwick Park Heart Study. Lancet 1993;342:1076–9 [DOI] [PubMed] [Google Scholar]
- 43.Heinrich J, Balleisen L, Schulte H, Assmann G. van de Loo J. Fibrinogen and factor VII in the prediction of coronary risk. Results from the PROCAM study in healthy men. Arterioscler Thromb 1994;14:54–9 [DOI] [PubMed] [Google Scholar]
- 44.Howard BV, Van HL, Hsia J, et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006;295:655–66 [DOI] [PubMed] [Google Scholar]