Abstract
Objectives
Data suggest that some licensed antiretroviral doses could be reduced. We assessed the safety, tolerability and pharmacokinetics of lopinavir/ritonavir at doses of 400/100, 200/150 and 200/50 mg twice daily in HIV-negative volunteers (http://clinicaltrials.gov/ct2/show/NCT00985543).
Methods
Male and female volunteers were administered lopinavir/ritonavir at doses of 400/100 mg (two lopinavir/ritonavir Meltrex 200/50 mg tablets, Regimen 1), 200/150 mg (one Meltrex tablet, one 100 mg ritonavir capsule, Regimen 2) and 200/50 mg (one Meltrex tablet, Regimen 3). Each dose was given twice daily for 7 days sequentially, separated by a 7 day wash-out period. Lopinavir/ritonavir steady-state pharmacokinetics was assessed over 12 h at the end of each phase (days 7, 21 and 35). Pharmacokinetic parameters were compared using the 400/100 mg twice daily dose as reference, by determining geometric mean ratios (GMRs) and 90% confidence intervals.
Results
Twenty-two subjects (eight females) completed the study. Lopinavir AUC0–12 (ng h/mL), Cmax (ng/mL) and the minimum concentration (Ctrough) (ng/mL) for the 400/100, 200/150 and 200/50 mg twice daily doses, respectively, were as follows: 99599, 73603 and 45146; 11965, 8939 and 6404; and 5776, 4293 and 1749. Lopinavir pharmacokinetic parameters were significantly lower for Regimens 2 and 3: GMR (90% CI) AUC0–12, 0.74 (0.65–0.84) and 0.45 (0.40–0.51); Cmax, 0.75 (0.66–0.85) and 0.54 (0.40–0.60); and Ctrough, 0.74 (0.62–0.89) and 0.30 (0.25–0.36), respectively. All subjects taking the 400/100 and 200/150 mg twice daily doses, and 19 (86%) subjects taking 200/50 mg twice daily had lopinavir concentrations above the suggested minimum effective concentration of 1000 ng/mL.
Conclusions
These pharmacokinetic data show that therapeutic plasma concentrations of lopinavir can be achieved with 200/150 mg of lopinavir/ritonavir twice daily (one Meltrex tablet and one 100 mg ritonavir capsule twice daily).
Keywords: antiretroviral therapy, HIV antiviral pharmacology, pharmacokinetics
Introduction
The approved dose of lopinavir/ritonavir is 400/100 mg twice daily. Originally, it was co-formulated in a soft gelatin capsule with 133 mg of lopinavir and 33 mg of ritonavir (three capsules twice daily). The more recent heat-stable formulation (Meltrex, 200/50 mg—two tablets twice daily) shows lower variability in plasma drug concentrations, no food dependence and does not require refrigeration.1 In addition, the new heat-stable (Meltrex) formulation of lopinavir/ritonavir (200/50 mg tablet) produces lopinavir concentrations 15%–30% higher than the original soft gelatin formulation. Ritonavir is currently only available as capsules of 100 mg.
During the development of lopinavir/ritonavir, lower drug concentrations have shown similar efficacy to the approved dose; the Abbott 720 trial2 evaluated the antiviral activity of lopinavir/ritonavir (plus stavudine and lamivudine) in antiretroviralnaive individuals. Patients were studied in two randomized sequential groups. In Group I (n=32), participants received 200/100 or 400/100 mg of lopinavir/ritonavir twice daily and Group II (n=68) received 400/100 or 400/200 mg twice daily. A rapid decline in HIV load from baseline to week 48 (mean 2.23 log10 copies/mL) was measured across all treatment arms. At week 48, in Group I, fewer patients achieved a plasma viral load of <50 copies/mL in the 400/100 mg dose arm than in the 200/100 mg dose arm [50% versus 100%, P=0.002]. This seemed to be driven by adverse events rather than antiviral efficacy. However, the 400/100 mg twice daily dose was selected for further clinical development, in order to maximize lopinavir concentrations while maintaining an acceptable tolerability profile.3
To further increase lopinavir exposure, co-formulated lopinavir/ritonavir tablets have been co-administered with additional ritonavir capsules.2 Studies have shown that both lopinavir concentration boosting and persistence are highly dependent on ritonavir concentrations.4 A meta-analysis of the dose-ranging trials of lopinavir/ritonavir suggested that the 200/150 mg twice daily dose (one 200/50 mg Meltrex and one 100 mg ritonavir capsule twice daily) would provide plasma lopinavir concentrations close to the 400/100 mg twice daily dose.5 A reduction in lopinavir plasma exposure of 20%–25% was predicted. However, the improved pharmacokinetics of the Meltrex formulation could compensate for this. The pharmacokinetics of the 200/50 mg twice daily dose of lopinavir/ritonavir (one Meltrex tablet twice daily) has not previously been evaluated.
This study aimed to evaluate the safety, tolerability and pharmacokinetic characteristics of alternative doses of lopinavir/ritonavir, and to assess whether therapeutic concentrations of lopinavir were maintained.
Methods
Subjects
Male and non-pregnant, non-lactating female subjects were eligible for enrolment if they provided written informed consent and met the following criteria: age between 18 and 65 years; and body mass index (BMI) 18–35 kg/m2. Subjects were excluded if they had: any significant acute or chronic medical illness; abnormal physical examination or electrocardiogram (ECG), or clinical laboratory abnormalities; HIV infection; hepatitis B or C infection; current or recent (within 3 months) gastrointestinal disease; clinically relevant alcohol or drug use that the investigator felt would adversely affect compliance with trial procedures; exposure to any investigational drug or placebo within 3 months of the first dose of the study drug; use of any other drugs, including over-the-counter medications and herbal preparations, within 2 weeks prior to the first dose of the study drug; and previous allergy to any of the constituents of the pharmaceuticals administered during the trial.
Study design
This was a 35 day, open-label, prospective, single arm pharmacokinetic study conducted at the Pharmacokinetic Unit of St Stephen’s Centre, Chelsea and Westminster Hospital, London. The study protocol was reviewed and approved by the Riverside Research Ethics Committee, UK. All subjects provided written informed consent, and the trial was conducted in accordance with Good Clinical Practice, the Declaration of Helsinki and applicable regulatory requirements (EudraCT 2009-012513-21). For clinical trial registration details, see http://clinicaltrials.gov/ct2/show/NCT00985543.
At screening, subjects had a clinical assessment, ECG and routine laboratory investigations performed. The safety and tolerability of study medications were evaluated throughout the study using the NIAID Division of AIDS table for grading the severity of adult and paediatric adverse events to characterize abnormal findings (published December 2004), based on vital signs, physical examinations and clinical laboratory investigations.
Following successful screening, subjects were administered 400/100 mg of lopinavir/ritonavir twice daily (two heat-stable 200/50 mg tablets twice daily; Regimen 1), followed by a wash-out period, followed by 200/150 mg of lopinavir/ritonavir twice daily (one heat-stable 200/50 mg tablet twice daily plus one ritonavir 100 mg capsule twice daily; Regimen 2), followed by a second wash-out period, followed by 200/50 mg of lopinavir/ritonavir twice daily (one heat-stable 200/50 mg tablet twice daily; Regimen 3). All regimen intake and wash-out periods lasted for 7 days.
Analytical methods
Concentrations of lopinavir and ritonavir in plasma were measured using a validated HPLC–tandem mass spectrometry method.6 Recovery of lopinavir and ritonavir was 110% and 98%, respectively. The lower limits of quantification for lopinavir and ritonavir were taken as the lowest point on the standard curve (16 and 5 ng/mL, respectively), with upper limits of quantification of 15083 and 5018 ng/mL, respectively. Intra-assay and interassay coefficients of variation at the low, medium and high quality controls did not exceed 10%.
Pharmacokinetic and statistical analyses
Lopinavir and ritonavir maximum plasma concentration (Cmax) and trough concentration (Ctrough) were derived from the plasma concentration–time profiles. The area under the curve from 0 to 12 h (AUC0–12) was calculated using WinNonLin version 5.2 (Mountain View, CA, USA), by non-compartmental trapezoidal methodology. Interindividual variability in plasma concentrations was assessed by measuring the coefficient of variation (CV=standard deviation/mean×100). Within-subject changes of drug concentrations were assessed by calculating geometric means (GMs), geometric mean ratios (GMRs) and 90% confidence intervals (CIs). GMRs were calculated using 400/100 mg of lopinavir/ritonavir twice daily (Regimen 1) as the reference group. GMRs and CIs were determined by using random effects maximum likelihood regression of the logarithms of the individual pharmacokinetic values, and then expressing the calculated values as linear values (back transforming). The differences in pharmacokinetic parameters between the regimens were considered significant when the interval between low and high CI did not include the value 1.0.
Results
Demographic and clinical characteristics
Twenty-two subjects (eight females) enrolled and completed the study. Median (range) age, weight and BMI were 33 (23–60) years, 74 (54–99) kg and 25 (21–32) kg/m2, respectively. Fifteen subjects were Caucasian, three were of Asian origin, three were black and one was Hispanic.
The study drugs were well tolerated and no grade 3 or 4 adverse events were reported. As expected, diarrhoea was the most frequent adverse event recorded, followed by fatigue, headache and nausea.
Pharmacokinetics
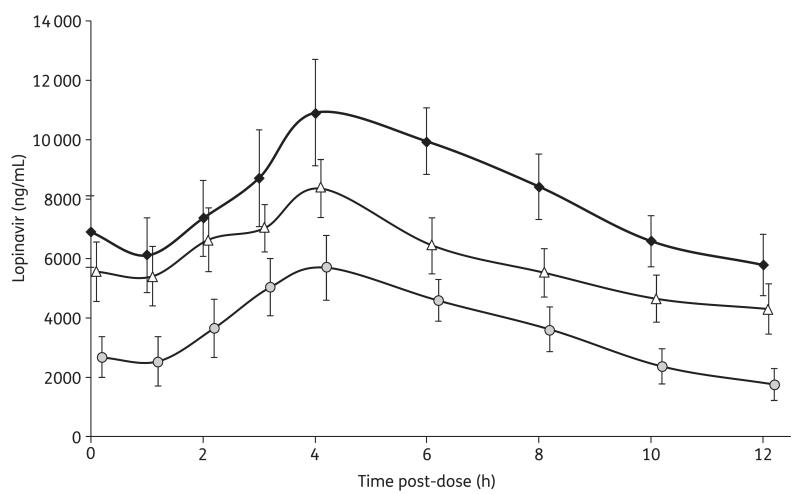
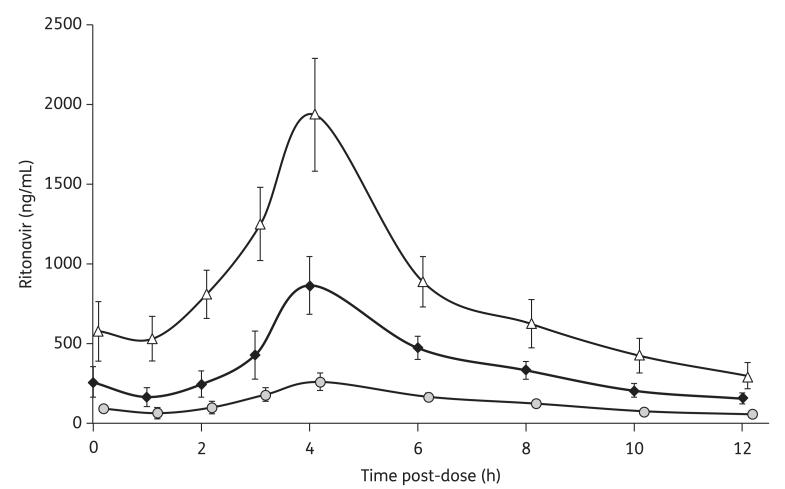
The lopinavir and ritonavir pharmacokinetic parameters of the three study regimens are illustrated in Table 1. The concentration profiles are shown in Figures 1 and 2.
Table 1.
Lopinavir and ritonavir pharmacokinetic parameters across the three study regimens
| Pharmacokinetic parameter | Regimen | CV% | GM | 90% CI | GMR | 90% CI | ||
|---|---|---|---|---|---|---|---|---|
| Lopinavir | ||||||||
| AUC0–12 (ng h/mL) | 1 | 35 | 99599 | 87180 | 113787 | |||
| 2 | 46 | 73603 | 65121 | 83191 | 0.74 | 0.65 | 0.84 | |
| 3 | 34 | 45146 | 39251 | 51927 | 0.45 | 0.40 | 0.51 | |
| Ctrough (ng/mL) | 1 | 46 | 5776 | 4884 | 6831 | |||
| 2 | 54 | 4293 | 3603 | 5115 | 0.74 | 0.62 | 0.89 | |
| 3 | 50 | 1749 | 1419 | 2156 | 0.30 | 0.25 | 0.36 | |
| Cmax (ng/mL) | 1 | 37 | 11965 | 10400 | 13766 | |||
| 2 | 50 | 8939 | 8047 | 9930 | 0.75 | 0.66 | 0.85 | |
| 3 | 29 | 6404 | 5648 | 7262 | 0.54 | 0.47 | 0.61 | |
| Ritonavir | ||||||||
| AUC0–12 (ng h/mL) | 1 | 41 | 4644 | 3808 | 5664 | |||
| 2 | 50 | 10462 | 8972 | 12200 | 2.25 | 1.96 | 2.59 | |
| 3 | 48 | 1625 | 1390 | 1899 | 0.35 | 0.30 | 0.40 | |
| Ctrough (ng/mL) | 1 | 66 | 154 | 126 | 189 | |||
| 2 | 74 | 297 | 236 | 374 | 1.93 | 1.64 | 2.26 | |
| 3 | 65 | 56 | 45 | 69 | 0.36 | 0.31 | 0.42 | |
| Cmax (ng/mL) | 1 | 46 | 887 | 708 | 1111 | |||
| 2 | 42 | 1963 | 1654 | 2330 | 2.21 | 1.85 | 2.65 | |
| 3 | 50 | 273 | 228 | 325 | 0.31 | 0.26 | 0.37 | |
GMRs are calculated using 400/100 mg of lopinavir/ritonavir twice daily (Regimen 1) as the referent group.
Figure 1.
Steady-state lopinavir plasma concentrations illustrated as GMs and 90% CIs measured during the intake of the three different regimens: 400/100 mg of lopinavir/ritonavir twice daily (diamonds); 200/150 mg of lopinavir/ritonavir twice daily (triangles); and 200/50 mg of lopinavir/ritonavir twice daily (circles).
Figure 2.
Steady-state ritonavir plasma concentrations illustrated as GMs and 90% CIs measured during the intake of the three different regimens: 400/100 mg of lopinavir/ritonavir twice daily (diamonds); 200/150 mg of lopinavir/ritonavir twice daily (triangles); and 200/50 mg of lopinavir/ritonavir twice daily (circles).
When lopinavir/ritonavir was administered at 200/150 mg twice daily, lopinavir total exposure was 26% lower than at the standard 400/100 mg twice daily dose (90% CI 0.65–0.84). The lopinavir Cmax and Ctrough were also significantly lower (25% and 26%, respectively). All subjects had lopinavir concentrations above the suggested minimum effective concentration (MEC) of 1000 ng/mL while taking either the 400/100 or 200/150 mg twice daily doses.
Ritonavir pharmacokinetic parameters were higher (125%) for the 200/150 mg than for the 400/100 mg twice daily doses.
The administration of 200/50 mg of lopinavir/ritonavir twice daily showed a lopinavir AUC0–12 that was 55% lower than that achieved by the standard 400/100 mg twice daily dose (90% CI 0.40–0.51). The lopinavir Cmax and Ctrough were also significantly lower (46% and 70%, respectively). Furthermore, three subjects (14%) receiving the 200/50 mg twice daily dose had lopinavir concentrations below the suggested MEC of 1000 ng/mL.
Ritonavir pharmacokinetic parameters were lower for the 200/50 mg twice daily dose versus the other two doses evaluated.
Although subjects received timed and witnessed drug doses after a standardized meal on the pharmacokinetic study days, the interpatient variability among AUC0–12, Cmax and Ctrough values was high (Table 1). The CV for lopinavir Ctrough was 46%, 54% and 50% during intake of the 400/100, 200/150 and 200/50 mg twice daily doses, respectively.
Discussion
This study investigated the steady-state pharmacokinetics of three different doses of lopinavir/ritonavir twice daily. The doses selected were: (i) the approved 400/100 mg twice daily dose that was used as a reference for intra-individual comparison; (ii) a lopinavir/ritonavir dose involving the administration of 200/150 mg twice daily (one Meltrex tablet and one 100 mg ritonavir capsule twice daily); and (iii) a lower lopinavir/ritonavir dose of 200/50 mg twice daily (one Meltrex tablet twice daily). The administration of the non-standard doses resulted in lower lopinavir pharmacokinetic parameters compared with the approved 400/100 mg twice daily dose. However, the decrease in the lopinavir Ctrough during the intake of the 200/150 mg twice daily dose was <30%, despite the 50% reduction in lopinavir dose. This was probably due to the presence of higher concentrations of ritonavir. Furthermore, all participants taking the 200/150 mg twice daily dose of lopinavir/ritonavir had lopinavir plasma concentrations above the MEC.
The 200/50 mg dose resulted in a lopinavir Ctrough above the MEC in all but three participants. The pharmacokinetics of this dose may not be sufficiently reliable to assess in future trials.
All three doses were well tolerated, with diarrhoea being the most common adverse event observed. The study was performed in HIV-negative volunteers and it was not powered to compare the different rates of adverse event reporting between the different phases. Moreover, the sequential design of the study may cause confusion when interpreting drug toxicity. Therefore, no comparison in the occurrence of lopinavir/ritonavir adverse events during the intake of the three different regimens is described.
The ritonavir concentrations of the 200/150 mg twice daily dose are higher than those of the 400/100 mg twice daily dose. However, the clinical significance of this is unknown. When combining lopinavir and ritonavir, the lopinavir concentrations are typically 20 times higher than the ritonavir concentrations. Higher lopinavir concentrations have been associated with lipid rises in previous studies.7,8 The 26% lower lopinavir AUC0–12 measured with the 200/150 mg twice daily dose might compensate for the higher ritonavir concentrations; however, short- and long-term toxicity data for this dose in HIV-infected individuals are not available. Therefore, studies in HIV-infected individuals on this dose should be performed to investigate the ritonavir-associated adverse events.
Furthermore, whether the pharmacokinetics of lopinavir differs in healthy volunteers and HIV-infected individuals is unclear,9 and, before establishing the possibility of changing lopinavir/ritonavir doses, formal pharmacokinetic/pharmacodynamic research in HIV-infected individuals is warranted.
Interestingly, previous studies either during drug development2 or recently performed in limited-resource countries7 have shown the effects of lower lopinavir/ritonavir doses.
In the Abbott 720 trial,2 more study patients showed HIV RNA reductions to <50 copies/mL in the 200/100 mg twice daily arm compared with the 400/100 mg twice daily arm. However, this was due to lopinavir/ritonavir side effects rather than antiviral efficacy and may suggest that in the presence of boosting ritonavir doses, the higher lopinavir concentrations achieved are responsible for the development of adverse events. Lopinavir plasma concentrations were higher for the 400/200 mg twice daily arm compared with the 400/100 mg twice daily arm, showing that the dose of ritonavir administered affects lopinavir exposure. Moreover, the increased lopinavir exposure was not dose proportional when comparing the 400/100 and 200/100 mg twice daily doses.2 In our study, 200/150 mg twice daily provided similar (only 26% lower) lopinavir exposures to 400/100 mg twice daily.
In the Thai HIVNAT 019 trial, 48 treatment-naive patients were randomized to lopinavir/saquinavir/ritonavir in four different dosing schedules in the absence of nucleoside reverse transcriptase inhibitors (400/1000/100 mg twice daily, 400/600/100 mg twice daily, 266/1000/66 mg twice daily and 266/600/66 mg twice daily). The efficacy rates were similar in the treatment arms using lower doses of lopinavir/ritonavir compared with those using higher doses.10
The above studies were performed with the old, soft gel capsule formulation of lopinavir/ritonavir. Importantly, the new Meltrex formulation, administered to the volunteers in our study, has shown an ~15% higher bioavailability than the soft gel formulation,1 allowing for slightly higher concentrations to be achieved when lower doses are selected.
The threshold therapeutic concentration of lopinavir in vivo is still a matter of debate. In protease inhibitor-naive HIV-infected patients, both the protein binding-adjusted 50% effective concentration of 40–180 ng/mL and the MEC of 1000 ng/mL have been proposed as cut-off concentrations, based on data from different studies.11–13 While the former has been calculated in vitro in the presence of 50% human serum against five HIV-1 laboratory strains, the latter is the result of a number of different studies published over the past 7 years that have provided information on the relationship between plasma concentrations and either virological response or toxicity.11
Our study results showed that when administering lower lopinavir doses (200 mg twice daily) with higher ritonavir doses (150 mg twice daily), therapeutic plasma concentrations of lopinavir can be achieved, supporting further exploration of lower lopinavir doses in properly designed randomized clinical trials. For lower lopinavir doses to be effective there would need to be evidence that the increased ritonavir dose does not lead to increased adverse effects.
Alternative doses of lopinavir/ritonavir might benefit funders by reducing drug costs and making prices a lesser factor in policy and clinical decision-making in limited-resource countries.
The advantages of antiretroviral dose reductions may translate into greater benefits for more individuals infected by HIV globally, since they may allow access programmes to reach higher numbers of infected patients, and compensate for the finite global manufacturing capacity and increasing demand.
In conclusion, these pharmacokinetic data support further consideration of the use of lower lopinavir doses in HIV-infected individuals. A new dose of lopinavir/ritonavir could lower costs and improve access in developing countries.
Acknowledgements
Some of the results of this study were presented at the Tenth International Congress on Drug Therapy in HIV Infection, Glasgow, UK, 2010, Thistle Poster 180.
We would like to thank Dr Stephen Becker from the Bill & Melinda Gates Foundation for reviewing the manuscript and providing his advice prior to submission.
Funding This study was funded from the following sources: the Australian Government Department of Health and Ageing; and an unrestricted grant from the Bill & Melinda Gates Foundation (Grant ID 51040).
NCHECR is affiliated with the Faculty of Medicine, University of New South Wales.
Footnotes
Transparency declarations A. J., D. B., S. K., S. E., R. M., B. G. and M. B. have received travel and research grants from and have been advisers for Tibotec, Roche, Pfizer, GlaxoSmithKline, Bristol-Myers Squibb, Merck, Abbott, Boehringer-Ingelheim, Chiron-Novartis and Virax Immunotherapeutics. All other authors: none to declare.
Disclaimer The views expressed in this publication do not necessarily represent the position of the Australian Government.
References
- 1.Klein CE, Chiu Y-L, Awni W, et al. The tablet formulation of lopinavir/ritonavir provides similar bioavailability to the soft-gelatin capsule formulation with less pharmacokinetic variability and diminished food effect. J Acquir Immune Defic Syndr. 2007;44:401–10. doi: 10.1097/QAI.0b013e31803133c5. [DOI] [PubMed] [Google Scholar]
- 2.Murphy RL, Brun S, Hicks C, et al. ABT-378/ritonavir plus stavudine and lamivudine for the treatment of antiretroviral-naive adults with HIV-1 infection: 48-week results. AIDS. 2001;15:F1–9. doi: 10.1097/00002030-200101050-00002. [DOI] [PubMed] [Google Scholar]
- 3.Gazzard BG on behalf of the BHIVA Treatment Guidelines Writing Group British HIV Association guidelines for the treatment of HIV-1-infected adults with antiretroviral therapy 2008. HIV Med. 2008;9:563–608. doi: 10.1111/j.1468-1293.2008.00636.x. [DOI] [PubMed] [Google Scholar]
- 4.Boffito M, Else L, Back D, et al. Pharmacokinetics of atazanavir/ritonavir once daily and lopinavir/ritonavir twice and once daily over 72 h following drug cessation. Antivir Ther. 2008;13:901–7. [PubMed] [Google Scholar]
- 5.Hill A, van der Lugt J, Sawyer W, et al. How much ritonavir is needed to boost protease inhibitors? Systematic review of 17 dose-ranging pharmacokinetic trials. AIDS. 2009;23:2237–45. doi: 10.1097/QAD.0b013e328332c3a5. [DOI] [PubMed] [Google Scholar]
- 6.Else L, Watson V, Tjia J, et al. Validation of a rapid and sensitive high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS) assay for the simultaneous determination of existing and new antiretroviral compounds. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:1455–65. doi: 10.1016/j.jchromb.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 7.González de Requena D, Blanco F, Garcia-Benayas T, et al. Correlation between lopinavir plasma levels and lipid abnormalities in patients taking lopinavir/ritonavir. AIDS Patient Care STDS. 2003;17:443–5. doi: 10.1089/108729103322395465. [DOI] [PubMed] [Google Scholar]
- 8.Gutiérrez F, Padilla S, Navarro A, et al. Lopinavir plasma concentrations and changes in lipid levels during salvage therapy with lopinavir/ritonavir-containing regimens. J Acquir Immune Defic Syndr. 2003;33:594–600. doi: 10.1097/00126334-200308150-00007. [DOI] [PubMed] [Google Scholar]
- 9.Dickinson L, Khoo S, Back D. Differences in the pharmacokinetics of protease inhibitors between healthy volunteers and HIV-infected persons. Curr Opin HIV AIDS. 2008;3:296–305. doi: 10.1097/COH.0b013e3282f82bf1. [DOI] [PubMed] [Google Scholar]
- 10.van der Lugt J, Autar RS, Ubolyam S, et al. Pharmacokinetics and short-term efficacy of a double-boosted protease inhibitor regimen in treatment-naive HIV-1-infected adults. J Antimicrob Chemother. 2008;61:1145–53. doi: 10.1093/jac/dkn050. [DOI] [PubMed] [Google Scholar]
- 11.La Porte CJ, Back DJ, Blaschke T, et al. Updated guideline to perform therapeutic drug monitoring for antiretroviral agents. Rev Antivir Ther. 2006;3:3–14. [Google Scholar]
- 12.Ananworanich J, Kosalaraksa P, Hill A, et al. Pharmacokinetics and 24-week efficacy/safety of dual boosted saquinavir/lopinavir/ritonavir in nucleoside-pretreated children. Pediatr Infect Dis J. 2005;24:874–9. doi: 10.1097/01.inf.0000180578.38584.da. [DOI] [PubMed] [Google Scholar]
- 13.Wateba MI, Billaud E, Dailly E, et al. Low initial trough plasma concentrations of lopinavir are associated with an impairment of virological response in an unselected cohort of HIV-1-infected patients. HIV Med. 2006;7:197–9. doi: 10.1111/j.1468-1293.2006.00354.x. [DOI] [PubMed] [Google Scholar]