Abstract
Purpose.
Primary open-angle glaucoma is characterized by increased resistance to aqueous humor outflow and a stiffer human trabecular meshwork (HTM). Two Yorkie homologues, Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif, encoded by WWTR1 (TAZ), are mechanotransducers of the extracellular-microenvironment and coactivators of transcription. Here, we explore how substratum stiffness modulates the YAP/TAZ pathway and extracellular matrix genes in HTM cells and how this may be play a role in the onset and progression of glaucoma.
Methods.
HTM cells from normal donors were cultured on hydrogels mimicking the stiffness of normal (5 kPa) and glaucomatous (75 kPa) HTM. Changes in expression of YAP/TAZ related genes and steroid responsiveness were determined. Additionally, transglutaminase-2 expression was determined after YAP silencing.
Results.
YAP and TAZ are both expressed in human trabecular meshwork cells. In vitro, YAP and TAZ were inversely regulated by substratum stiffness. YAP and 14-3-3σ were downregulated to different extents on stiffer substrates; TAZ, tissue transglutaminase (TGM2), and soluble frizzled-related protein-1 (sFRP-1) were significantly upregulated. CTGF expression appeared to be altered differentially by both YAP and TAZ. Myocilin and angiopoietin-like 7 expression in response to dexamethasone was more pronounced on stiffer substrates. We demonstrated a direct effect by YAP on TGM2 when YAP was silenced by small interfering RNA.
Conclusions.
The expression of YAP/TAZ and ECM-related-genes is impacted on physiologically relevant substrates. YAP was upregulated in cells on softer substrates. Stiffer substrates resulted in upregulation of canonical Wnt modulators, TAZ and sFRP-1, and thus may influence the progression of glaucoma. These results demonstrate the importance of YAP/TAZ in the HTM and suggest their role in glaucoma.
This paper demonstrates the importance of biophysical properties in trabecular meshwork cells and mechanotransducers YAP/TAZ in glaucoma.
Introduction
Glaucoma, a leading cause of blindness, afflicts approximately 80 million patients.1 Increased intraocular pressure is the primary risk factor for primary open angle glaucoma (POAG), the most common form of glaucoma.2 It is thought that aqueous humor outflow through the trabecular meshwork (TM) is the major site of intraocular pressure (IOP) regulation.3–5 The juxtacanalicular (JCT) meshwork and adjacent inner walls of the Schlemm's canal are believed to be responsible for this regulation. Changes in the structural organization of the extracellular matrix (ECM) of the JCT in patients with POAG have been correlated to damage to the optic nerve.6 Expanding on these morphologic findings, our laboratory recently reported that human TM (HTM) from glaucomatous donors was at least 20-fold stiffer than HTM from normal donors.7 These alterations to the attributes of the HTM suggest an intriguing biophysical component to glaucoma and IOP regulation.
Biophysical cues such as structural topography and stiffness are known to be potent modulators of cell behaviors. Numerous studies from our laboratory8–11 and others12–19 have demonstrated that modulation of substratum stiffness can alter phenotypic characteristics of cells, gene expression, or response to drugs. However, the primary molecular mechanisms by which the biophysical attributes influence cell behaviors remain unknown. Additionally, the molecular consequences of such altered biophysical cues on the onset and/or progression of glaucoma are not understood.
The specialized anatomy of the HTM has prevented development of suitable animal models of glaucoma for in vivo studies. For this reason, HTM cells have been used in vitro to investigate mechanisms by which HTM cells are altered during disease. A commonly used in vitro model for glaucoma is treatment of HTM cells with glucocorticoids (GCs),20 a family of steroid hormones. Approximately 30% to 40% of people treated with GCs develop elevated IOP (referred to as steroid responders), and this is a causal risk factor for glaucoma.21,22 Dexamethasone (DEX) has been widely used in vitro to investigate the mechanisms underlying GC-induced glaucoma23–27 in the HTM. Myocilin (MYOC) and angiopoietin like protein 7 (ANGPTL7) are two ECM proteins significantly increased in expression in glaucoma as well as in response to GC treatment of HTM cells.28–34
Recently, two Yorkie homologues YAP (Yes-associated protein) and TAZ (transcriptional coactivator with PDZ-binding motif, encoded by WWTR1) were identified as nuclear relays of mechanical signals exerted by ECM rigidity.35 While YAP and TAZ are proteins traditionally associated with the Hippo pathway to limit organ size,36,37 their role in mechanotransduction is thought to be outside of the canonical Hippo pathway.35 The functions of YAP/TAZ are primarily dictated by their spatial localization within the cell (i.e., cytoplasmic or nuclear).38 In the cytoplasm, YAP/TAZ are sequestered via phosphorylation and can bind to 14-3-3σ (encoded by SFN),39,40 as well as interact with a number of other proteins. Of specific interest to the current work, cytoplasmically localized TAZ can antagonize the Wnt pathway,41 similar to extracellular soluble frizzled related protein (sFRP-1).42,43
In the nucleus, YAP/TAZ modulate the activity of transcription factors (TEAD and RUNX family),44,45 and SMADS.46,47 Importantly, among the YAP transcription targets are connective tissue growth factor (CTGF) and TGF-β, growth factors that regulate the extent of remodeling of tissue architecture35 and have been shown to be associated with glaucoma.48–51 Transglutaminase-2 (TGM2), also regulated by YAP, is also important to ECM deposition and turnover and has been reported to be significantly upregulated in glaucoma or with glucocorticoid treatment of HTM cells.52 YAP/TAZ are localized to the nucleus when cells are cultured on stiffer substrates.35 The nuclear localization of YAP/TAZ and subsequent transcriptional activity on such genes as CTGF, TGF-β, and TGM2 can thus be hypothesized to play a significant role in the initiation and progression of glaucoma. In this study, we investigated the impact of substratum stiffness (normal/soft: homeomimetic; and glaucomatous/stiffer: pathomimetic) on genes that are involved with ECM changes observed in glaucomatous HTM as well as genes that transduce matrix changes in HTM cells. Since cytoplasmic YAP/TAZ can be targeted for degradation, the mRNA expression pattern for 14-3-3σ, the protein thought necessary for YAP/TAZ degradation, was also determined. A better understanding of the impact of homeomimetic and pathomimetic substratum compliance could inform the identification and development of novel glaucoma therapeutics.
Materials and Methods
Preparation of Substrates
Polyacrylamide hydrogels mimicking the elastic modulus of normal (5 kPa) and glaucomatous (75 kPa) HTM were fabricated and characterized by atomic force microscopy as described previously.8–10 Briefly, the hydrogels were sterilized with ultraviolet light and rinsed thoroughly three times for 24 hours each in Dulbecco's PBS (DPBS) to remove any toxic monomeric acrylamide and hydrate the hydrogels completely. The polymer hydrogels (20-mm diameter) were then allowed to adhere to tissue cultured plastic (TCP), stored in growth medium for 24 hours, and coated with a proprietary mixture of fibronectin/collagen (FNC; Athena ES, Baltimore, MD) prior to cell culture. Atomic force microscopy was used to validate the elastic modulus of the fully hydrated hydrogels, and the elastic modulus of the hydrogels used were 4 ± 2 kPa and 71 ± 5 kPa for 5 and 75 kPa, respectively.8,11
Isolation and Culture of HTM Cells
Primary human trabecular meshwork cells were isolated from donor corneoscleral rims (Heartland Lions Eye Bank, St. Louis, MO) as described previously.9,11,53 All experiments were performed in compliance with the tenets of the Declaration of Helsinki. Cells isolated from four donors (designated as HTM631, HTM265, HTM211, and HTM516) were used in this study. Isolated cells were maintained in DMEM/F-12 with 2.5 mM L-glutamine (Thermo Scientific HyClone, Logan, UT) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrence, GA) and 1% penicillin/streptomycin/amphotericin B (Lonza, Walkerville, MD). Cells in passages 3 to 7 were used for all experiments. For RNA extraction, cells were seeded on FNC coated polyacrylamide hydrogel substrates, in six well dishes at 2 × 105 (75 kPa) or 3 × 105 (5 kPa) cells/well. For protein extraction, cells were seeded on FNC-coated polyacrylamide hydrogels in one well plate (110 cm2) at approximately 2 × 106 (75 kPa) or 3 × 106 (5 kPa) cells/plate. A higher density of cells were seeded on the 5 kPa hydrogels because HTM cells proliferate more slowly on softer versus stiffer substrates.9
In addition, HTM cells were treated with DEX to investigate the influence of substratum stiffness on GC-stimulated HTM cells. HTM cells were treated with DEX. HTM cells, 24 hours after plating, were treated with 10−7 M DEX in ethanol (EtOH) or the vehicle only (equivalent volume of EtOH i.e. at a 1:1000 dilution). The media was changed after 3 days. Seven days after the first treatment, the cells were rinsed with DPBS and RNA or protein was extracted.
RNA Isolation and Quantitative Real-Time PCR
RNA was isolated using an RNA purification kit (RNeasy Mini Kit; Qiagen, Valencia, CA) following the manufacturer's instructions and equal amounts were used for the quantitative real time PCR (qPCR) reactions. The qPCR was performed using a reagent kit (TaqMan One-Step RT-PCR Master Mix Reagents Kit; Applied Biosystems, Carlsbad, CA) and commercially available primers for 18S (Hs99999901_s1); ANGPTL7 (Hs00221727_m1); CTGF (Hs00170014_m1); MYOC (Hs00165354_m1); sFRP1 (Hs00610060_m1); SFN (Hs00968567_s1); TGM2 (Hs01096681_m1); YAP (Hs00371735_m1); and WWTR1 (Hs00210007_m1) in total volumes of 10 μL per reaction (Applied Biosystems). The TAZ protein is encoded by the gene WWTR1. In this study, both the mRNA and the protein encoded by the WWTR1 gene are termed TAZ. The reverse transcription reaction was performed in a qPCR machine (StepOne; Applied Biosystems) with the following parameters: 30 minutes at 50°C followed by 10 minutes at 95°C; 40 cycles of 60°C for 1 minute followed by 95°C for 15 seconds. Relative expression levels of the mRNAs of interest were normalized to the expression of the ribosomal RNA 18S. At least three reactions were run for each sample, and the experiments were performed for HTM cells from three individual donors. Gene expression was normalized relative to the expression of mRNA from HTM cells on homeomimetic (5 kPa) hydrogels treated with vehicle (EtOH), which was given a value of 1.0. In brief, equal amounts of RNA (60 ng) were loaded for all PCR reactions to account for variations in cell density. The Ct values obtained represent logarithmic changes in gene expression. The difference in Ct (i.e., Δ Ct) between the gene of interest (e.g., YAP, TAZ) and the calibrator gene (18S) were calculated. Next, the Δ Ct values between the 75 kPa data was normalized with the control (5 kPa, EtOH treated) data. Control data was normalized for each experiment and the logarithmic changes in gene expression were expressed as relative expression to the control data.
Gene Downregulation by siRNA Transfection
At 60% to 80% confluence of HTM cells, siRNA transfections were performed using a transfection reagent (DharmaFECT 4; Dharmacon, Lafayette, CO) following the manufacturer's instructions with final concentrations of 28.5 nM of YAP siRNA (Hs_YAP1_5) or control siRNA (ON-TARGETplus Non-targeting siRNA #3, Dharmacon). At 48 and 72 hours posttransfection, cells were harvested for RNA isolation. Knockdown to expression levels below 30% was achieved as determined by qPCR analyses. The gene knockdown was done three times with cells from different donors.
Immunohistochemistry
The corneoscleral rim from a 57-year-old donor with no history of disease was fixed overnight in 10% neutral buffered formalin, paraffin embedded, and sectioned. Sections were deparaffinized in xylene, subjected to citrate antigen retrieval, peroxidase blocked, and incubated overnight at 4°C with mouse anti-human YAP-H9 (Santa Cruz Biotechnologies, Santa Cruz, CA) and TAZ (Abnova, Walnut, CA) antibodies. Sections were then treated with horse anti-mouse biotinylated secondary antibody, followed by streptavidin-horseradish peroxidase, and developed with Vector Red chromogen prior to counterstaining with hematoxylin and coverslipping.
Protein Isolation and Western Blotting
Cell monolayers cultured on polyacrylamide hydrogels were washed once in PBS and lysed and scraped into RIPA buffer (ThermoScientific, Waltham, MA) supplemented with protease and phosphatase inhibitors (Fisher Scientific, Hampton, NH) on ice. The cells were then homogenized and centrifuged at 1000g for 1 minute to remove any cell debris. Protein was quantified using a modified Lowry assay (DC assay; Bio-Rad Laboratories, Hercules, CA) with bovine serum albumin as the standard. Protein homogenate was then denatured in Laemmli buffer (Sigma-Aldrich, St. Louis, MO) by boiling for 10 minutes. Approximately 10 μg protein was loaded per well for each sample. Protein was separated on precast gels (NuPAGE 10% Bis-Tris; Invitrogen, Carlsbad, CA) and transferred onto nitrocellulose membranes. Immunoblotting was done against anti-human YAPH9 (Santa Cruz Biotechnologies, Santa Cruz, CA); TAZ (Abnova); and beta-actin (Abcam, Cambridge, MA) overnight at 4°C. This was followed by incubation with secondary antibodies conjugated with horseradish peroxidase (HRP; Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD) for 1 hour at 37°C. The bands were then amplified and detected colorimetrically following protocols detailed using a substrate kit (Amplified Opti-4CN kit; Bio-Rad Laboratories). Blots were then imaged using an imaging system (ImageQuant 350; GE Healthcare Life Sciences, Pittsburgh, PA). The optical densities of the protein bands were quantified using a Java-based image processing program (ImageJ; National Institutes of Health, Bethesda, MD).54
Statistics
All statistics were performed using graphing software (SigmaPlot 11; Systat Software, Inc., San Jose, CA). Pairwise comparisons were assessed using Student's t-test. Throughout the paper, statistically significant differences are denoted with *P < 0.05, **P < 0.01, ***P < 0.001 unless stated otherwise.
Results
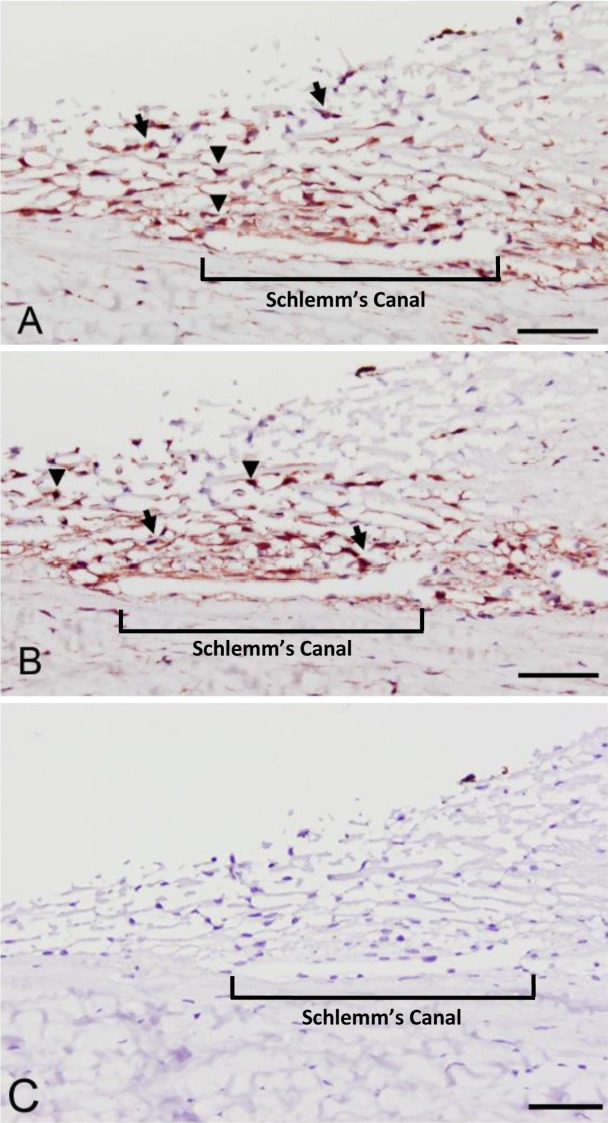
Immunohistochemical staining of HTM tissue from a normal donor showed both YAP and TAZ were present in HTM cells as well as in Schlemm's canal cells (Fig. 1). Both YAP and TAZ were found to be broadly localized in the cytoplasm (black arrows), with some cells having a strong nuclear localization (black arrowheads).
Figure 1. .
YAP (A) and TAZ (B) are expressed in human trabecular meshwork tissue. Strong cytoplasmic localization (black arrows) was observed for YAP and TAZ in all cells with multifocal nuclear localization in some cells (black arrowheads). No protein expression was observed in sections stained without primary antibody (C). Schlemm's canal is indicated with a bracket. Scale bar represents 50 μm.
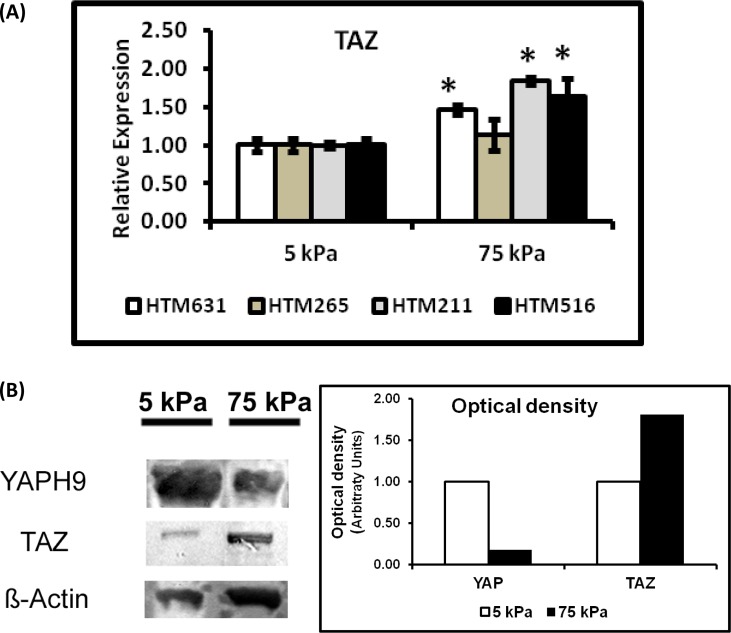
To determine the effects of substrate stiffness on the expression of YAP/TAZ, HTM cells were grown on homeomimetic (5 kPa) and pathomimetic (75 kPa) hydrogels for 7 days in the absence of DEX. The mRNA expression of YAP was significantly downregulated in three of the four donor HTM cells (HTM631, HTM265, and HTM211 cells) cultured on stiffer substrates (Fig. 2A). YAP protein expression was also markedly decreased in HTM631 cells on the 75 kPa hydrogels (Fig. 3B). In contrast, TAZ mRNA expression in three of the four donors was greater in cells cultured on stiffer substrates. For example, in HTM516 and HTM211 cells, gene expression of TAZ was ∼1.8 fold greater in cells cultured on 75 kPa versus 5 kPa hydrogels (Fig. 3A). CTGF mRNA expression was also influenced by the substrate stiffness, but the effect was donor dependent; whereas cells isolated from one donor (HTM631) demonstrated a 1.6-fold decrease in CTGF. HTM211 cells showed a dramatic increase (>2-fold) in mRNA expression on the stiffer substrates compared with the homeomimetic ones. There was no significant difference in the cells isolated from the other two donors (HTM265 and HTM516), but decreases in CTGF followed decreases in YAP (Fig. 2B versus Fig. 2A). Donor-dependent variability in expression patterns was also observed with SFN mRNA expression (encoding for 14-3-3σ). In HTM631 and HTM211 donor cells, SFN mRNA was 2-fold lower (Fig. 2C); but with other donors (HTM265 and HTM516), no significant differences were seen. Together, the trend suggested that a decrease in expression of SFN in cells on the stiffer hydrogels followed YAP (Fig. 2C versus Fig. 2A). In contrast to the trends shown by YAP, CTGF, and SFN, SFRP-1 and TGM2 expression tended to increase on stiffer substrates. SFRP-1 was significantly increased in cells from at least three donors (Fig. 4A). TGM2 followed a similar trend in cells from three of four donors (HTM265, HTM211 and HTM516), consistent with previous findings.11 For example, in HTM516 and HTM211 cells, TGM2 increased more than 2-fold on the stiffer hydrogels (Fig. 4B).
Figure 2. .
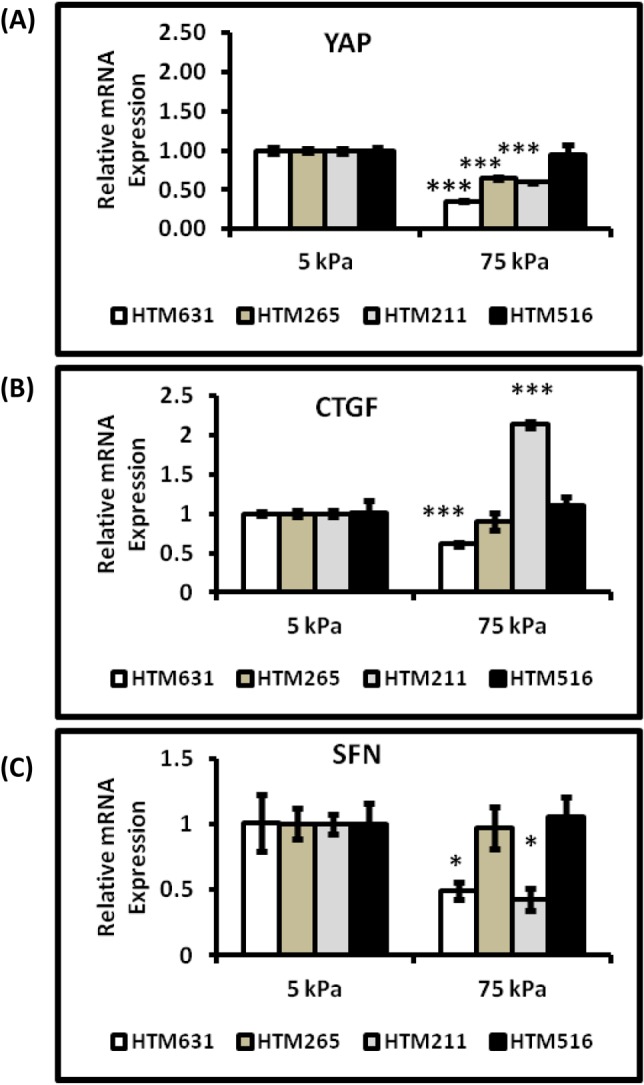
YAP, CTGF, and SFN expression were differentially modulated significantly by substratum stiffness in HTM cells. Graphs demonstrate significant downregulation of (A) YAP, (B) CTGF, and (C) SFN in HTM631 cells that were cultured on the pathomimetic substrate. Expression of CTGF and SFN genes in the other cells closely followed the expression of YAP on the 75 kPa substrate, but was not significantly different than the 5 kPa substrate. Donor lineage is indicated by HTM631, HTM265, HTM211 and HTM516. Results are mean ± SD, n = 3; *P < 0.05, ***P < 0.001 with respect to 5 kPa values, t-test.
Figure 3. .
(A) Expression of TAZ was significantly elevated in HTM cells when cultured on stiffer substrates. TAZ expression on the 75 kPa substrates was elevated in HTM516 and HTM631 cells. This increase in gene expression was also accompanied by an increase in its protein expression. Donor lineage is indicated by HTM631, HTM265, HTM211, and HTM516. Results are mean ± SD, n = 3, *P < 0.05 with respect to values on 5 kPa, t-test. (B) Western blot analyses of YAP and TAZ normalized to β-actin in control HTM631 cells. The desired representative blots were cut and aligned together as is shown here. The band for YAP occurred at a molecular weight between 65 to 75 kDa, TAZ around 75 kDa, and β-actin around 42 kDa. YAP is downregulated while TAZ is upregulated on stiffer substrates consistent with the mRNA expression of YAP and TAZ. Graph demonstrates the optical density of the bands normalized to β-actin.
Figure 4. .
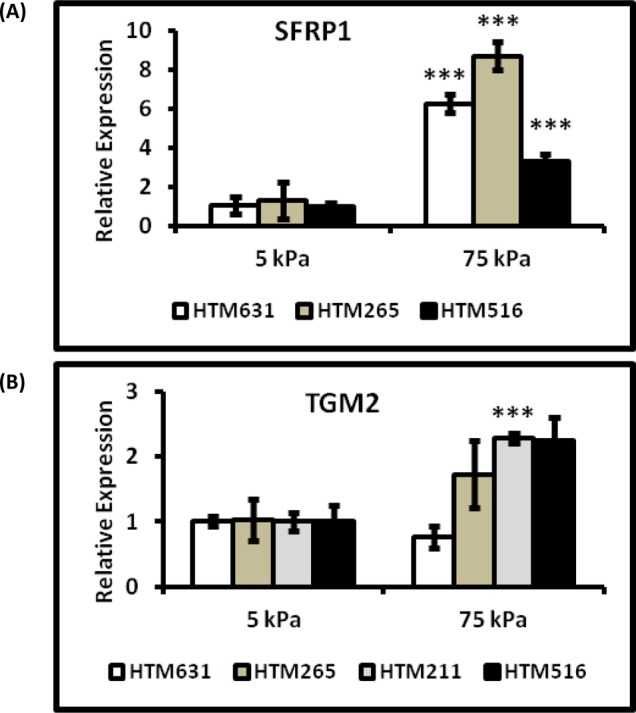
SFRP-1 and TGM2 expression were upregulated on the pathomimetic substrates. SFRP-1 (A) and TGM2 (B) were markedly increased in HTM cells grown on the 75 kPa substrates in comparison to the 5 kPa substrates. Donor lineage is indicated by HTM631, HTM265, HTM211, and HTM516. Results are mean ± SD, n = 3, ***P < 0.001 with respect to 5 kPa values, t-test; for TGM2 expression in HTM516 cells (P = 0.052).
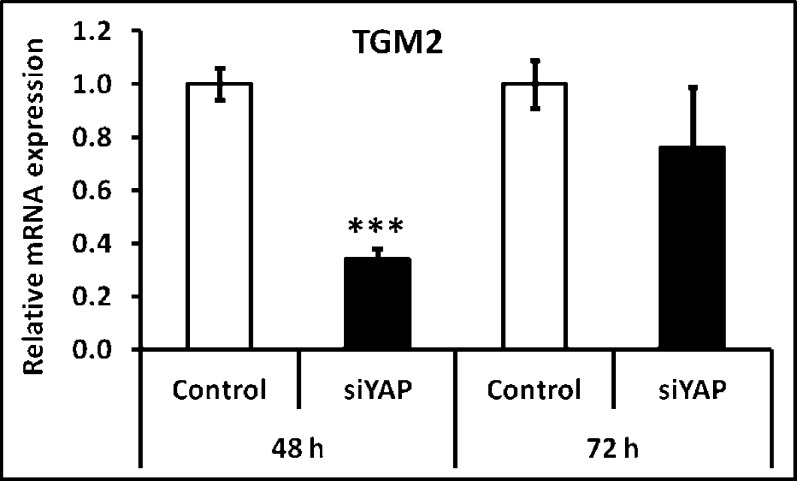
To determine a specific role of YAP in TGM2 expression, we knocked down YAP expression in HTM cells using siRNA. Forty-eight hours after transfection, YAP knockdown was confirmed at 13% of control and TGM2 expression dropped to 34% of control (Fig. 5). However, this loss was transient. Seventy-two hours after transfection, YAP was still effectively silenced (15% of control), but TGM2 expression had mostly recovered (76%).
Figure 5. .
TGM2 was also regulated by YAP. To investigate the relationship between YAP and TGM2, YAP was knocked-down, using siRNA specific to YAP, to levels <20% of control siRNA. YAP knockdown was associated with a dramatic inhibition of TGM2 expression (<30% when compared with control) within 48 hours. However, TGM2 mRNA levels appeared to recover to approximately 80% expression in control cells after 72 hours, although YAP expression remained below 20%. Results are mean ± SD, n = 3, ***P < 0.001 with respect to 5 kPa values, t-test.
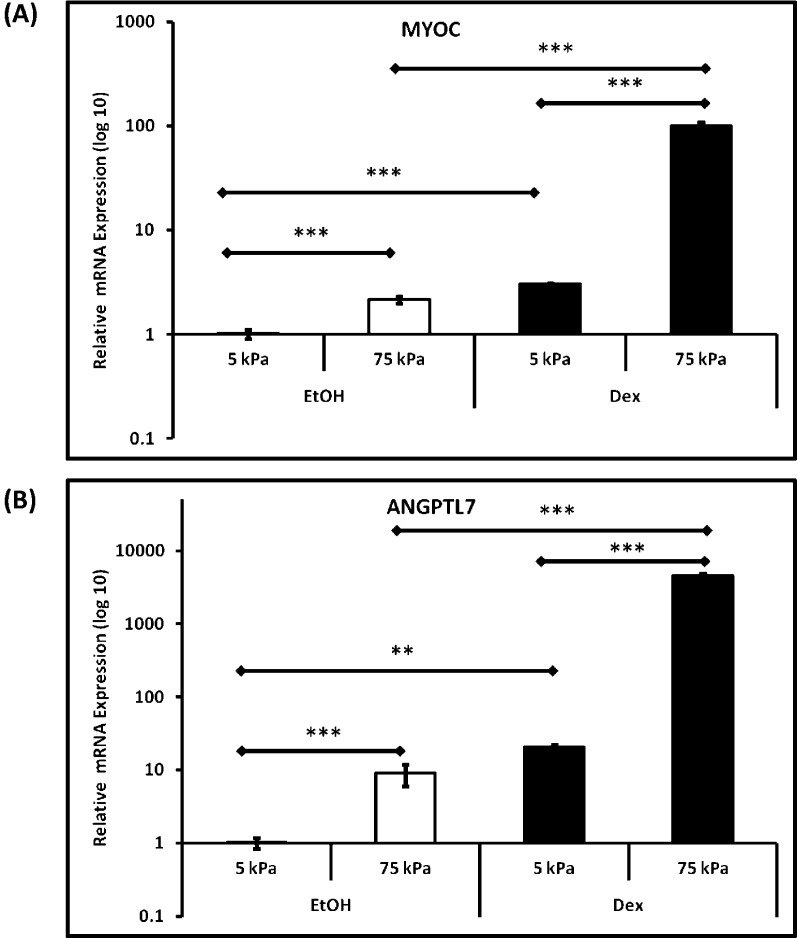
Finally, to determine the influence of substrate stiffness of HTM cells to dexamethasone, we treated cells from all donors with DEX over 7 days. In our expression panel, we included two ECM-related genes, MYOC and ANGPTL7, known to be upregulated with dexamethasone. Substratum stiffness had a marked effect on the mRNA expression of MYOC and ANGPTL7, with upregulation of both genes found in all three donors after 7 days on the pathomimetic polyacrylamide hydrogels compared with the homeomimetic substrates (Fig. 6). However, the relative response to MYOC and ANGPTL7 were suppressed when cells were cultured on the softer 5 kPa substrates. For example, in HTM631 cells, MYOC expression increased 3-fold on 5 kPa hydrogels on treatment with DEX, but it was elevated more than 100-fold on the 75 kPa hydrogels, compared with vehicle control on 5 kPa hydrogels (Fig. 6). In HTM631 cells, ANGPTL7 expression was increased 20-fold on 5 kPa hydrogels and greater than 4500-fold on 75 kPa hydrogels with DEX treatment (Fig. 6). No trends in other genes were noted with the DEX treatment (data not shown).
Figure 6. .
Myocilin (MYOC) and ANGPTL7 expression in HTM cells were modulated significantly by substratum stiffness. Representative graphs to illustrate the trends in expression of (A) Myocilin and (B) ANGPTL7 in HTM631 cells clearly demonstrate that both genes were significantly upregulated when cultured on stiffer substrates. A similar trend was demonstrated for HTM cells from two other donors (data not shown). Treatment with 10−7 M dexamethasone (DEX) accentuated this response. Results are mean ± SD (n = 3), **P < 0.001, ***P < 0.01, t-test.
Discussion
Alterations in trabecular meshwork ECM have long been correlated with glaucoma.55,56 In addition to the morphologic changes documented with light and electron microscopy, atomic force microscopy studies have recently documented that the meshwork is stiffer with glaucoma.7 The altered mechanical properties of the HTM in glaucoma7 and the changes in the substratum biophysical properties have dramatic effects on HTM cell phenotype.9,11 In this study, we report that two of the proteins known to be involved with the ECM, MYOC, and ANGPTL-7 were significantly upregulated when HTM cells were grown on substrates with stiffness similar to the glaucomatous meshwork in vivo. It is important to note that elevated stiffness has previously been reported to downregulate ANGPTL-711; however, those experiments used a much shorter time point (24-hours). Taken together, these reports revealed a temporal component to ANGPTL-7 regulation. Both MYOC and ANGPTL-7 have previously been reported to be upregulated by GCs such as DEX.57,58 In the present study, DEX added to HTM cells grown on hydrogels resulted in marked increases in mRNA expression of these two genes. Additionally, this DEX stimulated response of MYOC and ANGPTL-7 was even more striking in HTM cells grown on the pathomimetic substrates in comparison with the homeomimetic substrates. This is particularly important to consider while using GCs as a model for glaucoma. The DEX response of MYOC and ANGPTL7 observed on stiffer substrates is significantly attenuated on softer substrates (typical of normal HTM), suggesting that stiff substrates, such as the cultureware used in many previous studies, will induce a dramatically different DEX phenotype than would be observed in vivo. Thus, growing HTM cells on the homeomimetic and pathomimetic substrates described in this report may be a better in vitro model for studying glaucoma than HTM cells grown on TCP. Furthermore, these data suggest feedback loops in tissue stiffening. An increase in ANGPTL7 can reduce the deposition of key ECM proteins,29 resulting in reduced ECM stiffness and therefore reduced ANGPTL7 expression.
While the prime event responsible for changing the biophysical attributes of the microenvironment surrounding cells remains unknown, an increasing body of literature suggests that various proteins are involved in mechanosensing and mechanotransduction. YAP and TAZ were identified as two mechanotransducers of substrate rigidity that also act as nuclear relays for activation of transcription factors.35 Additionally, recent studies have demonstrated that YAP/TAZ expression plays a critical role in tumorigenesis, and renal and pulmonary diseases.36,59,60 We thus explored the expression of YAP/TAZ in HTM cells as well as other genes related to YAP/TAZ and the ECM. This is the first study to report that YAP and TAZ were present in all layers of HTM including the JCT, which is thought to provide the majority of resistance to aqueous humor outflow.5 Undoubtedly, it would be very interesting to compare YAP/TAZ expression in the HTM of normal and glaucomatous donors, but HTM tissue from patients with glaucoma is difficult to obtain. We investigated the regulation of YAP/TAZ in HTM cells grown on biologically relevant substrates. Indeed, altered expression of YAP/TAZ in response to substrate properties was observed. YAP mRNA and protein expression was elevated on the softer hydrogels, while TAZ mRNA and protein was elevated on the stiffer hydrogels demonstrating that YAP/TAZ are actively regulated in the HTM and therefore may serve as mechanotransducers in HTM cells. This inverse relation between YAP/TAZ expression—if conserved in vivo—would point to divergent influences of the two proteins, with YAP being more important during normal functioning of the HTM and TAZ growing in influence as the HTM is altered in glaucoma and contrasted with the Dupont study, which demonstrated that both YAP and TAZ were upregulated on stiffer substrates in mammary epithelial cells.35 Consistent with a recent publication,61 these results demonstrate that YAP and TAZ may respond differently in various cell types and it is important to investigate their role in the cell type of interest.
In line with this, it is important to keep in mind other biophysical or biochemical stimuli capable of modulating YAP/TAZ expression and localization may be present in the HTM. Iyer and colleagues have recently shown that the activity of autotaxin, the enzyme that produces lysophosphatidic acid (LPA), is upregulated in glaucoma and inhibition of LPA production led to a reduction in IOP.62 While the previous study did not describe a complete mechanism, Yu et al.63 and Miller et al.61 have identified LPA as an activator of YAP/TAZ. These reports, considered together, point to a novel autotaxin-LPA-YAP/TAZ signaling axis. Thus, further studies of alterations of autotaxin in HTM cells on normal and glaucomatous substrates are warranted.
CTGF is highly expressed in the HTM,64 modulates the expression of ECM proteins relevant to POAG,65 and has been linked to elevated IOP.66 Importantly, YAP67,68 and TAZ69 are both capable of directly regulating CTGF. In the present study, CTGF expression closely mirrored YAP expression in three of the four donor cells. In cells from one donor (HTM631), CTGF was strongly inhibited on the stiffer substrates while there was minimal change in cells from the other two donors (HTM265 and HTM516). However, in cells from HTM211, CTGF expression was markedly elevated. Concurrently it was also observed that TAZ expression was elevated in three of the four donor cells (HTM631, HTM211, and HTM516). These data suggest that there is high interindividual variability in CTGF expression. The data also suggest differential regulation by YAP or TAZ following exposure to different substrates. This result is especially intriguing. It must be noted that most data published on YAP/TAZ and gene regulation do not individually distinguish the specificity of YAP or TAZ. A more nuanced mechanism is suggested by our studies. We infer, from our data, that CTGF expression may be determined by the relative dominance of YAP or TAZ. Cautiously, we speculate that TAZ expression may be more dominant under glaucomatous or pathomimetic conditions to drive CTGF expression, while YAP may be the dominant regulator of CTGF under normal conditions or homeostasis.
TGM2 is an enzyme which crosslinks ECM proteins and thus increases their resistance to mechanical or proteolytic degradation.70 It is upregulated in glaucoma and has been speculated to contribute to the decreased outflow facility of glaucomatous TM.52 Importantly, it is regulated by YAP/TAZ,35 and our results suggest that it is influenced particularly by TAZ in HTM cells, as silencing YAP only resulted in a transient reduction in TGM2; specifically, TGM2 expression levels recovered in the cells 72 hours after siRNA treatment while YAP expression levels remained below 20%. Similar to TAZ expression, our results show increased TGM2 expression on pathomimetic substrates, consistent with our previous demonstration of increased TGM2 after acute (24 hours) exposure to pathomimetic substrates.11 These data show a persistent effect of substrate stiffness on TGM2 expression, concordant with data showing increased TGM2 expression in glaucomatous tissue.52 In vivo, this association could result in positive feedback of HTM stiffening with elevated stiffness leading to increased TGM2 expression, which in turn crosslinks the ECM, resulting in further stiffening of the tissue. Thus, decreasing the expression of TAZ, and thus downstream TGM2, is a novel therapeutic target in the treatment of glaucoma.
A key regulator of YAP/TAZ, SFN (14-3-3σ), was also modulated by substrate stiffness. Interestingly, the trend for expression of SFN across three of the four donors followed that of YAP. SFN sequesters both YAP and TAZ in the cytoplasm, thus inhibiting their transcriptional activity.38,40 The ability of SFN to regulate YAP/TAZ in HTM cells is important to consider in the development of new therapeutic targets for glaucoma.
The Wnt signaling pathway, an important pathway in glaucoma,43,71 is also influenced by YAP/TAZ. The results from the present study also implicate substratum stiffness in Wnt regulation. SFRP-1, a canonical Wnt antagonist,72,73 was significantly increased on pathomimetic substrates. These data were consistent with previous reports of SFRP-1 being overexpressed in glaucomatous HTM cells and increased expression resulting in elevated IOP.43,71 TAZ, also upregulated on pathomimetic substrates, has been identified as a Wnt antagonist.41 Our results suggest that the overexpression of SFRP-1 and TAZ in cells cultured on pathomimetic substrates inhibits Wnt signaling in glaucoma. These data are consistent with the view that antagonism of Wnt signaling was a causative factor in increased IOP43 or maybe constitutive Wnt expression is important in maintaining a normal IOP in the HTM.
In conclusion, our data show the potential importance of changes in the ECM and the mechanosensors YAP/TAZ in glaucoma. Additionally, we highlight the importance of incorporating substratum biophysical properties into an in vitro study design for investigating molecular mechanisms of diseases such as glaucoma. Our study points to YAP/TAZ mechanotransduction as a central pathway in mediating HTM cell response to the increased stiffness observed in glaucoma. This is particularly exciting as it identifies novel molecular targets for the development of glaucoma therapeutics.
Footnotes
Supported by National Institutes of Health Grants R01EY019475, R01EY019970, K08 EY021142, P30EY12576, a grant by National Glaucoma Research, a program of the American Health Assistance Foundation, and unrestricted funds from Research to Prevent Blindness.
These authors contributed equally to the work presented here and should therefore be regarded as equivalent authors.
Disclosure: V.K. Raghunathan, None; J.T. Morgan, None; B. Dreier, None; C.M. Reilly, None; S.M. Thomasy, None; J.A. Wood, None; I. Ly, None; B.C. Tuyen, None; M. Hughbanks, None; C.J. Murphy, None; P. Russell, None
References
- 1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90: 262–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Quigley HA. Open-angle glaucoma. N Engl J Med. 1993; 328: 1097–1106 [DOI] [PubMed] [Google Scholar]
- 3. Johnson M.‘ What controls aqueous humour outflow resistance?' Exp Eye Res. 2006; 82: 545–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnstone MA, Grant WG. Pressure-dependent changes in structures of the aqueous outflow system of human and monkey eyes. Am J Ophthalmol. 1973; 75: 365–383 [DOI] [PubMed] [Google Scholar]
- 5. Maepea O, Bill A. Pressures in the juxtacanalicular tissue and Schlemm's canal in monkeys. Exp Eye Res. 1992; 54: 879–883 [DOI] [PubMed] [Google Scholar]
- 6. Gottanka J, Johnson DH, Martus P, Lutjen-Drecoll E. Severity of optic nerve damage in eyes with POAG is correlated with changes in the trabecular meshwork. J Glaucoma. 1997; 6: 123–132 [PubMed] [Google Scholar]
- 7. Last JA, Pan T, Ding Y, et al. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest Ophthalmol Vis Sci. 2011; 52: 2147–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McKee CT, Wood JA, Shah NM, et al. The effect of biophysical attributes of the ocular trabecular meshwork associated with glaucoma on the cell response to therapeutic agents. Biomaterials. 2011; 32: 2417–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wood JA, McKee CT, Thomasy SM, et al. Substratum compliance regulates human trabecular meshwork cell behaviors and response to latrunculin B. Invest Ophthalmol Vis Sci. 2011; 52: 9298–9303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wood JA, Shah NM, McKee CT, et al. The role of substratum compliance of hydrogels on vascular endothelial cell behavior. Biomaterials. 2011; 32: 5056–5064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thomasy SM, Wood JA, Kass PH, Murphy CJ, Russell P. Substratum stiffness and latrunculin B regulate matrix gene and protein expression in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2012; 53: 952–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saha K, Keung AJ, Irwin EF, et al. Substrate modulus directs neural stem cell behavior. Biophys J. 2008; 95: 4426–4438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pelham RJ, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997; 94: 13661–13665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chew SY, Low WC. Scaffold-based approach to direct stem cell neural and cardiovascular differentiation: An analysis of physical and biochemical effects. J Biomed Mater Res A. 2011; 97A: 355–374 [DOI] [PubMed] [Google Scholar]
- 15. Leipzig ND, Shoichet MS. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials. 2009; 30: 6867–6878 [DOI] [PubMed] [Google Scholar]
- 16. Georges PC, Janmey PA. Cell type-specific response to growth on soft materials. J Appl Physiol. 2005; 98: 1547–1553 [DOI] [PubMed] [Google Scholar]
- 17. Wong JY, Velasco A, Rajagopalan P, Pham Q. Directed movement of vascular smooth muscle cells on gradient-compliant hydrogels. Langmuir. 2003; 19: 1908–1913 [Google Scholar]
- 18. Reinhart-King CA, Dembo M, Hammer DA. Cell-cell mechanical communication through compliant substrates. Biophys J. 2008; 95: 6044–6051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Han H, Wecker T, Grehn F, Schlunck G. Elasticity-Dependent Modulation of TGF-β Responses in Human Trabecular Meshwork Cells. Invest Ophthalmol Vis Sci. 2011; 52: 2889–2896 [DOI] [PubMed] [Google Scholar]
- 20. Gasiorowski JZ, Russell P. Biological properties of trabecular meshwork cells. Exp Eye Res. 2009; 88: 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stokes J, Walker BR, Campbell JC, Seckl JR, O'Brien C, Andrew R. Altered peripheral sensitivity to glucocorticoids in primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2003; 44: 5163–5167 [DOI] [PubMed] [Google Scholar]
- 22. Clark AF, Morrison JC. Steroid-induced glaucoma. Glaucoma. 2003; 197–206 [Google Scholar]
- 23. Zhuo YH, He Y, Leung KW, et al. Dexamethasone disrupts intercellular junction formation and cytoskeleton organization in human trabecular meshwork cells. Mol Vis. 2010; 16: 61–71 [PMC free article] [PubMed] [Google Scholar]
- 24. Knepper PA, Collins JA, Frederick R. Effects of dexamethasone, progesterone, and testosterone on IOP and GAGs in the rabbit eye. Invest Ophthalmol Vis Sci. 1985; 26: 1093–1100 [PubMed] [Google Scholar]
- 25. Clark AF, Brotchie D, Read AT, et al. Dexamethasone alters F-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell Motil Cytoskeleton. 2005; 60: 83–95 [DOI] [PubMed] [Google Scholar]
- 26. Clark AF, Wilson K, McCartney MD, Miggans ST, Kunkle M, Howe W. Glucocorticoid-induced formation of cross-linked actin networks in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1994; 35: 281–294 [PubMed] [Google Scholar]
- 27. Russell P, Gasiorowski JZ, Nealy PF, Murphy CJ. Response of human trabecular meshwork cells to topographic cues on the nanoscale level. Invest Ophthalmol Vis Sci. 2008; 49: 629–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rozsa FW, Reed DM, Scott KM, et al. Gene expression profile of human trabecular meshwork cells in response to long-term dexamethasone exposure. Mol Vis. 2006; 12: 125–141 [PubMed] [Google Scholar]
- 29. Comes N, Buie LK, Borrás T. Evidence for a role of angiopoietin-like 7 (ANGPTL7) in extracellular matrix formation of the human trabecular meshwork: implications for glaucoma. Genes Cells. 2011; 16: 243–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fingert JH, Stone EM, Sheffield VC, Alward WLM. Myocilin Glaucoma. Surv Ophthalmol. 2002; 47: 547–561 [DOI] [PubMed] [Google Scholar]
- 31. Polansky J. Current perspectives on the TIGR/MYOC gene (Myocilin) and glaucoma. Ophthalmol Clin North Am. 2003; 16: 515 [DOI] [PubMed] [Google Scholar]
- 32. Tamm ER. Myocilin and glaucoma: facts and ideas. Prog Retin Eye Res. 2002; 21: 395–428 [DOI] [PubMed] [Google Scholar]
- 33. Kuchtey J, Kallberg ME, Gelatt KN, Rinkoski T, Komaromy AM, Kuchtey RW. Angiopoietin-like 7 secretion is induced by glaucoma stimuli and its concentration is elevated in glaucomatous aqueous humor. Invest Ophthalmol Vis Sci. 2008; 49: 3438–3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Polansky JR, Fauss DJ, Zimmerman CC. Regulation of TIGR/MYOC gene expression in human trabecular meshwork cells. Eye. 2000; 14: 503–514 [DOI] [PubMed] [Google Scholar]
- 35. Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011; 474: 179–183 [DOI] [PubMed] [Google Scholar]
- 36. Wang K, Degerny C, Xu M, Yang XJYAP. TAZ, and Yorkie: a conserved family of signal-responsive transcriptional coregulators in animal development and human disease. Biochem Cell Biol. 2009; 87: 77–91 [DOI] [PubMed] [Google Scholar]
- 37. Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011; 13: 877–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao B, Li L, Guan KL. Hippo signaling at a glance. J Cell Sci. 2010; 123: 4001–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007; 130: 1120–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kanai F, Marignani PA, Sarbassova D, et al. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000; 19: 6778–6791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Varelas X, Miller BW, Sopko R, et al. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell. 2010; 18: 579–591 [DOI] [PubMed] [Google Scholar]
- 42. Finch PW, He X, Kelley MJ, et al. Purification and molecular cloning of a secreted, Frizzled-related antagonist of Wnt action. Proc Natl Acad Sci U S A. 1997; 94: 6770–6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang WH, McNatt LG, Pang IH, et al. Increased expression of the WNT antagonist sFRP-1 in glaucoma elevates intraocular pressure. J Clin Invest. 2008; 118: 1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vassilev A, Kaneko KJ, Shu H, Zhao Y, DePamphilis ML. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 2001; 15: 1229–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cui CB, Cooper LF, Yang X, Karsenty G, Aukhil I. Transcriptional coactivation of bone-specific transcription factor Cbfa1 by TAZ. Mol Cell Biol. 2003; 23: 1004–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hill CS. Nucleocytoplasmic shuttling of Smad proteins. Cell Res. 2008; 19: 36–46 [DOI] [PubMed] [Google Scholar]
- 47. Varelas X, Sakuma R, Samavarchi-Tehrani P, et al. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol. 2008; 10: 837–848 [DOI] [PubMed] [Google Scholar]
- 48. Tripathi RC, Li J, Chan WF, Tripathi BJ. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp Eye Res. 1994; 59: 723–727 [DOI] [PubMed] [Google Scholar]
- 49. Kottler UB, Junemann AG, Aigner T, Zenkel M, Rummelt C, Schlotzer-Schrehardt U. Comparative effects of TGF-beta 1 and TGF-beta 2 on extracellular matrix production, proliferation, migration, and collagen contraction of human Tenon's capsule fibroblasts in pseudoexfoliation and primary open-angle glaucoma. Exp Eye Res. 2005; 80: 121–134 [DOI] [PubMed] [Google Scholar]
- 50. Junglas B, Kuespert S, Seleem AA, et al. Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. Am J Pathol. 2012; 180: 2386–2403 [DOI] [PubMed] [Google Scholar]
- 51. Taylor AW. Primary open-angle glaucoma: a transforming growth factor-beta pathway-mediated disease. Am J Pathol. 2012; 180: 2201–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tovar-Vidales T, Roque R, Clark AF, Wordinger RJ. Tissue transglutaminase expression and activity in normal and glaucomatous human trabecular meshwork cells and tissues. Invest Ophthalmol Vis Sci. 2008; 49: 622–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rhee DJ, Tamm ER, Russell P. Donor corneoscleral buttons: a new source of trabecular meshwork for research. Exp Eye Res. 2003; 77: 749–756 [DOI] [PubMed] [Google Scholar]
- 54. Schneider CA, Rasband WS, Eliceiri KWNIH. Image to ImageJ: 25 years of image analysis. Nat Methods. 2012; 9: 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yue BY. The extracellular matrix and its modulation in the trabecular meshwork. Surv Ophthalmol. 1996; 40: 379–390 [DOI] [PubMed] [Google Scholar]
- 56. Acott TS, Kelley MJ. Extracellular matrix in the trabecular meshwork. Exp Eye Res. 2008; 86: 543–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tamm ER, Russell P, Epstein DL, Johnson DH, Piatigorsky J. Modulation of myocilin/TIGR expression in human trabecular meshwork. Invest Ophthalmol Vis Sci. 1999; 40: 2577–2582 [PubMed] [Google Scholar]
- 58. Kuchtey J, Kallberg ME, Gelatt KN, Rinkoski T, Komaromy AM, Kuchtey RW. Angiopoietin-like 7 secretion is induced by glaucoma stimuli and its concentration is elevated in glaucomatous aqueous humor. Invest Ophthalmol Vis Sci. 2008; 49: 3438–3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Makita R, Uchijima Y, Nishiyama K, et al. Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am J Physiol Renal Physiol. 2008; 294: F542–F553 [DOI] [PubMed] [Google Scholar]
- 60. Zhao B, Li L, Lei Q, Guan KL. The Hippo–YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010; 24: 862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Miller E, Yang J, Deran M, et al. Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chem Biol. 2012; 24: 955–962 [DOI] [PubMed] [Google Scholar]
- 62. Iyer P, Lalane R 3rd, Morris C, Challa P, Vann R, Rao PV. Autotaxin-lysophosphatidic Acid axis is a novel molecular target for lowering intraocular pressure. PloS One. 2012; 7: e42627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yu FX, Zhao B, Panupinthu N, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012; 150: 780–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tomarev SI, Wistow G, Raymond V, Dubois S, Malyukova I. Gene expression profile of the human trabecular meshwork: NEIBank sequence tag analysis. Invest Ophthalmol Vis Sci. 2003; 44: 2588–2596 [DOI] [PubMed] [Google Scholar]
- 65. Junglas B, Yu AH, Welge-Lussen U, Tamm ER, Fuchshofer R. Connective tissue growth factor induces extracellular matrix deposition in human trabecular meshwork cells. Exp Eye Res. 2009; 88: 1065–1075 [DOI] [PubMed] [Google Scholar]
- 66. Junglas B, Kuespert S, Seleem AA, et al. Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. Am J Pathol. 2012; 180: 2386–2403 [DOI] [PubMed] [Google Scholar]
- 67. Zhao B, Ye X, Yu J, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008; 22: 1962–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Huntoon CJ, Nye MD, Geng L, et al. Heat shock protein 90 inhibition depletes LATS1 and LATS2, two regulators of the mammalian hippo tumor suppressor pathway. Cancer Res. 2010; 70: 8642–8650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang H, Liu C-Y, Zha Z-Y, et al. TEAD transcription factors mediate the function of TAZ in cell growth and epithelial-mesenchymal transition. J Biol Chem. 2009; 284: 13355–13362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Beninati S, Piacentini M. The transglutaminase family: an overview: minireview article. Amino Acids. 2004; 26: 367–372 [DOI] [PubMed] [Google Scholar]
- 71. Mao W, Millar JC, Wang WH, et al. Existence of the canonical Wnt signaling pathway in the human trabecular meshwork. Invest Ophthalmol Vis Sci. 2012; 53: 7043–7051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Katoh Y, Katoh M. WNT antagonist, SFRP1, is Hedgehog signaling target. Int J Mol Med. 2006; 17: 171 [PubMed] [Google Scholar]
- 73. Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003; 116: 2627–2634 [DOI] [PubMed] [Google Scholar]