Abstract
Background. Severe acute pancreatitis (SAP) is associated with serious morbidity and mortality. Our objective was to describe the case mix, management, and outcome of patients with SAP receiving modern critical care in the Intensive Care Unit (ICU). Methods. Retrospective analysis of patients with SAP admitted to the ICU in a single tertiary care centre in the UK between January 2005 and December 2010. Results. Fifty SAP patients were admitted to ICU (62% male, mean age 51.7 (SD 14.8)). The most common aetiologies were alcohol (40%) and gallstones (30%). On admission to ICU, the median Acute Physiology and Chronic Health Evaluation (APACHE) II score was 17, the pancreatitis outcome prediction score was 8, and the median Computed Tomography Severity Index (CTSI) was 4. Forty patients (80%) tolerated enteral nutrition, and 46% received antibiotics for non-SAP reasons. Acute kidney injury was significantly more common among hospital nonsurvivors compared to survivors (100% versus 42%, P = 0.0001). ICU mortality and hospital mortality were 16% and 20%, respectively, and median lengths of stay in ICU and hospital were 13.5 and 30 days, respectively. Among hospital survivors, 27.5% developed diabetes mellitus and 5% needed long-term renal replacement therapy. Conclusions. The outcome of patients with SAP in ICU was better than previously reported but associated with a resource demanding hospital stay and long-term morbidity.
1. Introduction
Acute pancreatitis affects 22.4 people per 100 000 of the general UK population per annum [1]. The incidence has risen by 46% over the last three decades with an epidemiological trend towards younger, female patients and alcohol as the main aetiology. Approximately 25% of patients with acute pancreatitis develop severe disease with associated organ dysfunction and require admission to the Intensive Care Unit (ICU) [2].
Although the mortality rate for the mild form of the disease is as low as 1%, severe acute pancreatitis (SAP) is still associated with high mortality and a prolonged stay in the ICU [3]. According to the Intensive Care National Audit & Research Centre (ICNARC), between 1995 and 2003 in the UK, 2677 patients with SAP were admitted to an ICU, and ICU mortality and hospital mortality were 31% and 42%, respectively [4].
There are several different scoring systems aimed at identifying patients with a high risk of a more complicated course. The Ranson and Glasgow (Imrie) criteria are the most commonly used [5, 6]. The Computed Tomography Severity Index (CTSI) is another score that has been shown to have good predictive value [7]. The Acute Physiology and Chronic Health Evaluation (APACHE) II and the Sequential Organ Failure Assessment (SOFA) scores are general severity of illness scoring systems that have also been shown to have good prognostic value in SAP [8, 9]. In 2007, Harrison et al. described the pancreatitis outcome prediction (POP) score which was derived from data in the ICNARC cohort and is based on arterial pH, age, serum urea, mean arterial pressure, pO2/FiO2 ratio, and total serum calcium (in order of decreasing impact) [10]. Although the original paper showed superiority over the aforementioned models, it has not yet been validated in other patient cohorts.
The objectives of our paper were to describe the case mix, current management, and outcome of patients with SAP in a large ICU in a tertiary care centre with a dedicated surgical pancreatitis team. In addition, we aimed to identify risk factors for mortality and to test the prognostic accuracy of commonly used scoring systems and the recently proposed POP score.
2. Materials and Methods
2.1. Setting
Guy's and St Thomas' NHS Foundation Trust is a tertiary referral centre for specialist services with 53 ICU beds and a dedicated surgical pancreatitis team.
2.2. Study Design
We retrospectively analysed available data between January 2005 and December 2010. In the absence of a consensus definition for SAP, we pragmatically included all patients with pancreatitis who were admitted to the ICU. Patients with chronic pancreatitis and patients who were transferred from other ICUs if their previous ICU stay was more than 48 hours were excluded. Clinical, laboratory, and imaging data were retrieved from the medical notes and electronic record system. We documented 6 conditions from the past medical history: history of pancreatitis, gallstones, diabetes mellitus, transplantation, chronic kidney disease, and liver cirrhosis. Detailed data regarding associated organ failure, need for organ support, type of nutrition, antibiotic use, complications, and radiological and surgical interventions throughout the whole stay in ICU were recorded. The criteria by the American-European Consensus Conference on ARDS were used to define Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS) [11]. Acute Kidney Injury (AKI) was defined according to the Acute Kidney Injury Network criteria [12], and intra-abdominal hypertension (IAH) was defined as a persistently raised intravesical pressure >20 mmHg as per criteria agreed at an International Conference of Experts in 2006. In all patients, we recorded length of stay (LOS) in ICU and ICU and hospital outcome. In hospital survivors, we also documented the presence of diabetes and dialysis dependent end-stage renal failure.
2.3. Scoring Systems and Definitions
We explored the predictive role of two general severity of illness scoring systems (APACHE II and SOFA scores) and two disease-specific scoring systems (POP score and Computed Tomography Severity Index (CTSI)). All scores were calculated using the worst values obtained in the first 24 hours after admission to ICU.
2.4. Statistics
Categorical variables were summarised using frequencies and proportions. Age was described as mean (standard deviation) and other continuous variables as median and interquartile range (IQR). Comparisons between subgroups were made using t- or Mann-Whitney tests for continuous variables, and the Chi-squared or Fisher's exact test, as appropriate, for categorical variables. The relationship between the number of computed tomography (CT) scans and CTSI was evaluated using Pearson's correlation. The correlation between APACHE II, SOFA, CTSI and POP scores and hospital outcome was assessed by receiver operating characteristic curve (ROC) analysis.
3. Results
3.1. Demographics
Between January 2005 and December 2010, 50 patients (31 male) were admitted to the ICU with SAP (Table 1). The mean age was 51.7 years (SD 14.8; range 16–85). Twenty patients (40%) had a previous episode of pancreatitis, and 8 patients (16%) were known to have gallstone disease, of whom 4 had previously undergone a cholecystectomy. The most common aetiologies of SAP were alcohol (40%) and gallstone disease (30%). On admission to the ICU, the median APACHE II score was 17 (IQR 12–19); median SOFA score, 5 (IQR 3–5); median POP score, 8 (IQR 5–12); and median CTSI 4 (IQR 2–7.5).
Table 1.
Baseline characteristics and outcomes.
| Parameter | Prevalence (n = 50) |
|---|---|
| Male gender (%) | 31 (62%) |
| Mean age (SD; range) | 51.7 (14.8; 16–85) |
| Past Medical History | |
| Pancreatitis | 40% |
| Diabetes mellitus | 24% |
| Gallstone disease | 16% |
| Liver cirrhosis | 8% |
| Chronic kidney disease | 8% |
| Transplantation | 4% |
| Aetiology of severe pancreatitis | |
| Alcohol | 40% |
| Gallstone disease | 30% |
| Drug induced | 6% |
| Hypocalcaemia | 4% |
| Post ERCP | 2% |
| Hypertriglyceridemia | 2% |
| Idiopathic | 16% |
| Transfer from other hospital | 48% |
| Severity of illness on admission to ICU | |
| APACHE II score, median (IQR) | 17 (12–19) |
| SOFA, median (IQR) | 5 (3–8) |
| POP, median (IQR) | 8 (5–12) |
| CTSI, median (IQR) | 4 (2–7.5) |
| Associated organ failure | |
| AKI | 54% |
| ALI | 56% |
| IAH | 20% |
| Need for respiratory support | 78% |
| Need for RRT | 44% |
| Treatment with vasoactive drugs | 62% |
| Nutrition | |
| TPN | 20% |
| Enteral nutrition only | 80% |
| Interventional treatment | |
| Drain insertion | 24% |
| Surgical intervention | 26% |
| Embolisation | 7.5% |
| Outcome | |
| ICU mortality | 16% |
| Hospital mortality | 20% |
| LOS in ICU, median (IQR) | 13.5 (6–30) |
| LOS in Hospital, median (IQR) | 30 (16–70) |
| Diabetes mellitus in hospital survivors | 11 of 40 survivors |
| End stage renal failure in hospital survivors | 2 of 40 survivors |
ICU: intensive care unit; ERCP: Endoscopic Retrograde Cholangiopancreatography; SD: standard deviation; IQR: interquartile range; APACHE: Acute Physiology and Chronic Health Evaluation; SOFA: sequential organ failure assessment; POP: pancreatitis outcome prediction; CTSI: Computed Tomography Severity Index; AKI: acute kidney injury; ALI: acute lung injury; IAH: intraabdominal hypertension; RRT: renal replacement therapy; TPN: total parenteral nutrition; LOS: length of stay.
3.2. Progress in ICU
During stay in ICU, 39 patients (78%) required respiratory support, 27 patients (54%) developed AKI of whom 22 (44%) received renal replacement therapy (RRT), and 31 patients (62%) needed treatment with vasoactive drugs (Table 1). The majority of patients (80%) tolerated enteral feeding via either a nasogastric tube (72%) or a nasojejunal tube (28%). Ten patients (20%) had to be converted to total parenteral nutrition (TPN). Patients on TPN had a longer median LOS in ICU (43 days versus 13 days, P = 0.0097) but there was no difference in ICU mortality (15% in patients on enteral nutrition compared to 30% in TPN group, P = 0.36).
There were no significant differences in APACHE II score on admission (P = 0.74) or length of stay in hospital (P = 0.23) between both groups. Forty-four patients were treated with antibiotics (empirical treatment for presumed sepsis (n = 23), confirmed sepsis (n = 21)). No patient received antibiotics prophylactically and no patient received treatment with octreotide.
All patients had at least one CT scan of the pancreas with oral and intravenous contrast (Figure 1). The median number of CT scans per patient during stay in ICU was 2 (IQR 1–4) ranging from 1 to 14 scans per patient. Ten patients (20%) had evidence of IAH and 2 patients (4%) developed pseudoaneurysms (gastroduodenal and splenic artery). Twelve patients (24%) required CT-guided drain insertion for pancreatic cysts/collections, of whom 5 patients subsequently needed a surgical intervention. A total of 13 patients (26%) had surgery (necrosectomy (n = 5), laparotomy for abdominal compartment syndrome, bowel obstruction or small bowel perforation (n = 8)). The episode of bowel perforation occurred spontaneously and was not related to any intervention. Three patients (6%) underwent CT-guided embolisation of bleeding intraabdominal blood vessels following complications from pseudoaneurysms.
Figure 1.
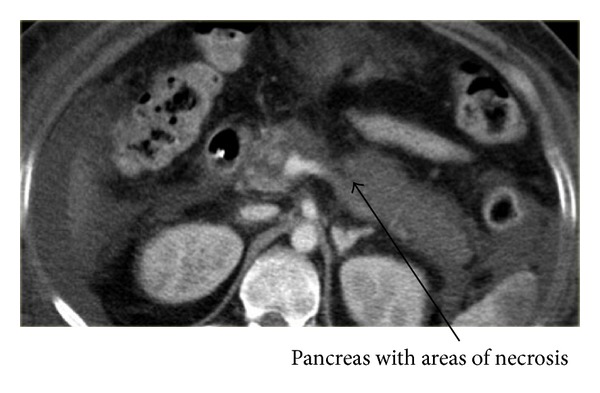
Representative CT scan of severe acute pancreatitis. CT scan with oral and intravenous contrast: >50% of the pancreas does not enhance consistent with necrotizing pancreatitis.
3.3. Outcome
Median LOS in ICU and hospital was 13.5 days (IQR 6–30) and 30 days (IQR 16–70), respectively. ICU mortality and hospital mortality were 16% and 20%, respectively. Among hospital survivors, 11 patients (27.5%) developed insulin dependent diabetes mellitus and 2 patients (5%) needed long-term RRT for end-stage renal failure.
3.4. Risk Factor Assessment
There was no statistically significant difference in gender, age, past medical history, aetiology of SAP, or length of ICU and hospital stay between survivors and nonsurvivors. All non-survivors had evidence of AKI in contrast to 42% of survivors (P < 0.001).
Patients who died in hospital had a significantly higher POP score on admission compared to patients who died (Table 2). There was no significant difference in APACHE II, SOFA, and CTSI scores between hospital survivors and non-survivors. The POP score had the highest area under the receiver operating curve but the difference between POP and APACHE was not statistically significant ((area under the receiver operating characteristics curve 0.84 versus 0.68 (P = 0.25)) (Figure 2).
Table 2.
Average scores of patients who survived and those who died in hospital.
| Score | Hospital survivors | Hospital nonsurvivors | P |
|---|---|---|---|
| APACHE II | 14.5 (11–18.5) | 18.5 (17–22) | 0.080 |
| POP | 7 (4.5–11) | 13 (11–19) | 0.001 |
| SOFA | 5 (3–7.5) | 5.5 (4–13) | 0.323 |
| CTSI | 3 (2–7) | 5.5 (2–8) | 0.508 |
All values are given as median (interquartile range).
ROC: receiver operator characteristic curve; AUC: area under curve; CI: confidence interval; APACHE: Acute Physiology and Chronic Health Evaluation; SOFA: sequential organ failure assessment; POP: pancreatitis outcome prediction; CTSI: Computed Tomography Severity Index.
Figure 2.
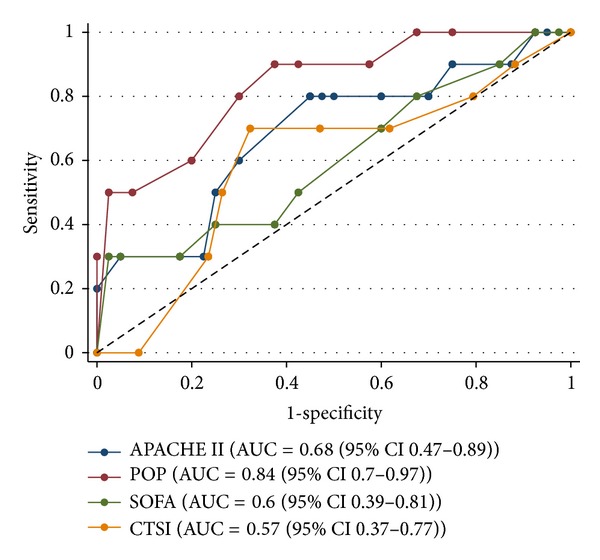
ROC curves for APACHE II, POP, SOFA, CTSI scores. ROC, receiver operator characteristic curve; AUC, area under curve; CI, confidence interval; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, sequential organ failure assessment; POP, pancreatitis outcome prediction; CTSI, Computed Tomography Severity Index.
4. Discussion
This retrospective analysis provides a detailed description of the case mix, management, and outcome of patients with SAP receiving modern critical care in a tertiary care centre in the UK. High morbidity with organ failure, multiple imaging requirements, invasive procedures, and long-term complications after discharge (diabetes and end-stage renal failure) are still the characteristics of the disease. In particular, the development of AKI is associated with a high risk of dying, as previously described [13].
The mortality rates described in this study are much lower than our group and others have previously reported [4, 14]. The likely explanation is due to differences in the patient cohorts and variations in clinical management, including criteria for admitting patients to the ICU. We note that in our own case series from the 1990s the median APACHE II score on admission to ICU was 18 compared to 17 in our more recent analysis, and the ICU mortality was 39%. Changes in medical management may have also contributed, including a less invasive approach as advocated over the last 7 years with focus on fluid resuscitation, early imaging, rational antibiotic use, use of enteral nutrition, and minimally invasive surgical procedures [15, 16].
Nutritional support has been the focus of numerous studies. Meta-analyses have suggested improved outcomes with enteral compared with parenteral nutrition [17]. The largest meta-analysis included a total of 27 randomized controlled trials [18]. Combined analysis of seven trials comparing enteral to parenteral nutrition showed a significant reduction in infectious morbidity and hospital length of stay (202 patients, mean difference 4 days between groups), and a trend towards reduced organ failure. Other benefits were seen in individual trials of enteral nutrition including a reduction in oxidative stress and hastening of resolution of the disease process. The majority of patients in our cohort tolerated enteral nutrition. This is a change from our own previous case series when only 25% of patients were fed enterally and a large proportion (33%) did not receive any nutritional support and were kept nil by mouth for >5 days [14].
Infection is a feared complication in patients with SAP. Recommendations on the role of antibiotics are conflicting [19, 20]. One systematic review concluded that prophylactic antibiotics decreased mortality in severe pancreatitis, but not the rate of infected pancreatic necrosis [19]. In contrast, a subsequent meta-analysis of seven trials detected no mortality benefit or reduction in the incidence of infected necrosis [20]. In our practice, broad spectrum antibiotics are not routinely administered to patients with SAP. However, the majority of patients received antibiotics during their stay in ICU empirically or for confirmed infections.
Fifty percent of our patients needed a surgical and/or radiological intervention. In the case of infected necrosis, our first line treatment was a minimally invasive approach as advocated by the literature [21]. Surgical debridement was reserved for patients in whom the minimally invasive methods failed to resolve the fluid collections or if the collections were not accessible by these methods. Of significance was the number of CT scans required per patient while in ICU, that is, a median number of 2 scans per patient ranging from 1 to 14. The associated high radiation exposure has been highlighted in previous studies [22]. The low CTSI scores in our cohort may be attributed to early patient scanning.
Several scoring systems are in use to estimate the prognosis of patients with SAP [5–10]. In the original study, the POP score was superior to other methods. In our cohort, we did not detect a significant difference between the APACHE II and POP scores. However, an adequately powered validation study is necessary to evaluate the role of the POP score in more detail.
It is important to acknowledge potential limitations of this analysis. The obvious weakness is the size of the cohort and the single centre setting. As a result, we cannot exclude any confounding influences specific to our clinical practice. We also acknowledge that univariate analysis of a relatively small sample needs to be interpreted with caution. Ideally we would have liked to perform a multivariate regression analysis but our sample size and number of events were too small. Finally, we were unable to calculate the Ransom and Glasgow Scores retrospectively due to missing values.
5. Conclusions
This paper describes improved ICU and hospital outcomes in a cohort of patients with SAP receiving modern critical care in a tertiary care centre at the cost of a prolonged, resource demanding stay in hospital and significant morbidity. Larger studies will be required to verify the presented findings, evaluate morbidity as well as quality of life after discharge from hospital, and, if possible, estimate the associated healthcare costs.
Ethical Approval
The need for individual informed consent was waived as this was a retrospective analysis of data collected prospectively for routine care, and there was no breach of privacy or anonymity (UK National Research Ethics Service).
Conflict of Interests
The authors declare that they have no conflict of interests.
References
- 1.Roberts SE, Williams JG, Meddings D, Goldacre MJ. Incidence and case fatality for acute pancreatitis in England: geographical variation, social deprivation, alcohol consumption and aetiology—a record linkage study. Alimentary Pharmacology and Therapeutics. 2008;28(7):931–941. doi: 10.1111/j.1365-2036.2008.03809.x. [DOI] [PubMed] [Google Scholar]
- 2.Neoptolemos JP, Raraty M, Finch M, Sutton R. Acute pancreatitis: the substantial human and financial costs. Gut. 1998;42(6):886–891. doi: 10.1136/gut.42.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uhl W, Warshaw A, Imrie C, et al. IAP guidelines for the surgical management of acute pancreatitis. Pancreatology. 2002;2(6):565–573. doi: 10.1159/000071269. [DOI] [PubMed] [Google Scholar]
- 4.Harrison DA, D’Amico G, Singer M. Case mix, outcome, and activity for admissions to UK critical care units with severe acute pancreatitis: a secondary analysis of the ICNARC case mix programme database. Critical Care. 2007;11(1, article S1) doi: 10.1186/cc5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surgery Gynecology and Obstetrics. 1974;139(1):69–81. [PubMed] [Google Scholar]
- 6.Wilson C, Heath DI, Imrie CW. Prediction of outcome in acute pancreatitis: a comparative study of APACHE II, clinical assessment and multiple factor scoring systems. The British Journal of Surgery. 1990;77(11):1260–1264. doi: 10.1002/bjs.1800771120. [DOI] [PubMed] [Google Scholar]
- 7.Balthazar EJ, Robinson DL, Megibow AJ, Ranson JHC. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174(2):331–336. doi: 10.1148/radiology.174.2.2296641. [DOI] [PubMed] [Google Scholar]
- 8.Larvin M, McMahon MJ. APACHE-II score for assessment and monitoring of acute pancreatitis. The Lancet. 1989;2(8656):201–205. doi: 10.1016/s0140-6736(89)90381-4. [DOI] [PubMed] [Google Scholar]
- 9.Vincent JL, Moreno R, Takala J, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Intensive Care Medicine. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 10.Harrison DA, D’Amico G, Singer M. The pancreatitis outcome prediction (POP) score: a new prognostic index for patients with severe acute pancreatitis. Critical Care Medicine. 2007;35(7):1703–1708. doi: 10.1097/01.CCM.0000269031.13283.C8. [DOI] [PubMed] [Google Scholar]
- 11.Bernard GR, Artigas A, Brigham KL, et al. The American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. American Journal of Respiratory and Critical Care Medicine. 1994;149(3 I):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 12.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical Care. 2007;11(2, article R31) doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Qian Z, Liu Z, Liu X, Han X, Kang H. Risk factors and outcome of acute renal failure in patients with severe acute pancreatitis. Journal of Critical Care. 2010;25(2):225–229. doi: 10.1016/j.jcrc.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Schneider H, Boyle N, McCluckie A, Beal R, Atkinson S. Acute severe pancreatitis and multiple organ failure: total parenteral nutrition is still required in a proportion of patients. The British Journal of Surgery. 2000;87(3):362–373. doi: 10.1046/j.1365-2168.2000.01383-22.x. [DOI] [PubMed] [Google Scholar]
- 15.Working Party of the British Society of Gastroenterology. UK guidelines for the management of acute pancreatitis. Gut. 2005;54(supplement 3):iii1–iii9. doi: 10.1136/gut.2004.057026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garg PK, Sharma M, Madan K, Sahni P, Banerjee D, Goyal R. Primary conservative treatment results in mortality comparable to surgery in patients with infected pancreatic necrosis. Clinical Gastroenterology and Hepatology. 2010;8(12):1089.e2–1094.e2. doi: 10.1016/j.cgh.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Al-Omran M, Albalawi ZH, Tashkandi MF, Al-Ansary LA. Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database of Systematic Reviews. 2010;(1) doi: 10.1002/14651858.CD002837.pub2.CD002837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McClave SA, Chang WK, Dhaliwal R, Heyland DK. Nutrition support in acute pancreatitis: a systematic review of the literature. Journal of Parenteral and Enteral Nutrition. 2006;30(2):143–156. doi: 10.1177/0148607106030002143. [DOI] [PubMed] [Google Scholar]
- 19.Villatoro E, Bassi C, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database of Systematic Reviews. 2006;(4) doi: 10.1002/14651858.CD002941.pub2.CD002941 [DOI] [PubMed] [Google Scholar]
- 20.Bai Y, Gao J, Zou DW, Li ZS. Prophylactic antibiotics cannot reduce infected pancreatic necrosis and mortality in acute necrotizing pancreatitis: evidence from a meta-analysis of randomized controlled trials. The American Journal of Gastroenterology. 2008;103(1):104–110. doi: 10.1111/j.1572-0241.2007.01575.x. [DOI] [PubMed] [Google Scholar]
- 21.van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH. A step-up approach or open necrosectomy for necrotizing pancreatitis. The New England Journal of Medicine. 2010;362:1491–1502. doi: 10.1056/NEJMoa0908821. [DOI] [PubMed] [Google Scholar]
- 22.Morgan DE, Ragheb CM, Lockhart ME, Cary B, Fineberg NS, Berland LL. Acute pancreatitis: computed tomography utilization and radiation exposure are related to severity but not patient age. Clinical Gastroenterology and Hepatology. 2010;8(3):303–308. doi: 10.1016/j.cgh.2009.10.021. [DOI] [PubMed] [Google Scholar]


