Abstract
Within a single-center prospective cohort study of neonatal encephalopathy that contained 315 subjects, 15 neonates were found to have a focal stroke on MRI. These 15 cases were matched on the basis of gender and degree of encephalopathy to 30 neonates from the same cohort without stroke. Using the Bayley Scales of Infant Development, the stroke group had Mental Developmental Index scores that were 1.7 standard deviations lower (P=0.007.) This association was no longer seen after adjusting for the presence of neonatal seizures (P=0.11.) Of the 15 cases of stroke, 5 had been treated with hypothermia. None of these 5 had seizures in the neonatal period, compared to 7 of the untreated 10. This is the first human study to demonstrate a potential treatment effect of therapeutic hypothermia upon perinatal stroke. In addition the presence of seizures is associated with worse cognitive outcomes for stroke that presents with encephalopathy.
Introduction
Currently no specific neuroprotective interventions are available for the management of acute perinatal stroke. Encephalopathy can be a presenting feature of perinatal stroke, which may initially lead to a diagnosis of birth asphyxia until neuroimaging reveals otherwise. Approximately 3-5% of neonates who present with encephalopathy are found to have focal infarcts on neuroimaging.1,2
Therapeutic hypothermia is used to treat birth asphyxia in the neonate and significantly reduces incidence of death and improves developmental outcomes.3 There are no published studies of hypothermia used to treat human perinatal stroke. In contrast, there are ongoing studies of hypothermia in the treatment of adult stroke.4 Animal data have shown compelling evidence that cooling may be neuroprotective in acute stroke. In a meta-analysis of over 3000 animals with focal ischemia, hypothermia reduced infarct size by 44% and improved outcomes by roughly one-third.5 The evidence from the animal and adult human literature, in addition to the success of hypothermia for the treatment of neonatal hypoxic-ischemic encephalopathy (HIE), raises the question of potential utility of hypothermia in perinatal stroke.
Newborns with ischemic stroke who present with encephalopathy provide a useful cohort to determine if therapies used to treat encephalopathy, such as hypothermia, may also have efficacy in the treatment of stroke. We sought to begin to answer this question by studying neonates with perinatal stroke who presented with encephalopathy at birth. An additional aim was to compare these neonates with encephalopathy and stroke to a matched group of neonates with encephalopathy from perinatal HIE (without evidence of focal infarct), as previous data has shown that neonates with stroke who present with encephalopathy tend to have significantly poorer outcomes.6
Methods
Case and Control Identification
All subjects were admitted to the intensive care nursery at University of California San Francisco. Cases of focal stroke and non-stroke subjects were identified from within a prospective cohort study of MRI predictors of outcome in hypoxic ischemic encephalopathy (HIE) that has enrolled patients from 1994 to the present. Inclusion criteria for this study consisted of: 1)umbilical artery pH <7.1, 2) umbilical artery base deficit >10, 3) 5-minute Apgar score </= 5, and 4) encephalopathy. Encephalopathy was defined as abnormality of mental status, respiratory status, feeding, tone or reflexes. Neonates with congenital malformation, infection or inborn error of metabolism were excluded.
Degree of encephalopathy was rated using a validated scoring system in HIE without therapeutic hypothermia,7 in which a score was assigned with one point each for abnormalities of the following characteristics: feeding, alertness, tone, respiratory status, reflexes and presence of seizures. The minimum score was 0 (indicating normal mental status) and the maximum was 6.
Neonates with encephalopathy and no evidence of focal infarct were selected using a random-number generator on the basis of subject identification number after being matched for gender and encephalopathy score. Two non-stroke subjects were matched for each case of stroke.
The presence of seizures was determined on the basis of witnessed clinical seizure activity and/or electrographic seizure activity on electroencephalogram (EEG) or cerebral function monitoring.
Hypothermia
Therapeutic hypothermia was initiated at University of California San Francisco in November 2007, with the eligibility criteria and protocol described previously.8 Eligibility for hypothermia required the presence of: (1) gestational age >/=36 weeks, (2) moderate to severe encephalopathy, and (3) one or more of the following: 10-minute Apgar score <5, prolonged resuscitation at birth, severe acidosis as defined by cord, arterial or venous pH<7.0 within 60 minutes of birth, or a base deficit of -12 from cord or arterial sample within 60 minutes of birth. Our protocol for therapeutic hypothermia required initiation of cooling within six hours of birth. Children born at outside institutions were passively cooled during transport. Active whole-body cooling was accomplished via a blanket cooling device (Cincinnati Subzero Blanketrol III, Cincinnati, Ohio.) Core temperatures as measured by a rectal probe were maintained at 33.5C for 72 hours. Both video EEG and amplitude-integrated EEG monitoring were used throughout the duration of cooling and the 24- hour period after rewarming.
Neuroimaging
All enrolled subjects were studied with MRI at a median of 4 days of life (interquartile range 4-7.) Images were obtained on a 1.5 T GE scanner (GE Healthcare, Milwaukee, Wisconsin), which consisted of T1-weighted sagittal and axial spin-echo images with repetition time (TR)/echo time (TE) of 500/11, 4-mm thickness, 1 excitation, 192×256 encoding matrix; T2-weighted axial dual echo, spin-echo with TR of 3 seconds, TE of 60 and 120 ms, 192×256 encoding matrix, 4-mm thickness. Most subjects also had DWI which was done using a spin-echo echo-planar imaging diffusion sequence with TE/TR 99/7000 ms, field of view 180 mm, 128×128, 3-mm thickness (no skip), b value of 700 s/m2, six directions, and three averages. 8 Diffusion-weighted imaging was performed on all subjects enrolled beginning in 1998. All MRI studies were read and interpreted by a pediatric neuroradiologist blinded to the patient's condition. All cases had identified strokes, which were defined as a focal infarct in an arterial or venous distribution.
Outcomes
All subjects who attended scheduled follow-up appointments were assessed by a trained child neurologist blinded to the patient's neonatal history. Subjects were also given a neuromotor score9: (0) normal, (1) abnormal tone or reflexes or primitive reflexes, (2) abnormal tone and reflexes, (3) decreased power and tone or reflex abnormality, (4) cranial nerve involvement and any motor abnormality, (5) cranial nerve involvement and spastic quadriparesis, and (6) death.
Subjects were administered the Bayley Scales of Infant Development (BSID.)( Bayley N. The Bayley scales of infant development II. New York: New York Psychological Corporation; 1993.) These were performed at 12 and 30 months of age. Though the majority of the cohort had the BSID II, subjects enrolled after 2005 were tested with the Bayley III. If the Bayley III was administered, then the cognitive composite score and language composite score were averaged to approximate the Mental Development Index (MDI) from the BSID II (which evaluated both cognition and language.)
Data Analysis
Statistical analysis was performed using Stata 11 (Stata Corp, College Station, TX.) Fisher exact test was employed for comparisons using categorical variables and t-test was used for continuous variables. Linear regression analysis was used to study the associations between stroke, seizures, therapeutic hypothermia, and outcome measures.
Results
Clinical characteristics of stroke and non-stroke subjects
Of 315 eligible subjects with neonatal encephalopathy, a total of fifteen neonates with focal stroke were identified. The subjects with and without stroke did not differ with regard to gestational age, birth weight or percentage of those receiving hypothermia (Table 1.) A high proportion of the stroke group (and the gender-matched non-stroke subjects) were male. The stroke group was significantly more likely to have been born via Cesarean section. Table 2 displays each stroke subject and their respective stroke locations. Of the non-stroke subjects, 14 had normal MRI scans, 7 had evidence of both watershed injury and basal ganglia injury, 3 had injury to the basal ganglia and 6 had watershed injury.
Table 1. Clinical characteristics of newborns with neonatal encephalopathy, with and without focal stroke.
Means were compared using the t-test, proportions using the Fisher's exact test, and medians using the Pearson chi-squared test.
| No stroke (n=30) | Stroke (n=15) | P value | |
|---|---|---|---|
| Male (n, %) | 22 (73%) | 11 (73%) | 1.0 |
| Gestational age (weeks) (mean ± SD) | 39.4 ± 1.65 | 39.6 ± 1.12 | 0.72 |
| Birth weight (grams) (mean ± SD) | 3553 ± 652 | 3482 ± 375 | 0.70 |
| Delivered by Caesarian section (n, %) | 9 (30%) | 10 (67%) | 0.02 |
| Apgar score at 5 minutes (median, IQR) | 4 (2-6) | 5 (3-7) | 0.40 |
| Uterine artery blood gas pH (mean ± SD) | 7.08 ± 0.18 | 7.02 ± 0.17 | 0.31 |
| Therapeutic hypothermia (n, %) | 8 (27%) | 5 (33%) | 0.68 |
| Encephalopathy score (median, IQR) | 4 (4-5) | 4 (4-5) | 1.0 |
Table 2.
Lesion locations, hypothermia treatment and occurrence of seizures in stroke subjects.
| Subject | Lesion Location | Hypothermia | Seizures |
|---|---|---|---|
| 1 | Left medial parietal cortex | Yes | No |
| 2 | Right posterior sylvian cortex | Yes | No |
| 3 | Left superolateral thalamus, left parietal operculum | Yes | No |
| 4 | Left caudate head | Yes | No |
| 5 | Right occipital pole | Yes | No |
| 6 | Right temporal operculum | No | No |
| 7 | Left insular cortex, bilateral sylvian fissures | No | Yes |
| 8 | Right medial parietal convexity | No | No |
| 9 | Right globus pallidus | No | Yes |
| 10 | Left caudate body | No | Yes |
| 11 | Right middle cerebral artery territory | No | Yes |
| 12 | Left middle cerebral artery territory | No | Yes |
| 13 | Left posterior frontal cortex | No | Yes |
| 14 | Left middle cerebral artery territory | No | Yes |
| 15 | Right parietal cortex | No | No |
Cooling and stroke
Of the fifteen neonates that had isolated focal infarcts on neuroimaging, five had been treated with hypothermia. None of the five children treated with hypothermia had seizures in the neonatal period, compared with seven of the ten non-treated children (p=0.01.) This relationship was not observed for the encephalopathy-matched non-stroke subjects; of the thirty matched subjects, three of the eight neonates treated with hypothermia had seizures, as did 10 of the 22 untreated neonates (p=0.73.) The proportion of untreated neonates with stroke who had seizures was 70%, higher than the proportion of seizures in the non-stroke group (45%) though this difference was not significant (p=0.27.)
Stroke and outcomes
All subjects who attended follow-up visits were assessed at 12 and 30 months of age. At 12 months, the MDI score was not significantly associated with perinatal stroke (-3.75, 95%CI 5.79, p=0.52.) At 30 months of age, MDI score was now significantly associated with perinatal stroke (-26.21, 95%CI 8.37, p=0.007, Figure 1.) Correcting for the presence of neonatal seizures, the relationship was no longer significant (-23.30, 95%CI 13.43, p=0.11.) The majority of children in both the stroke and non-stroke groups had not been administered the PDI portion of the Bayley. Neuromotor scores were not significantly different between the stroke and non-stroke groups at either 12 months (-0.14, 95%CI 0.41, p=0.73) or 30 months (0.45, 95%CI 0.43, p=0.32.) Of note, as the children treated with therapeutic hypothermia are more recent, none of the children old enough to have 30 month assessments at this time were treated with hypothermia.
Figure 1.
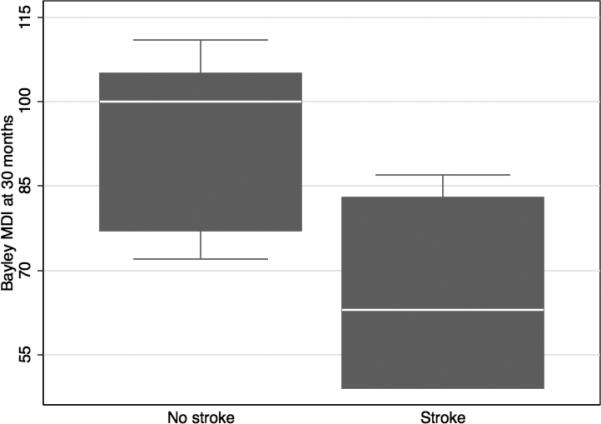
Bayley MDI scores at 30 months after neonatal encephalopathy with (n=5) and without (n=11) focal stroke on MRI. The boxplot shows the median as a white line, the 25th and 75th percentiles in the shaded box, and the 5th and 95th percentiles in the whiskers. Any outliers are plotted outside the whiskers (none).
Discussion
Perinatal stroke, neonatal seizures and cognitive outcomes
Neonates with a focal stroke who present with encephalopathy may not have a favorable neurodevelopmental outcome when compared to neonates who only have encephalopathy. In 2004 Ramaswamy and colleagues characterized a cohort of six patients presenting with encephalopathy who also had evidence of focal stroke on magnetic resonance imaging (MRI.)6 All six stroke patients had seizures and abnormal cognitive outcomes at 30 months of age. The neurodevelopmental outcomes of the stroke group were much worse than encephalopathic term neonates but the study was not sufficiently powered to achieve statistical significance. It was our objective to re-evaluate this finding by comparing outcomes for all the neonates in our study with stroke and encephalopathy to neonates matched for the same degree of encephalopathy. When all the encephalopathic neonates with stroke were compared to matched subjects without stroke, the stroke group had significantly worse cognitive outcomes at 30 months of age, performing an average of 26 points lower on the MDI. Though Ramaswamy and colleagues noted this phenomenon qualitatively, this is the first study to provide supportive quantitative data. (This comparison did not include any of the neonates treated with hypothermia, as none of them were yet old enough for the 30-month assessment.) When this association was adjusted for the presence of neonatal seizures, it was no longer significant, suggesting that the presence of seizures in the setting of perinatal stroke adversely affects outcomes to a greater degree than seizures in HIE.
The question of how much neonatal seizures affect cognitive outcomes is not entirely clear and may be an issue fundamental to the clinical setting in which they occur. Recent outcome data from a large hypothermia trial for neonatal HIE showed no significant association between seizures and poorer cognitive outcomes.10 This does not contradict our findings, but does support the assertion that the long-term outcomes of neonatal seizures may be driven by etiology. If this assertion is true then there are likely to be clinical scenarios such as perinatal stroke in which seizure cessation should be more aggressively pursued. This would be an intriguing and necessary avenue for new research.
Perinatal stroke and hypothermia
This is the first human study to suggest associations between therapeutic hypothermia and improved seizures after perinatal stroke. Hypothermia was associated with the absence of seizures in neonates presenting with encephalopathy and a focal infarct on neuroimaging. This association was not observed in the group of non-stroke controls that were matched for degree of encephalopathy, even though a similar proportion of the control group received hypothermia. Although no significant difference in mean cognitive performance was found between stroke subjects treated and not treated with hypothermia, small sample size may have limited this result. Small sampling is virtually unavoidable for neonates with stroke who present with encephalopathy. Fifteen total neonates with focal stroke were found out of approximately 300 children enrolled in the overall study of HIE to date. This number is consistent with previous work showing that roughly four to five percent of neonates presenting with encephalopathy will have evidence of focal infarct on neuroimaging.2 Even though a difference in mean cognitive performance was observed that was not significant at 12 months of age, this difference may become significant at 30 months of age or later, as previous work has shown that cognitive and motor deficits in children with perinatal stroke become more apparent with age.11,12
Though the small sample size was the primary limitation of this study, opiate administration during hypothermia presented an additional difficulty, as morphine was used in all cooled subjects to control shivering. Given that mild sedation is a well-known side effect of morphine, it is possible that the sedation falsely increased the encephalopathy scores of the cooled subjects. However this should not confound any comparison between the stroke and control groups, as a similar proportion of each group received hypothermia. Another concern is that opiates have potentially neuroprotective properties in neonates.13 This difficulty has also been encountered in the literature establishing the efficacy of cooling in neonates with HIE.14 Indeed this is an issue in any trial of therapeutic hypothermia and to date there have been no studies of cooling that have included a separate arm for the assessment of medication effect. Additional limitations of this study include the lack of continuous EEG monitoring prior to 2008 (which increases the likelihood of seizure detection) and the lack of a validated method of comparing the BSID II scores to those of the Bayley III.
We found that hypothermia was associated significantly with a lower frequency of seizures in perinatal stroke. As previously mentioned there is significant animal data that has demonstrated the benefit of hypothermia in stroke.5 This work has been limited to animal models of adult stroke. To our knowledge a parallel study in the animal literature to evaluate hypothermia in a model of neonatal stroke has not been performed. The results of this study are the first to demonstrate that hypothermia may also be of benefit in perinatal stroke and much more research is necessary to expand upon this finding, particularly given the present lack of therapeutic interventions in this arena. In addition we provided the first evidence that children who present with encephalopathy and are found to have a focal infarct are at significantly higher risk of compromised cognitive outcomes than children with encephalopathy. This phenomenon disappears when corrected for the presence of seizures. Because hypothermia is associated with a decrease in seizures, it is a reasonable hypothesis that hypothermia may improve outcomes for children with perinatal stroke, and certainly worth future exploration.
Supplementary Material
Acknowledgments
This study was performed at University of California San Francisco and was supported by the National Institutes of Health [P50 NS35902, UL1 RR024131, NINDS 1K23NS066137 (HCG)]. EWYT is a Cerebral Palsy International Research Foundation Ethel & Jack Hausman Clinical Research Scholar.
Footnotes
All authors deny any conflicts of interest.
References
- 1.Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the National Institute of Neurological Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109:116–23. doi: 10.1542/peds.109.1.116. [DOI] [PubMed] [Google Scholar]
- 2.Cowan F, Rutherford M, Groenendaal F, et al. Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet. 2003;361:736–42. doi: 10.1016/S0140-6736(03)12658-X. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs S, Hunt R, Tarnow-Mordi W, Inder T, Davis P. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2007:CD003311. doi: 10.1002/14651858.CD003311.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Hemmen TM, Raman R, Guluma KZ, et al. Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke (ICTuS-L): final results. Stroke. 2010;41:2265–70. doi: 10.1161/STROKEAHA.110.592295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Worp HB, Sena ES, Donnan GA, Howells DW, Macleod MR. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain. 2007;130:3063–74. doi: 10.1093/brain/awm083. [DOI] [PubMed] [Google Scholar]
- 6.Ramaswamy V, Miller SP, Barkovich AJ, Partridge JC, Ferriero DM. Perinatal stroke in term infants with neonatal encephalopathy. Neurology. 2004;62:2088–91. doi: 10.1212/01.wnl.0000129909.77753.c4. [DOI] [PubMed] [Google Scholar]
- 7.Miller SP, Latal B, Clark H, et al. Clinical signs predict 30-month neurodevelopmental outcome after neonatal encephalopathy. Am J Obstet Gynecol. 2004;190:93–9. doi: 10.1016/s0002-9378(03)00908-6. [DOI] [PubMed] [Google Scholar]
- 8.Bonifacio SL, Glass HC, Vanderpluym J, et al. Perinatal events and early magnetic resonance imaging in therapeutic hypothermia. J Pediatr. 2011;158:360–5. doi: 10.1016/j.jpeds.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hajnal BL, Sahebkar-Moghaddam F, Barnwell AJ, Barkovich AJ, Ferriero DM. Early prediction of neurologic outcome after perinatal depression. Pediatr Neurol. 1999;21:788–93. doi: 10.1016/s0887-8994(99)00101-0. [DOI] [PubMed] [Google Scholar]
- 10.Kwon JM, Guillet R, Shankaran S, et al. Clinical Seizures in Neonatal Hypoxic-Ischemic Encephalopathy Have No Independent Impact on Neurodevelopmental Outcome: Secondary Analyses of Data From the Neonatal Research Network Hypothermia Trial. J Child Neurol. 2010 doi: 10.1177/0883073810380915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westmacott R, MacGregor D, Askalan R, deVeber G. Late emergence of cognitive deficits after unilateral neonatal stroke. Stroke. 2009;40:2012–9. doi: 10.1161/STROKEAHA.108.533976. [DOI] [PubMed] [Google Scholar]
- 12.Lanska MJ, Lanska DJ, Horwitz SJ, Aram DM. Presentation, clinical course, and outcome of childhood stroke. Pediatr Neurol. 1991;7:333–41. doi: 10.1016/0887-8994(91)90062-p. [DOI] [PubMed] [Google Scholar]
- 13.Angeles DM, Ashwal S, Wycliffe ND, et al. Relationship between opioid therapy, tissue-damaging procedures, and brain metabolites as measured by proton MRS in asphyxiated term neonates. Pediatr Res. 2007;61:614–21. doi: 10.1203/pdr.0b013e318045bde9. [DOI] [PubMed] [Google Scholar]
- 14.Simbruner G, Mittal RA, Rohlmann F, Muche R. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics. 2010;126:e771–8. doi: 10.1542/peds.2009-2441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


