Abstract
The mechanisms by which cells accurately distinguish between DNA double-strand break (DSB) ends and telomeric DNA ends remain poorly defined. Recent investigations have revealed intriguing interactions between DNA repair and telomeres. We were the first to report a requirement for the non-homologous end-joining (NHEJ) protein DNA-dependent protein kinase (DNA-PK) in the effective end-capping of mammalian telomeres. Here, we report our continued characterization of uncapped (as opposed to shortened) dysfunctional telomeres in cells deficient for the catalytic subunit of DNA-PK (DNA-PKcs) and shed light on their consequence. We present evidence in support of our model that uncapped telomeres in this repair-deficient background are inappropriately detected and processed as DSBs, and so participate not only in spontaneous telomere-telomere fusion, but importantly, also in ionizing radiation (IR)-induced telomere-DSB fusion events. We demonstrate that phosphorylation of DNA-PKcs itself (Thr-2609 cluster) is a critical event for proper telomere end-processing and that ligase IV (NHEJ) is required for uncapped telomere fusion. We also find uncapped telomeres in cells from the BALB/c mouse, which harbors two single-nucleotide polymorphisms (SNPs) that result in reduced DNA-PKcs abundance and activity, most markedly in mammary tissue, and is both radiosensitive and susceptible to radiogenic mammary cancer. Our results suggest mechanistic links between uncapped/dysfunctional telomeres in DNA-PKcs repair-deficient backgrounds, radiation-induced instability and breast cancer. These studies provide the first direct evidence of genetic susceptibility and environmental insult interactions leading to a unique and on-going form of genomic instability capable of driving carcinogenesis.
Keywords: Telomeres, DNA-PKcs, Carcinogenesis, Genomic Instability, Ionizing Radiation
Introduction
Telomeres are specialized nucleoprotein structures that contribute to genomic stability by protecting natural chromosomal termini, an essential function inferred cytogenetically from the end-to-end fusions that result when telomeric end-capping fails. In striking contrast to telomeres, broken chromosome ends resulting from DNA DSBs are highly recombinogenic and represent a major threat to genomic integrity. These lesions have the potential for involvement in chromosomal rearrangements that contribute to genomic instability and, ultimately, tumorigenesis. Previously, we demonstrated that effective end-capping of mammalian telomeres requires proteins more commonly regarded as being involved in DSB repair by NHEJ; i.e., Ku70, Ku80 and DNA-PKcs (1). Initially this requirement appeared paradoxical, but it is now better appreciated that a plethora of DNA repair proteins interact with telomeres (2).
DNA-PK, composed of a catalytic subunit (DNA-PKcs) and a heterodimeric regulatory subunit (Ku70/Ku80), is a caretaker of genomic stability, regulating access to DNA ends (3). Recent studies investigating the role of DNA-PKcs in NHEJ have provided valuable insight, demonstrating that regulation occurs through phosphorylation-mediated conformational changes (3, 4). Two clusters of auto-phosphorylation sites, one situated around Threonine 2609 (Thr-2609) and the other around Serine 2056 (Ser-2056), are the only known targets of DNAPKcs’ robust kinase activity essential for in vivo NHEJ (5–7). Phosphorylation of the Thr-2609 cluster promotes an “open” configuration of DNA-PKcs, thereby promoting accessibility to, and processing of, DNA ends (3, 4); e.g., phosphorylation of this site facilitates Artemis-mediated DNA end-processing (8). Auto-phosphorylation of the Thr-2609 cluster can occur in trans-, an event made possible by the presence of two DNA-PKcs molecules, one bound at each side of a DSB (9). Further, it has been suggested that ATM may contribute to phosphorylation of the Thr-2609 cluster (10), although work from our laboratories indicates this contribution (if any) is minimal (9). ATM does contribute to NHEJ (11), however its precise function remains undefined. The Ser-2056 cluster remains exclusively a DNA-PKcs auto-phosphorylation site, with phosphorylation resulting in a “closed” state, blocking further end-processing (3).
Dysfunctional telomeres arising from DNA-PKcs deficiencies participate in spontaneous chromosomal end-to-end fusions that maintain large blocks of telomere sequence at the point of fusion (telomere fusion) (1, 12–14), which are not the result of telomere shortening, nor are they telomere associations (telomeres in unusually close proximity). These uncapped telomeres mis-join not only with one another, they also inappropriately fuse to IR-induced DSBs (telomere-DSB fusion) (15). SCID mouse cell lines totally deficient in DNA-PKcs exposed to graded doses of gamma (γ)-rays and examined via the strand-specific fluorescence in situ hybridization (FISH) technique of Chromosome Orientation-FISH (CO-FISH) exhibited telomere-DSB fusion in a dose-dependent fashion (15–17). Out-of-place interstitial (TTAGGG)n sequences are expected to have unusual properties whose consequences for the cell are not well understood. Cytogenetic studies in mammalian cells correlating interstitial telomere sequences (ITS) with sites of spontaneous and radiation-induced chromosomal rearrangements, suggest they may destabilize chromosomes (18). ITS might accumulate telomere proteins (TRF1, TRF2), and so may represent special challenges for replication (2, 19). It is also noteworthy that a telomere-DSB rejoining reaction results in an “orphaned” chromosomal fragment containing an “open” DSB, a situation similar to that known to promote on-going genomic instability triggered by the loss of a single telomere (20).
Recent investigation of radiation-related human breast cancer risk in the United States Radiologic Technologists cohort found three different SNPs in the DNA-PKcs gene (PRKDC) that significantly increased breast cancer risk from occupational and medical diagnostic IR exposures (21). The BALB/c mouse, which is both radiosensitive and susceptible to radiogenic mammary cancer, provides a relevant model in which to test the hypothesis that SNPs and the resulting partial deficiencies of DNA-PKcs result in uncapped telomeres and IR-induced telomere-DSB fusions. The BALB/c phenotype has been attributed to a variant allele of the DNA-PKcs gene, PrkdcBALB, which possesses two naturally occurring SNPs that result in reduced DNA-PKcs abundance and activity, most markedly in mammary gland tissue (22–24). No individuals null for DNA-PKcs have been identified; therefore, pertinent human DNA-PKcs deficiencies likely involve haploinsufficiencies or SNPs (rather than complete loss of function), and low penetrance genes fixed in the human population at relatively high frequencies. Reduced function resulting from such alterations might be expected to lead to subtle phenotypes, perhaps only being revealed following insult (e.g., IR exposure). Also relevant in this regard are reports of decreased DNA-PKcs expression associated with invasive carcinoma of the breast (25) and significantly lower DNA-PKcs activity in peripheral blood lymphocytes from breast cancer patients versus controls (26).
Here, we sought to further characterize telomere dysfunction in DNA-PKcs-deficient backgrounds, including the BALB/c mouse. Our results provide evidence supporting our hypothesis that uncapped telomeres in DNA-PKcs-deficient backgrounds are inappropriately recognized and processed as DSBs. The consequences of uncapped telomeres include on-going telomere-DSB fusion events in the progeny of irradiated BALB/c cells that precedes and continues during the expression of cytogenetic instability and the emergence of pre-neoplastic mammary epithelial clones. By providing a link between a genetic defect (partial DNA-PKcs deficiency), the cellular dysfunction associated with this defect (telomere uncapping), and its consequence (radiation-induced genomic instability), our studies suggest a unique mechanism of individual genetic susceptibility to genomic instability resulting from environmental insult.
Materials and Methods
Cells
Sf19 (DNA-PKcs deficient) mouse cells expressing vector, wt DNA-PKcs, auto-phosphorylation mutant ABCDE (T2609A, S2612A, S2602A, T2638A, and T2638A), and auto-phosphorylation mutant PQR (S2023A, S2029A, S2041A, S2053A, and S2056A) were constructed as previously reported (6). Mouse embryonic fibroblasts (MEFs) deficient in p53 (p53−/−) or doubly deficient for p53 and ligase IV (p53−/− ligIV−/−) have been described (27). The kinase activity of DNA-PKcs was inhibited via exposure to 55µM NU7026 (2-(morpholin-4-yl)-benzo[h]chomen-4-one; Sigma-Aldrich, Inc., St. Louis, MO) for a single cell cycle. Mammary epithelial and fibroblast cells were extracted from virgin female BALB/cByJ and C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) and maintained as described previously (28, 29). BALB/c p53−/− mammary fibroblasts and epithelial cells have been described elsewhere (30), as have the SCID p53−/− fibroblasts (1). Radiation-induced growth variants, IRO, 6-1, and 6-2 were isolated during studies of IR-induced instability in primary mouse mammary epithelial cells ((31) and Supplemental Table 2).
Irradiations
Gamma-ray exposures were delivered at a dose rate of 3.9 Gy/min in a calibrated, sealed source Mark I 137Cs γ-irradiator (J.L. Shepherd and Associates, Glendale, CA).
Fluorescence in situ hybridization (FISH)
Following irradiation, cultures were incubated for various times and processed for telomere FISH (32). Specialized applications were also utilized: Chromosome-Orientation Fluorescence in situ hybridization (CO-FISH) (17) and Spectral Karyotyping (SKY)-CO-FISH; both were optimized and side-by-side images evaluated. (17, 33, 34)
Image Analysis
Preparations were examined using a Zeiss fluorescence microscope (Axioplan 2ie MOT). DAPI and Cy3 exicitor/dichroic/barrier filter sets (Carl Zeiss Microimaging, Inc., Thornwood, NY) were used to detect counterstained chromosomes and telomere signals, respectively. Images of chromosomes were captured with a CCD camera (model CV-M4+CL, JAI PULNiX Inc., San Jose, CA, USA), controlled by a Dell precision 360 workstation running Isis FISH imaging software (Metasystems, Altlussheim, Germany).
Scoring Criteria
Telomere fusion necessitates that telomeres of adjoining chromosomes fuse into a single FISH signal and the DAPI signal remain continuous (1). Telomere-DSB fusion appears as single-sided (only on one chromatid of a mitotic chromosome) interstitial blocks of CO-FISH telomere signal. Telomere association is defined as telomeres of adjacent chromosomes touching or in very close proximity (≤ 1/4 width of a chromatid), yet remaining as separate signals. Unless noted, at least 25 metaphases were scored for each condition.
Immuno-FISH
Cells were grown and irradiated on two-well chamber slides (Nalgene Nunc International, Rochester, NY), fixed in 4% formaldehyde, permeabilized in 0.2% triton X-100 in PBS, and blocked in 5% milk. Mouse anti-γH2AX antibody (Millipore Corporation, Bellerica, MA) was mixed in 5% milk and incubated for 1 h at room temperature. Cells were rinsed in PBS and secondary antibody (Alexa fluor 488 goat anti-mouse, Invitrogen, Carlsbad, CA) diluted in 5% milk was added for 1 h. Cells were “post-fixed” in 4% formaldehyde for 10 min, chambers removed, slides denatured and telomere FISH performed.
Results
Characterization of telomere uncapping with DNA-PKcs deficiency
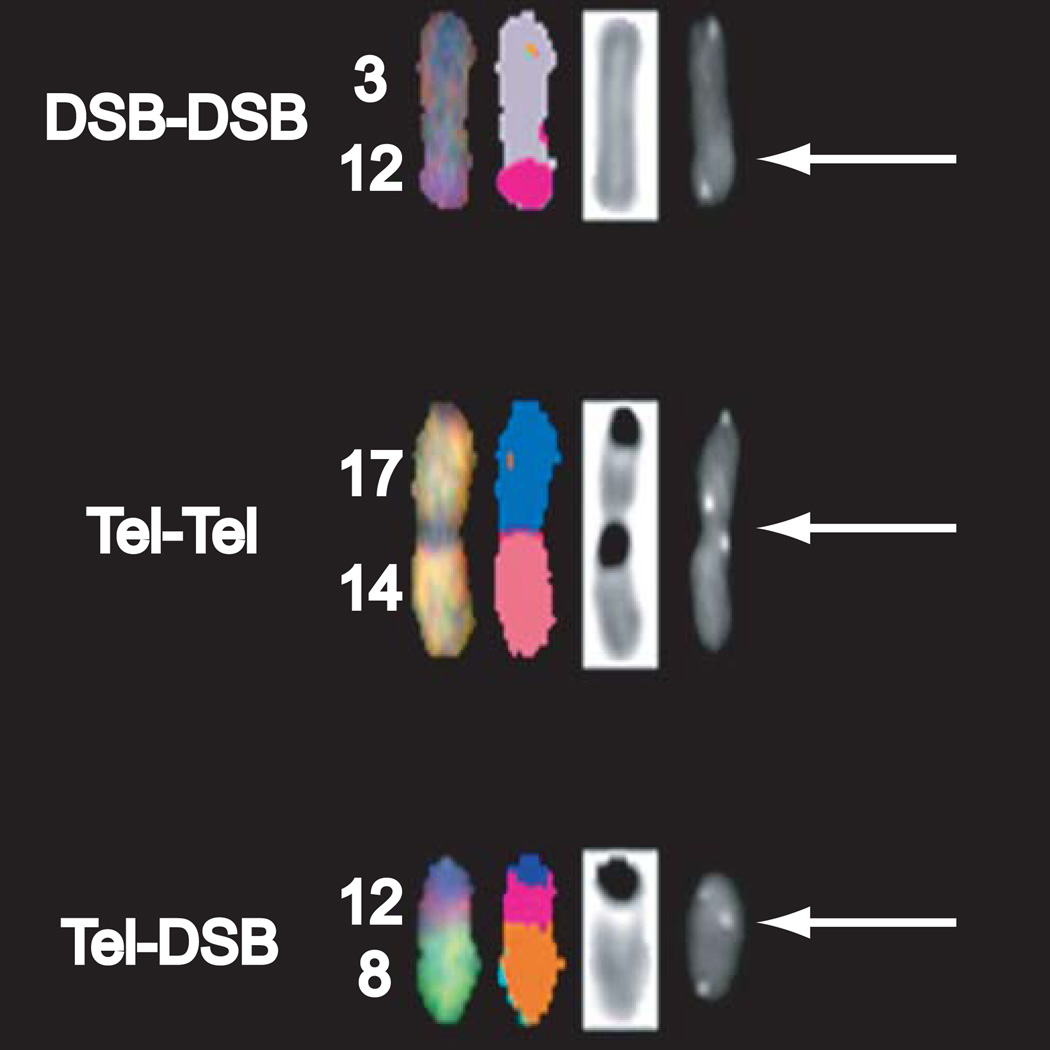
We have previously reported telomere-DSB fusion frequencies in DNA-PKcs-deficient backgrounds utilizing CO-FISH (15). To unambiguously demonstrate that telomere-DSB fusion occurs at chromosomal translocation breakpoints, we combined SKY (33) with telomere COFISH (17) (SKY-CO-FISH). SCID p53−/− and BALB/c p53−/− fibroblasts exposed to IR (1, 2 Gy; 137Cs γ-rays) were collected for analyses; absence of p53 facilitated continued cycling of mouse cells despite telomere dysfunction (35). Visualization of SKY and CO-FISH telomere patterns revealed the presence of single-sided ITS (telomere-DSB fusion) (15) at chromosomal translocation breakpoints (Figure 1). Consistent with previous G-banding studies (Bouffler and Bailey, unpublished), SKY-CO-FISH confirmed both that any chromosome can suffer telomeric end-capping failure (i.e., not chromosome-specific) and the presence of clonal rearrangements (e.g., T8;12), supporting telomere-DSB fusions as covalent linkages and therefore potentially transmissible. These results provide direct evidence that uncapped telomeres in DNA-PKcsdeficient backgrounds are detected and processed as DSBs, and thus participate in chromosomal rearrangements following IR exposure.
Figure 1. Telomeric repeats are present at translocation breakpoints.
SKY-CO-FISH distinguishes between telomere independent (DSB-DSB) and uncapped telomere dependent (Tel-Tel and Tel-DSB) chromosomal rearrangements. Telomere-DSB rearrangement shown, translocation between chromosomes 8 and 12, was a clonal event.
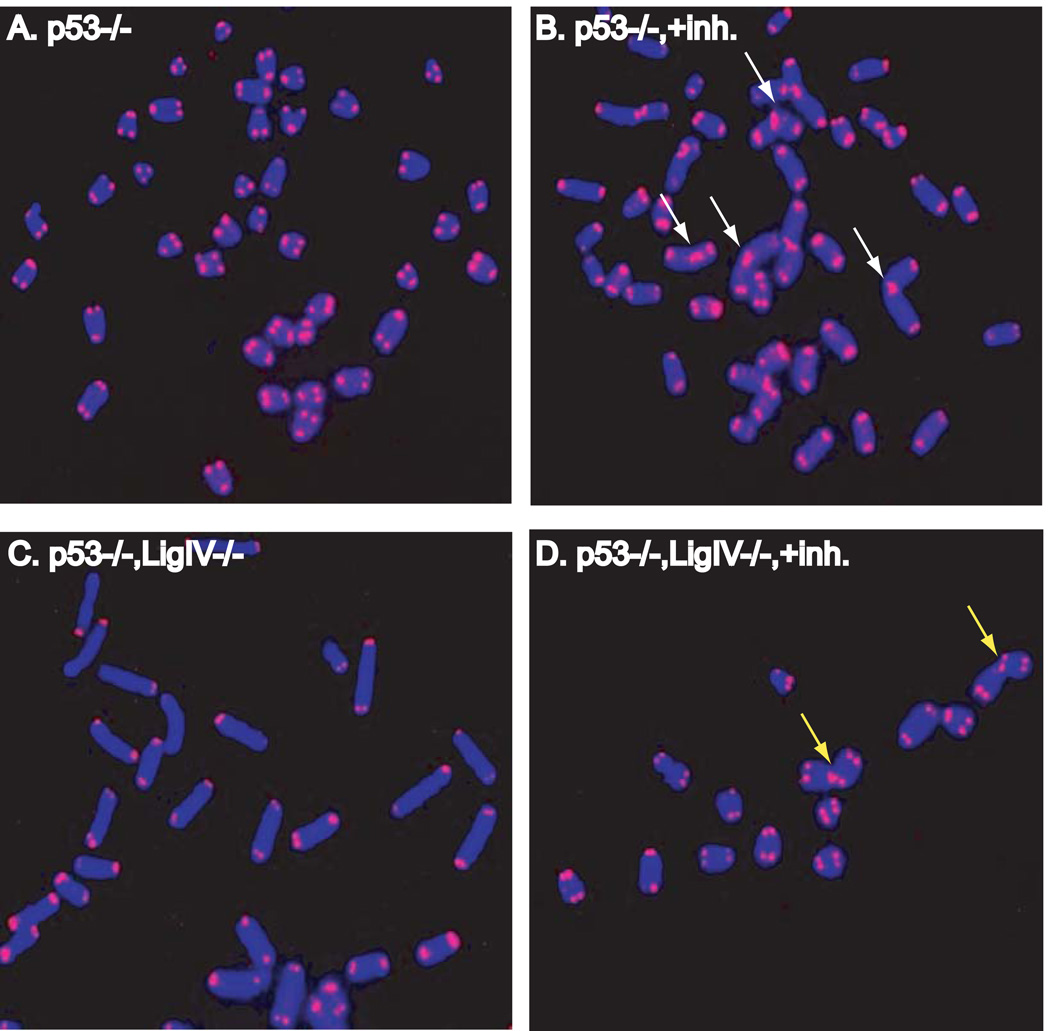
To provide additional evidence that uncapped telomeres in DNA-PKcs-deficient backgrounds are mis-identified as DSBs, we investigated the role of the NHEJ repair pathway in telomere fusion formation. MEFs deficient in p53 (p53−/−) or doubly deficient for p53 and ligase IV (p53−/− lig IV−/−) (27) were exposed to NU7026, a specific chemical inhibitor of the kinase activity of DNA-PKcs (36); DNA ligase IV is essential for NHEJ and the lack of p53 rescues ligase IV deficiency lethality without affecting NHEJ (37, 38). Following a single cell cycle in the presence of NU7026, cells were collected and analyzed by telomere FISH. A dramatic induction of telomere fusion was observed in p53−/− MEFs grown in the presence of inhibitor (Figure 2 Supplemental Table 1). All telomere fusions were of the chromatid-type, i.e, they involved only one chromatid of a metaphase chromosome, indicating that failure of end-capping occurred in the cell cycle of collection, during or after replication. These results are consistent with our previous works demonstrating differential post-replicative processing of mammalian telomeres (39) and that inhibition of DNA-PKcs kinase activity is a potent inducer of telomere uncapping and fusion (40). In striking contrast, p53−/− lig IV−/− MEFs displayed significantly fewer telomere fusions following NU7026 exposure. Failure to generate telomere fusion despite inhibition of DNA-PKcs kinase activity in p53−/− lig IV−/− MEFs implies a requirement for ligase IV in creating telomere fusion in DNA-PKcs deficient cells. These results further support the notion that uncapped telomeres are inappropriately identified as DSBs and subsequently processed by NHEJ.
Figure 2. Ligase IV (NHEJ) is required for uncapped telomere fusion formation.
A. and B. Telomere fusion (white arrows) in cells expressing ligase IV following chemical inhibition of DNA-PKcs kinase activity with Nu7026 (panels labeled “+inh”). C. and D. Nu7026 exposure in the absence of ligase IV results in telomere association (yellow arrows), not fusion.
The mechanism of action of DNA-PKcs in telomeric end-processing following replication was examined by evaluating the role of DNA-PKcs auto-phosphorylation. Telomere FISH was performed to assess telomere dysfunction in DNA-PKcs mutant mouse cells expressing a) wild type DNA-PKcs, b) kinase-dead DNA-PKcs, c) DNA-PKcs with six Thr-2609 cluster auto-phosphorylation sites mutated to Alanine (“ABCDE” mutant), or d) DNA-PKcs with five Ser-2056 cluster auto-phosphorylation sites mutated (“PQR” mutant).
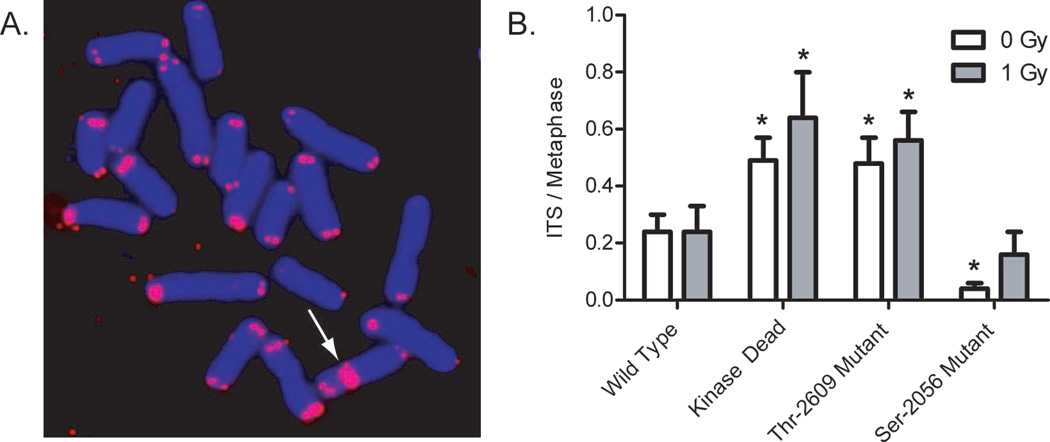
Cells expressing Thr-2609 auto-phosphorylation mutations display increased numbers of ITS compared to cells expressing WT DNA-PKcs (Figure 3). Moreover, the frequency of ITS in the Thr-2609 mutant was similar to ITS observed in cells expressing kinase dead DNA-PKcs, consistent with an essential role for DNA-PKcs Thr-2609 auto-phosphorylation in proper telomere function. Cells expressing Ser-2056 mutations displayed a decreased number of ITS compared to WT, in concordance with the proposed reciprocal action of these two auto phosphorylation clusters (3). The presence of ITS in cells expressing WT DNA-PKcs could represent incomplete rescue of the parental SCID phenotype by WT DNA-PKcs expression, or more likely, transmissible fusions formed previously in the SCID background. Additionally, analysis of telomere-induced foci (TIFs), a marker for dysfunctional telomeres (41), revealed a significant increase of TIFs in cells expressing the Thr-2609 mutations and not the Ser-2056 mutations (Supplemental Figure 1), lending further support to the notion that the Thr-2609 auto-phosphorylation cluster is an important in vivo target for proper mammalian telomeric end-capping function.
Figure 3. Auto-phosphorylation of DNA-PKcs at Thr-2609 cluster is required for telomeric end-capping function.
A. Representative image of an internal telomere sequence observed in cells unable tophosphorylate DNA-PKcs at the Thr-2609 cluster. B. Similar increases in internal telomere signals are observed in kinase dead mutants and Thr-2609 mutants indicating a critical role for this site in telomeric end-capping.
Characterization of telomere uncapping in the DNA-PKcs-deficient BALB/c mouse
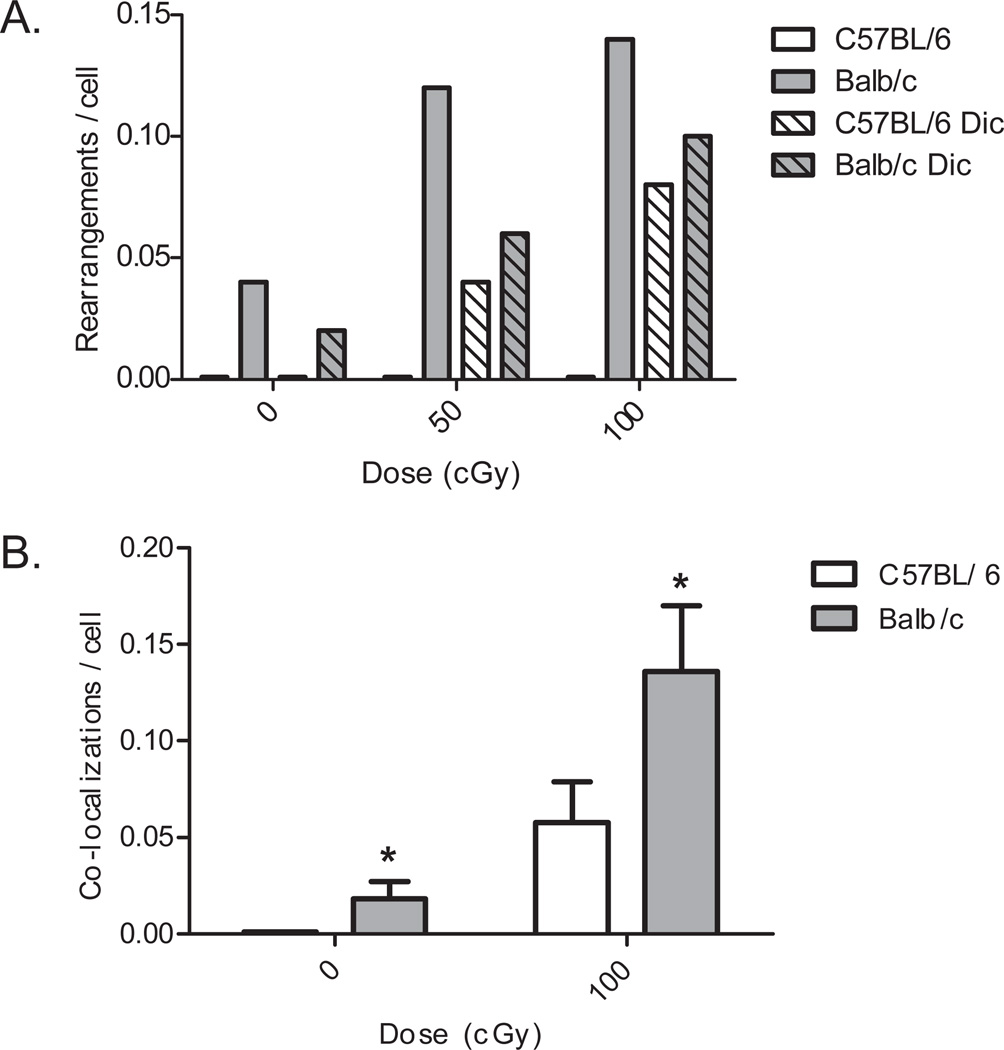
Having firmly established that uncapped telomeres in DNA-PKcs−/− (null) and SCID mice (lacking DNA-PKcs protein) participate in inappropriate telomere fusion events (1), we next examined telomeric end-capping failure resulting from partial DNA-PKcs deficiency in mammary fibroblasts derived from BALB/c versus C57BL/6 mice. Previous studies have identified a unique variant of Prkdc, the gene encoding DNA-PKcs, in BALB/c mice (31). The PrkdcBALB variant is associated with decreased DNA-PKcs abundance and activity, increased radiation sensitivity, and susceptibility to IR-induced genomic instability and mammary carcinogenesis. We observed a significant increase in the frequency of telomere fusion in cells derived from BALB/c (variant Prkdc) compared to C57BL/6 (wild-type Prkdc) (Figure 4A). Following IR exposure, the frequency of telomere-DSB fusion in BALB/c cells increased as a function of dose, indicating that indeed, uncapped telomeres are fusing to IR-induced DSBs. TIF frequencies were also elevated in BALB/c mammary fibroblasts compared to C57BL/6, regardless of IR exposure (Figure 4B), evidence that uncapped telomeres are triggering a damage response. While an increase of TIFs in C57BL/6 following IR was observed, that seen in BALB/c was nearly twice that in C57BL/6. Interestingly, the prevalence of telomere-DSB fusion in BALB/c was higher than that of dicentric chromosomes, suggesting that telomere-DSB fusion is relatively frequent in DNA-PKcs deficient backgrounds. We have also shown that partial deficiency of DNA-PKcs in human cells result in telomere-DSB fusion frequencies that plateau with increasing dose (42).
Figure 4. Telomere dysfunction in BALB/c (variant Prkdc allele) compared to C57BL/6 (WT Prkdc allele) mouse mammary fibroblasts.
A. Telomere-DSB fusions are observed following IR exposure only in BALB/c, and increase as a function of dose; frequencies rival that of dicentrics. B. Increased TIF formation, indicative of a damage response, is observed in BALB/c vs. C57BL/6. (*p < 0.05 versus C57BL/6 by student’s t-test
Consequence of telomere uncapping in the DNA-PKcs-deficient BALB/c mouse
We have previously shown that BALB/c is sensitive to radiation-induced genomic instability compared to C57BL/6 mice and others (31). This has been attributed to the variant Prkdc gene expressed in BALB/c resulting in decreased abundance and activity of DNA-PKcs (23). However, the mechanisms responsible for generating this radiation-induced genomic instability are unknown. Our experiments demonstrating that BALB/c experiences telomeric uncapping lead us to examine the contribution of this telomere dysfunction in the observed radiation-induced genomic instability. It is important to note again that the telomeric uncapping phenotype seen in BALB/c is expressed primarily as telomere-DSB fusion following insult, not as spontaneous telomere-telomere fusion, and so is more subtle than when end-capping completely fails, as in cells expressing dominant negative alleles of TRF2 (43), SCID cells (1), or cells in which the kinase activity of DNA-PKcs has been inhibited (40) (Figure 2B).
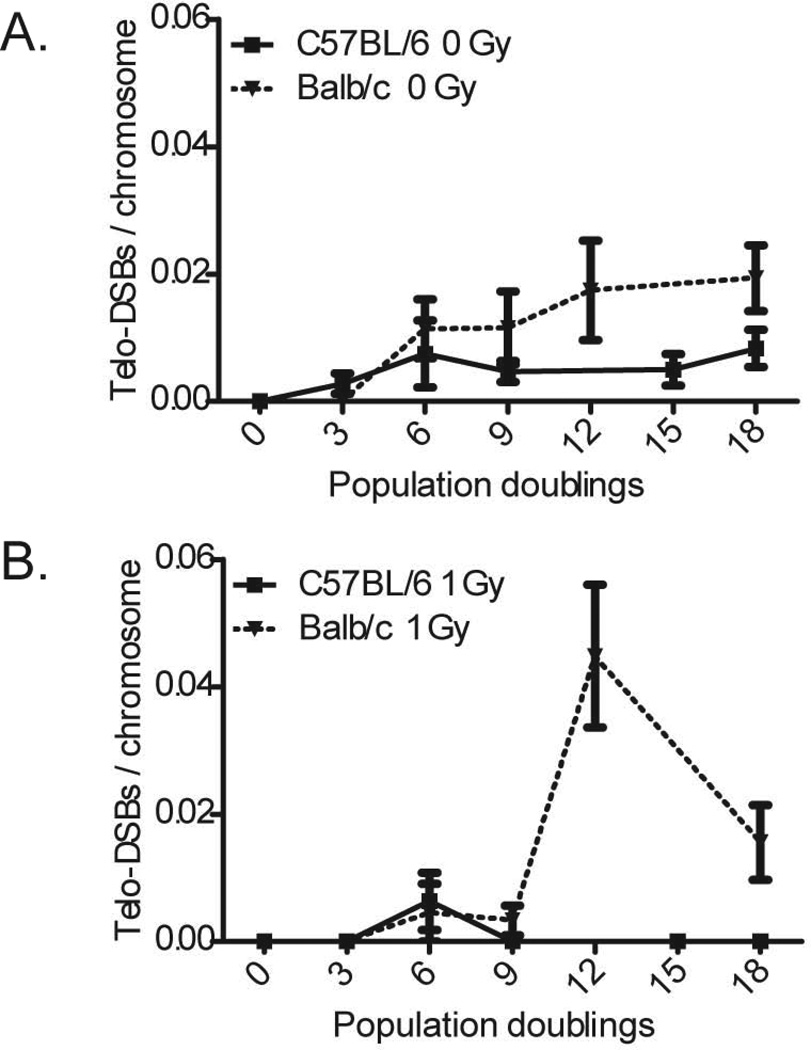
Primary mammary epithelial cells derived from BALB/c and C57BL/6 mice were examined for evidence of telomere-telomere and telomere-DSB fusion every three population doublings (PD) following irradiation (1 Gy) up to 18 PD (approximately 23 days). Frequencies of telomere-telomere fusion remained essentially zero in cells derived from both mouse strains, indicating no overt telomere uncapping is occurring following IR. However, the frequency of telomere- DSB fusion in BALB/c epithelial cells increased at 12 PD following IR and remained elevated through 18 PD, whereas C57BL/6 levels remained at baseline (Figure 5). This sudden increase in the frequency of telomere-DSB fusion underscores several important points. First, it reiterates our observation that cells derived from BALB/c mice display increased telomere dysfunction compared to those derived from C57BL/6. Second, the timing of the increase of telomere-DSB fusion precedes or is concomitant with our previously reported delayed chromatid-type chromosomal instability in the same experimental system (31). The presence of telomere-DSB fusion, but not telomere-telomere fusion, leads us to conclude that telomere uncapping is not causative of the delayed instability in BALB/c, but rather a contributing factor in driving genomic instability forward; i.e., telomere-DSB fusion, by virtue of their nature and transmissibility, may contribute to on-going genomic instability.
Figure 5. On-going telomere instability is present in BALB/c mammary epithelial cells.
Elevated levels of telomere-DSB fusion, indicative of on-going telomere dysfunction, is evident in BALB/c, but not C57BL/6. A. This on-going telomere instability can be seen in unirradiated BALB/c cells as a gradual increase in telomere-DSB fusion compared to C57BL/6. B. The progeny of irradiated BALB/c cells show a striking increase in telomere-DSB fusion compared to C57BL/6 at 12PD.
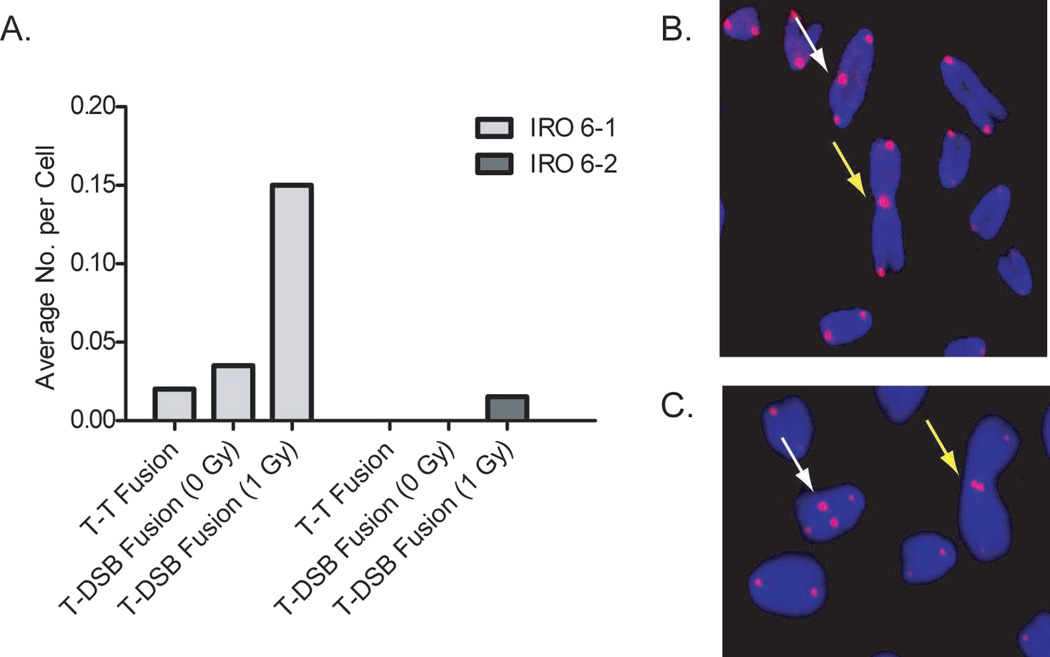
We also examined telomere dysfunction in two immortalized subclones clones (6-1 and 6-2), which were isolated from an altered growth variant (IRO) that emerged from a population of irradiated BALB/c mammary epithelial cells (Supplemental Table 2). Although immortalized, IRO remained diploid and expressed increased levels of chromatid-type aberrations compared with non-irradiated controls, a clear indication of continued chromosomal instability. At low passages neither 6-1 nor 6-2 clones were tumorigenic, and 6-2 remained non-tumorigenic through at least 27 passages. In striking contrast, injection of 6-1 at passages 21 and higher resulted in tumor formation, clearly demonstrating the preneoplastic nature of clone 6-1 (44). Intriguingly, following IR exposure we observed an elevated frequency of telomere-DSB fusion in 6-1 (tumorigenic) with no evidence of such fusions in 6-2 (Figure 6). The presence of telomere-DSB fusion in the preneoplastic 6-1 clone, particularly in the unirradiated samples, is reflective of ongoing instability. This is consistent with our evaluation of chromatid-type aberrations in these clones, which revealed elevated levels in the tumorigenic clone, but not in the non-tumorigenic clone (31).
Figure 6. Telomere dysfunction/uncapping in BALB/c clones.
A. Immortalized BALB/c IRO 6-1 and 6-2 clones, derived from an irradiated cell population, display different levels of telomere dysfunction. The pre-neoplastic clone, IRO 6-1, displays telomere dysfunction represented by both telomere fusion and telomere-DSB fusion in unirradiated and irradiated cells (1 Gy). The non-tumorigenic clone, IRO 6-2, displays no telomere dysfunction in unirradiated cells, and only a slight increase following exposure. B. Telomere CO-FISH in BALB/c shows telomere-DSB fusion: Dicentric (white arrow) and Robertsonian-like translocation (yellow arrow) with single-sided telomere signal. C. Telomere CO-FISH reveals telomere-telomere fusion in BALB/c: Interstitial (white arrow) and Robertsonian (yellow arrow) with two CO-FISH telomere signals.
Discussion
Maintenance of genomic stability obligates cells to correctly distinguish between and properly process two very different types of double-stranded DNA ends, telomeres and DSBs. A better understanding of this fundamental discrimination provides insight into two critical cellular pathways, the DNA damage response and telomere end-capping function. The aim of the present study was to further elucidate and characterize the role of the NHEJ repair protein DNA-PKcs in mammalian telomeric end-capping, as well as to identify the consequences of telomere dysfunction in DNA-PKcs-deficient backgrounds. The BALB/c mouse provided an especially relevant model in this regard, as it possesses partial deficiency of DNA-PKcs due to two SNPs in Prkdc, is radiosensitive and susceptible to IR-induced mammary carcinogenesis.
We conclusively demonstrate that uncapped telomeres in DNA-PKcs deficient backgrounds mistakenly fuse to IR-induced DSBs, supporting our hypothesis that such dysfunctional telomeres behave as DSBs. SKY-CO-FISH provided the first direct evidence of interstitial telomere-DSB fusion at translocation breakpoints between chromosomes. These characteristic telomere-DSB fusions, which maintain large blocks of displaced interstitial telomere sequence, can involve any chromosome; uncapping is not chromosome specific, but rather depends on the presence of an uncapped telomere and a suitable substrate, whether it be a DSB or another dysfunctional telomere. SKY-CO-FISH also confirmed the presence of clonal rearrangements, supporting telomere-DSB fusion as covalent linkages and, therefore, potentially transmissible.
BALB/c mammary fibroblasts and epithelial cells exhibit telomere-DSB fusions following IR exposure that increase in frequency in a dose-dependent manner. The presence of TIFs in BALB/c irrespective of IR exposure reveals inherent telomere instability, providing evidence that the cell improperly identifies uncapped telomeres in this background as DSBs, then misjoins them to either other uncapped telomeres (spontaneous telomere fusion), or to IR-induced DSBs (telomere-DSB fusion). DNA-PKcs-deficient cells can (and do) improperly fuse uncapped telomeres to DSBs at levels consistent with, or even higher than, more common chromosomal rearrangements such as dicentrics. This scenario suggests that uncapped telomeres are being improperly identified as DSBs, as they trigger a DNA damage response and participate in DSB repair pathways.
The requirement for DNA Ligase IV, an essential NHEJ protein, in telomere fusion formation establishes that uncapped telomeres do indeed participate in DSB repair pathways, namely NHEJ. Strikingly, p53−/− ligIV−/− MEFs in which the kinase activity of DNA-PKcs was inhibited did not display telomere fusion events. However, they did display an abundance of telomere association, intimating that uncapped telomeres are processed for ligation via NHEJ, but are unable to re-establish the phosphodiester linkage without ligase IV. It must be appreciated that ample telomere sequence is present at the points of fusion in DNA-PKcs deficienct backgrounds, as previous studies have shown that ligase IV is dispensable for telomere fusion of critically shortened telomeres in telomerase-deficient mouse cells (45). Distinctions between chromosome fusions arising from shortened (loss of sequence) vs. uncapped (loss of structure) telomeres have been drawn previously (46). Uncapped telomere fusions resulting from depletion of the telomere protein TRF2 have also been shown to be generated by DNA ligase IV-dependent NHEJ (47). Circumstance and cause of telomere dysfunction may govern the choice of repair pathway for end fusion, as recently proposed (48).
Our demonstration that telomeres in DNA-PKcs-deficient backgrounds are identified as DSBs and that NHEJ mediates telomere fusion formation in these instances, led us to hypothesize that DNA-PKcs acts in similar fashion at telomeres as it does at DSBs. Recent investigations examining the role of DNA-PKcs in NHEJ have highlighted the importance of two clusters of auto-phosphorylation sites (5–7). Our results provide the first evidence that phosphorylation of DNA-PKcs itself at the Thr-2609 cluster is an important target for effective telomeric end-capping function and are consistent with the concept that phosphorylation of DNA-PKcs at telomeres mimics that occurring at sites of DSBs, which then causes conformational change that facilitates protein accessibility to the DNA end and subsequent critical end-processing (3, 4, 8).
Still, key differences must exist between DNA-PKcs presence at telomeres vs. DSBs. Perhaps the most relevant – and most obvious – may be that there is only one telomeric DNA end, whereas at DSBs there are two DNA ends in close proximity. It was recently demonstrated that the auto-phosphorylation of DNA-PKcs can transpire in trans-(9), an occurrence requiring juxtaposition of two DNA-PKcs molecules at the synapse. At telomeric ends, however, presumably only one DNA-PKcs molecule is present, thus the ability to auto-phosphorylate in trans-would be compromised. Interestingly, ATM was recently shown to preferentially phosphorylate DNA-PKcs at the Thr-2609, but not serine-2056 cluster, in response to IR (10). Taken together with evidence of reciprocal action of these two auto-phosphorylation sites, our data demonstrating that the Thr-2609 cluster, but not the Ser-2056 cluster, is critical for telomere end-capping function suggests that ATM, not DNA-PKcs, may be the kinase responsible for phosphorylation of this cluster at telomeres. However, it must also be appreciated that in addition to debate over the role of ATM in Thr-2609 phosphorylation (9), the kinase activity of ATM is attenuated at telomeres through interactions with TRF2 (49).
Our data support a model in which DNA-PKcs rapidly binds to newly replicated telomeres, perhaps preferentially to those produced by leading-strand DNA synthesis where it is absolutely required (39). The reality of strand-specific differences in telomeric end-processing is now supported by several studies (50, 51). We envision that once DNA-PKcs loads onto newly replicated, essentially blunt-ended leading-strand telomeres, the huge protein acts to sterically block the telomeric end from further processing. Subsequent auto-phosphorylation of the DNA-PKcs Thr-2609 cluster induces conformational change that allows access to as yet unidentified factors that properly process the end and generate an extensive 3′ single-stranded overhang, thereby distinguishing it from a DSB. The notion of DNA-PKcs physically blocking telomeric DNA ends is also supported by our work with a specific chemical inhibitor of DNA-PKcs kinase activity. We have shown that kinase-inhibited DNA-PKcs more severely impedes telomere end-capping than absence of DNA-PKcs (40), an observation reminiscent of a previous report demonstrating that cells expressing an unphosphorylatable form of DNA-PKcs are more radiosensitive than cells completely lacking DNA-PKcs (4).
When contemplating the consequence of the role of DNA-PKcs at telomeres, a critical determinant for significance is the frequency at which telomere fusions form in DNA-PKcs-deficient backgrounds, especially those relevant to the human condition, such as partial deficiencies resulting from SNPs or haploinsufficiency. While there has been some discrepancy as to the reported frequencies of these events, with immortalization status and manner of DNA-PKcs depletion among the contributing factors to these inconsistencies, telomere fusions have consistently been reported in DNA-PKcs-deficient backgrounds (1, 13, 14). The lack of a dramatic telomere phenotype, of the sort seen when the critical telomere protein TRF2 is depleted for example (43), could indicate either that DNA-PKcs is not strictly required for end-capping function, or more likely, that its importance is underestimated due to the fact that NHEJ is required for the very pathway required for fusion formation.
In the present study, we expand our characterization of DNA-PKcs-deficient telomere dysfunction by examining the relationship between telomere uncapping and IR-induced genomic instability, a key intermediate process on the path of tumorigenesis. To this end, we utilized primary mammary epithelial cells derived from the DNA-PKcs-deficient BALB/c mouse, which experiences increased IR-induced genomic instability and susceptibility to mammary tumorigenesis, and, as we demonstrate here, telomere uncapping. A marked increase in telomere-DSB fusion in BALB/c cells was observed following exposure. We also find evidence of delayed formation of telomere-DSB fusion, suggestive of a role for these events in on-going IR-induced genomic instability.
It is important to appreciate that telomere-DSB fusions represent sensitive markers of delayed instability, as they require an uncapped telomere (occurs with replication) and the presence of DSBs at long times post exposure. Telomere-DSB fusion may perpetuate instability in several ways including resulting in an “open” DSB end (20). ITS themselves have been shown to be a source of chromosomal instability (18), which may encounter inordinate replication stalling (2, 19), a circumstance that can also drive genomic instability. In this regard, it is intriguing that the observed IR-induced telomere instability occurred immediately preceding our previously reported IR-induced cytogenetic instability in BALB/c (31); i.e., replication fork stalling occurring on one chromatid encountering ITS, may well contribute to chromatid-type breaks observed in subsequent cell cycles. Additionally, the slow rejoining kinetics of DSBs associated with DNA-PKcs deficiency following IR exposure has been shown to contribute to genomic instability, a significant source of which was identified as sister chromatid fusion (52). Delayed rejoining kinetics (more time) combined with postreplicative mis-joining not only of DSBs, but also of uncapped telomeres (more opportunity) as we demonstrate here, most certainly adds up to increased probability of incorrect end-joining.
While telomere shortening has been associated with radiosensitivity, instability and cancer, here we provide the first evidence that telomere uncapping associated with even partial DNA repair deficiency may be involved in driving IR-induced instability and thus carcinogenesis via inappropriate telomere-DSB fusion. Further evidence for this linkage comes from the observation that telomere instability was observed in a pre-neoplastic mammary epithelial clone, but not in a non-neoplastic clone, derived from the same growth variant that emerged from an unstable population of irradiated mammary epithelial cells.
In conclusion, we have expanded our characterization of telomere dysfunction with DNA-PKcs deficiency and established the presence of significant telomere uncapping in the BALB/c mouse. These studies begin to uncover the manner by which DNA-PKcs interacts with telomeres, and we present evidence supporting the critical role of phosphorylation of DNA-PKcs itself for its interaction with telomeric DNA ends. Analysis of consequence and time course for expression of telomere-DSB fusion and IR-induced genomic instability is suggestive of a role for telomere dysfunction in driving this instability. Importantly, subtle repair deficiencies, such as result from SNPs or haploinsufficiencies relevant to the human condition, can result in subtle telomeric uncapping, which may not present much of a problem most of the time (52). However, as we demonstrate here, following IR exposure (e.g., accidental, therapeutic), the resulting telomere-DSB fusions can fuel on-going instability and emergence of pre-neoplasia, ultimately driving carcinogenesis.
Supplementary Material
Acknowledgements
The authors thank Drs. Penelope Jeggo and Daniel Medina for cell lines and Edwin H. Goodwin for helpful discussions and critical reading of the manuscript. Support from the NIH/NCI, grants CA-09236-30 (RLU) and CA-043322-20 (RLU and SMB), DOE grant DE-FG02-01ER63239 (RLU and SMB), and NASA grant NNJ04HD83G (SMB) is gratefully acknowledged.
References
- 1.Bailey SM, Meyne J, Chen DJ, et al. DNA double-strand break repair proteins are required to cap the ends of mammalian chromosomes. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:14899–14904. doi: 10.1073/pnas.96.26.14899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verdun RE, Karlseder J. Replication and protection of telomeres. Nature. 2007;447:924–931. doi: 10.1038/nature05976. [DOI] [PubMed] [Google Scholar]
- 3.Cui X, Yu Y, Gupta S, Cho YM, Lees-Miller SP, Meek K. Autophosphorylation of DNA-dependent protein kinase regulates DNA end processing and may also alter double-strand break repair pathway choice. Mol Cell Biol. 2005;25:10842–10852. doi: 10.1128/MCB.25.24.10842-10852.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Block WD, Yu Y, Merkle D, et al. Autophosphorylation-dependent remodeling of the DNA-dependent protein kinase catalytic subunit regulates ligation of DNA ends. Nucleic Acids Res. 2004;32:4351–4357. doi: 10.1093/nar/gkh761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan DW, Chen BP, Prithivirajsingh S, et al. Autophosphorylation of the DNA-dependent protein kinase catalytic subunit is required for rejoining of DNA double-strand breaks. Genes Dev. 2002;16:2333–2338. doi: 10.1101/gad.1015202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding Q, Reddy YV, Wang W, et al. Autophosphorylation of the catalytic subunit of the DNA-dependent protein kinase is required for efficient end processing during DNA double-strand break repair. Mol Cell Biol. 2003;23:5836–5848. doi: 10.1128/MCB.23.16.5836-5848.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soubeyrand S, Pope L, Pakuts B, Hache RJ. Threonines 2638/2647 in DNA-PK are essential for cellular resistance to ionizing radiation. Cancer Res. 2003;63:1198–1201. [PubMed] [Google Scholar]
- 8.Douglas P, Sapkota GP, Morrice N, et al. Identification of in vitro and in vivo phosphorylation sites in the catalytic subunit of the DNA-dependent protein kinase. Biochem J. 2002;368:243–251. doi: 10.1042/BJ20020973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meek K, Douglas P, Cui X, Ding Q, Lees-Miller SP. trans Autophosphorylation at DNA-dependent protein kinase's two major autophosphorylation site clusters facilitates end processing but not end joining. Mol Cell Biol. 2007;27:3881–3890. doi: 10.1128/MCB.02366-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen BP, Uematsu N, Kobayashi J, et al. Ataxia telangiectasia mutated (ATM) is essential for DNA-PKcs phosphorylations at the Thr-2609 cluster upon DNA double strand break. J Biol Chem. 2007;282:6582–6587. doi: 10.1074/jbc.M611605200. [DOI] [PubMed] [Google Scholar]
- 11.Bredemeyer AL, Sharma GG, Huang CY, et al. ATM stabilizes DNA double-strand-break complexes during V(D)J recombination. Nature. 2006;442:466–470. doi: 10.1038/nature04866. [DOI] [PubMed] [Google Scholar]
- 12.Bailey SM, Goodwin EH. DNA and telomeres: beginnings and endings. Cytogenet Genome Res. 2004;104:109–115. doi: 10.1159/000077474. [DOI] [PubMed] [Google Scholar]
- 13.Goytisolo FA, Samper E, Edmonson S, Taccioli GE, Blasco MA. The absence of the DNA-dependent protein kinase catalytic subunit in mice results in anaphase bridges and in increased telomeric fusions with normal telomere length and G-strand overhang. Molecular And Cellular Biology. 2001:3642–3651. doi: 10.1128/MCB.21.11.3642-3651.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilley D, Tanaka H, Hande MP, et al. DNA-PKcs is critical for telomere capping. Proc Natl Acad Sci U S A. 2001;98:15084–15088. doi: 10.1073/pnas.261574698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey SM, Cornforth MN, Ullrich RL, Goodwin EH. Dysfunctional mammalian telomeres join with DNA double-strand breaks. DNA Repair (Amst) 2004;3:349–357. doi: 10.1016/j.dnarep.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Bailey SM, Cornforth MN. Telomeres and DNA double-strand breaks: ever the twain shall meet? Cell Mol Life Sci. 2007 doi: 10.1007/s00018-007-7242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey SM, Goodwin EH, Cornforth MN. Strand-specific fluorescence in situ hybridization: the CO-FISH family. Cytogenet Genome Res. 2004;107:14–17. doi: 10.1159/000079565. [DOI] [PubMed] [Google Scholar]
- 18.Kilburn AE, Shea MJ, Sargent RG, Wilson JH. Insertion of a telomere repeat sequence into a mammalian gene causes chromosome instability. Mol Cell Biol. 2001;21:126–135. doi: 10.1128/MCB.21.1.126-135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohki R, Ishikawa F. Telomere-bound TRF1 and TRF2 stall the replication fork at telomeric repeats. Nucleic Acids Res. 2004;32:1627–1637. doi: 10.1093/nar/gkh309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabatier L, Ricoul M, Pottier G, Murnane JP. The loss of a single telomere can result in instability of multiple chromosomes in a human tumor cell line. Mol Cancer Res. 2005;3:139–150. doi: 10.1158/1541-7786.MCR-04-0194. [DOI] [PubMed] [Google Scholar]
- 21.Bhatti P, Struewing JP, Alexander BH, et al. Polymorphisms in DNA repair genes, ionizing radiation exposure and risk of breast cancer in U.S. Radiologic technologists. Int J Cancer. 2008;122:177–182. doi: 10.1002/ijc.23066. [DOI] [PubMed] [Google Scholar]
- 22.Yu Y, Okayasu R, Weil MM, et al. Elevated breast cancer risk in irradiated BALB/c mice associates with unique functional polymorphism of the Prkdc (DNA-dependent protein kinase catalytic subunit) gene. Cancer Res. 2001;61:1820–1824. [PubMed] [Google Scholar]
- 23.Okayasu R, Suetomi K, Yu Y, et al. A deficiency in DNA repair and DNA-PKcs expression in the radiosensitive BALB/c mouse. Cancer Res. 2000;60:4342–4345. [PubMed] [Google Scholar]
- 24.Ullrich RL, Bowles ND, Satterfield LC, Davis CM. Strain-dependent susceptibility to radiation-induced mammary cancer is a result of differences in epithelial cell sensitivity to transformation. Radiat Res. 1996;146:353–355. [PubMed] [Google Scholar]
- 25.Moll U, Lau R, Sypes MA, Gupta MM, Anderson CW. DNA-PK, the DNA-activated protein kinase, is differentially expressed in normal and malignant human tissues. Oncogene. 1999;18:3114–3126. doi: 10.1038/sj.onc.1202640. [DOI] [PubMed] [Google Scholar]
- 26.Someya M, Sakata K, Matsumoto Y, et al. The association of DNA-dependent protein kinase activity with chromosomal instability and risk of cancer. Carcinogenesis. 2006;27:117–122. doi: 10.1093/carcin/bgi175. [DOI] [PubMed] [Google Scholar]
- 27.Riballo E, Kuhne M, Rief N, et al. A pathway of double-strand break rejoining dependent upon ATM, Artemis, and proteins locating to gamma-H2AX foci. Mol Cell. 2004;16:715–724. doi: 10.1016/j.molcel.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 28.Ethier SP, Mahacek ML, Gullick WJ, Frank TS, Weber BL. Differential isolation of normal luminal mammary epithelial cells and breast cancer cells from primary and metastatic sites using selective media. Cancer Res. 1993;53:627–635. [PubMed] [Google Scholar]
- 29.Ethier SP, Ullrich RL. Detection of ductal dysplasia in mammary outgrowths derived from carcinogen-treated virgin female BALB/c mice. Cancer Res. 1982;42:1753–1760. [PubMed] [Google Scholar]
- 30.Medina D, Kittrell FS. p53 function is required for hormone-mediated protection of mouse mammary tumorigenesis. Cancer Res. 2003;63:6140–6143. [PubMed] [Google Scholar]
- 31.Ponnaiya B, Cornforth MN, Ullrich RL. Radiation-induced chromosomal instability in BALB/c and C57BL/6 mice: The difference is as clear as black and white. Radiation Research. 1997;147:121–125. [PubMed] [Google Scholar]
- 32.Zhang Y, Lim CU, Williams ES, et al. NBS1 knockdown by small interfering RNA increases ionizing radiation mutagenesis and telomere association in human cells. Cancer Res. 2005;65:5544–5553. doi: 10.1158/0008-5472.CAN-04-4368. [DOI] [PubMed] [Google Scholar]
- 33.Schrock E, Zschieschang P, O'Brien P, et al. Spectral karyotyping of human, mouse, rat and ape chromosomes--applications for genetic diagnostics and research. Cytogenet Genome Res. 2006;114:199–221. doi: 10.1159/000094203. [DOI] [PubMed] [Google Scholar]
- 34.Schrock E, du Manoir S, Veldman T, et al. Multicolor spectral karyotyping of human chromosomes. Science. 1996;273:494–497. doi: 10.1126/science.273.5274.494. [DOI] [PubMed] [Google Scholar]
- 35.Smogorzewska A, De Lange T. Different telomere damage signaling pathways in human and mouse cells. Embo J. 2002;21:4338–4348. doi: 10.1093/emboj/cdf433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veuger SJ, Curtin NJ, Richardson CJ, Smith GC, Durkacz BW. Radiosensitization and DNA repair inhibition by the combined use of novel inhibitors of DNA-dependent protein kinase and poly(ADP-ribose) polymerase-1. Cancer Res. 2003;63:6008–6015. [PubMed] [Google Scholar]
- 37.Frank KM, Sharpless NE, Gao Y, et al. DNA ligase IV deficiency in mice leads to defective neurogenesis and embryonic lethality via the p53 pathway. Mol Cell. 2000;5:993–1002. doi: 10.1016/s1097-2765(00)80264-6. [DOI] [PubMed] [Google Scholar]
- 38.Ferguson DO, Sekiguchi JM, Chang S, et al. The nonhomologous end-joining pathway of DNA repair is required for genomic stability and the suppression of translocations. Proc Natl Acad Sci U S A. 2000;97:6630–6633. doi: 10.1073/pnas.110152897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bailey SM, Cornforth MN, Kurimasa A, Chen DJ, Goodwin EH. Strand-specific postreplicative processing of mammalian telomeres. Science. 2001;293:2462–2465. doi: 10.1126/science.1062560. [DOI] [PubMed] [Google Scholar]
- 40.Bailey SM, Brenneman MA, Halbrook J, Nickoloff JA, Ullrich RL, Goodwin EH. The kinase activity of DNA-PK is required to protect mammalian telomeres. DNA Repair (Amst) 2004;3:225–233. doi: 10.1016/j.dnarep.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 41.Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr Biol. 2003;13:1549–1556. doi: 10.1016/s0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Zhou J, Cao X, et al. Partial deficiency of DNA-PKcs increases ionizing radiation-induced mutagenesis and telomere instability in human cells. Cancer Lett. 2007;250:63–73. doi: 10.1016/j.canlet.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 43.van Steensel B, Smogorzewska A, de Lange T. TRF2 protects human telomeres from end-to-end fusions. Cell. 1998;92:401–413. doi: 10.1016/s0092-8674(00)80932-0. [DOI] [PubMed] [Google Scholar]
- 44.Selvanayagam CS, Davis CM, Cornforth MN, Ullrich RL. Latent expression of p53 mutations and radiation-induced mammary cancer. Cancer Res. 1995;55:3310–3317. [PubMed] [Google Scholar]
- 45.Maser RS, Wong KK, Sahin E, et al. DNA-dependent protein kinase catalytic subunit is not required for dysfunctional telomere fusion and checkpoint response in the telomerase-deficient mouse. Mol Cell Biol. 2007;27:2253–2265. doi: 10.1128/MCB.01354-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Capper R, Britt-Compton B, Tankimanova M, et al. The nature of telomere fusion and a definition of the critical telomere length in human cells. Genes Dev. 2007;21:2495–2508. doi: 10.1101/gad.439107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smogorzewska A, Karlseder J, Holtgreve-Grez H, Jauch A, de Lange T. DNA Ligase IV-Dependent NHEJ of Deprotected Mammalian Telomeres in G1 and G2. Curr Biol. 2002;12:1635. doi: 10.1016/s0960-9822(02)01179-x. [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Baumann P. Chromosome fusions following telomere loss are mediated by single-strand annealing. Mol Cell. 2008;31:463–473. doi: 10.1016/j.molcel.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 49.Karlseder J, Hoke K, Mirzoeva OK, et al. The telomeric protein TRF2 binds the ATM kinase and can inhibit the ATM-dependent DNA damage response. PLoS Biol. 2004;2:E240. doi: 10.1371/journal.pbio.0020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang RC, Smogorzewska A, de Lange T. Homologous recombination generates T-loop-sized deletions at human telomeres. Cell. 2004;119:355–368. doi: 10.1016/j.cell.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 51.Crabbe L, Verdun RE, Haggblom CI, Karlseder J. Defective telomere lagging strand synthesis in cells lacking WRN helicase activity. Science. 2004;306:1951–1953. doi: 10.1126/science.1103619. [DOI] [PubMed] [Google Scholar]
- 52.Martin M, Genesca A, Latre L, et al. Postreplicative joining of DNA double-strand breaks causes genomic instability in DNA-PKcs-deficient mouse embryonic fibroblasts. Cancer Res. 2005;65:10223–10232. doi: 10.1158/0008-5472.CAN-05-0932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.