Abstract
The Src family kinases (SFKs) Src and Fyn are implicated in hypoxic–ischemic (HI) injury in the developing brain. However, it is unclear how these particular SFKs contribute to brain injury. Using neuron-specific Fyn overexpressing (OE) mice, we investigated the role of neuronal Fyn in neonatal brain HI. Wild type (WT) and Fyn OE mice were subjected to HI using the Vannucci model at postnatal day 7. Brains were scored five days later for evaluation of damage using cresyl violet and iron staining. Western blotting with postsynaptic density (PSD)-associated synaptic membrane proteins and co-immunoprecipitation with cortical lysates were performed at various time points after HI to determine NMDA receptor tyrosine phosphorylation and Fyn kinase activity. Fyn OE mice had significantly higher mortality and brain injury compared to their WT littermates. Neuronal Fyn overexpression led to sustained NR2A and NR2B tyrosine phosphorylation and enhanced NR2B phosphorylation at tyrosine (Y) 1472 and Y1252 in synaptic membranes. These early changes correlated with higher calpain activity 24 h after HI in Fyn OE mice relative to WT animals. Our findings suggest a role for Fyn kinase in neuronal death after neonatal HI, possibly via up-regulation of NMDA receptor tyrosine phosphorylation.
Keywords: Fyn, Neonatal brain, Hypoxia-ischemia, NR2B
Introduction
Neonatal hypoxic–ischemic encephalopathy (HIE) occurs 2 in 1000 live births and is a leading cause of morbidity and mortality in infants. However, there are limited treatments (Ferriero, 2004). The Src family kinases (SFKs) have recently been implicated in a rodent model of neonatal hypoxia–ischemia (HI). SFKs Src and Fyn are expressed in the developing brain and are activated in the postsynaptic densities (PSD) in response to neonatal HI (Jiang et al., 2008). Specific inhibition of SFKs is protective (Jiang et al., 2008) suggesting that SFKs are involved in neonatal HI brain injury.
One of the proposed mechanisms by which SFKs contribute to brain damage following HI is through enhanced tyrosine phosphorylation of the N-Methyl-d-aspartate receptor (NMDAR) (Jiang et al., 2008; Takagi et al., 1999). The NMDAR is an important determinant of survival and cell death in the developing brain (Ikonomidou et al., 1999; McDonald et al., 1987). It is a heteromeric glutamate receptor composed of an obligatory NR1 subunit and modulatory subunits NR2A-D. NR1-NR2B receptors predominate in the neonatal brain, whereas NR1–NR2A receptors predominate at later stages of development. Excessive glutamate release in the setting of ischemia and subsequent overactivation of the NMDAR cause an influx of calcium ions that damages neurons leading to excitotoxicity (Choi, 1987; Olney, 1969; Olney et al., 1971). Increased levels of intracellular calcium (Vexler and Ferriero, 2001) and recruitment of signaling molecules to the NMDAR (Ferriero et al., 1995; Jiang et al., 2003, 2008) are critical for the evolution of brain injury in the neonate.
Tyrosine phosphorylationof NR2A and NR2B by Src or Fyn enhances NMDAR channel conductance (Köhr and Seeburg, 1996), prevents NMDAR internalization via phosphorylation at tyrosine (Y) 1472 of NR2B (Goebel-Goody et al., 2009; Nakazawa et al., 2006; Prybylowski et al., 2005; Roche et al., 2001) and controls calpain-mediated NR2B cleavage via phosphorylation at Y1336 (Wu et al., 2007). Since NR2B is mainly phosphorylated by Fyn in the PSD, we determined the specific contribution of Fyn in neonatal HI brain injury by using mice with neuronal Fyn overexpression (OE).
Materials and methods
Animals
Animal study was approved by the University of California San Francisco (UCSF) institutional animal care and use committee. C57BL/6 wildtype (WT) and Fyn OE mice (N8 transgenic mouse line overexpressing the native form of Fyn, generously provided by Dr. Nobuhiko Kojima, Gunma University School of Medicine, Japan, as described in Kojima et al., 1998) were bred at the Laboratory Animal Resource Center (LARC) of UCSF. Both sexes were used for these studies at postnatal day 7 (P7). Fyn OE mice express Fyn under the control of the CaMKIIα promoter, overexpressing Fyn postnatally in excitatory neurons in the forebrain (Kojima et al., 1998). The Fyn OE mice are indistinguishable from their WT littermates with normal general behaviors and brain morphological structures (Kojima, et al., 1998; Lu et al., 1999). Neuronal Fyn overexpression does not affect neural development (Lu et al., 1999).
Hypoxic–ischemic brain injury
HI was induced with an adaptation of the Vannucci procedure (Rice et al., 1981). At P7, pups were anesthetized with isoflurane (2–3% isoflurane/balance oxygen) and the right common carotid artery was ligated. Animals were allowed to recover for 1.5 h with their dam and then exposed to 40 min of hypoxia in a humidified chamber at 37 °C with 8% oxygen/balance nitrogen. Sham-operated control animals received isoflurane anesthesia and exposure of the right common carotid artery without ligation or hypoxia. HI and sham animals were returned to their dams until they were euthanized.
Evaluation of brain injury
Five days after the HI procedure, brains were examined histologically with cresyl violet and Perl's stain to assess the degree of damage as previously described (Sheldon et al., 1998). Briefly, animals were anesthetized with pentobarbital (50 mg/kg) and perfused with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (pH 7.4). Brains were postfixed in the same solution for 4 h, and then transferred to 30% sucrose in 0.1 M phosphate buffer. Coronal sections were cut through the forebrain at 50 μm intervals with a vibratome. Alternate sections were stained with cresyl violet for morphology or with Perl's stain enhanced with diaminobenzidine to localize iron deposition. Brain sections were scored as described (Jiang et al., 2008).
Western blotting from cortical whole cell lysates
Cortical tissue from naïve WT and Fyn OE mice at different developmental stages (P3, P7, P14, P21 and P48) or from sham-operated and the ipsilateral side of HI-injured animals at P7 was homogenized in modified radioimmunoprecipitation assay buffer (RIPA buffer, 1× sodium phosphate buffer with 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, protease and phosphatase inhibitors). 30 μg protein was applied to 4–12% Bis–Tris SDS polyacrylamide gel electrophoresis (Invitrogen, Carlsbad, CA) and transferred to polyvinyl difluoride membrane (Bio-Rad, Hercules, CA) as described elsewhere (Jiang et al., 2008). The membranes were probed with the following primary antibodies overnight at 4 °C: Fyn (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA), Src (1:1000; Millipore, Billerica, MA), NR2B (1:1000; BD Transduction Laboratories, San Jose, CA), NR2A (1:1000; Cell Signaling), α-spectrin (1:2000, Millipore) and β-actin (1:3000; Santa Cruz). Appropriate secondary horseradish peroxidase-conjugated antibodies (1:2000, Santa Cruz) were used and signal was visualized with enhanced chemiluminescence (Amersham, Buckinghamshire, UK). Image J software was used to measure the optical densities (OD) and areas of protein signal on radiographic film after scanning.
Subcellular fractionation
Purification of synaptic membrane proteins was performed according to Goebel-Goody et al.'s (2009) procedure using a subcellular fractionation approach followed by extraction with Triton X-100. In brief, cortical tissue was homogenized in ice-cold sucrose buffer containing 0.32 M sucrose, 10 mM Tris–HCl (pH 7.4), 1 mM EDTA, 1 mM EGTA and protease and phosphatase inhibitors (Complete mini and Phospho-Stop cocktail tablets, Roche, Indianapolis, IN). A low-speed (1000 ×g) centrifugation was performed to remove the nuclear fraction and tissue debris. The resulting supernatant (S1) was spun at 10,000 ×g for 15 min to yield a crude membrane fraction (P2). The supernatant (S2) was then centrifuged at 100,000 ×g for 60 min to separate cytoplasmic protein (S3) and intracellular light membrane fraction (P3). The P2 was subsequently resuspended in 120 μl sucrose buffer, and mixed with 8 volumes of 0.5% Triton X-100 buffer containing 10 mM Tris–HCl (pH 7.4), 1 mM EDTA, 1 mM EGTA and protease and phosphatase inhibitors. The mixture was homogenized again with 30 pulses of a glass pestle and rotated at 4 °C for 30 min followed by centrifugation at 32,000 ×g for 30 min in a TL-100 tabletop ultracentrifuge (Beckman). The resultant pellet containing Triton X-insoluble postsynaptic density (PSD) proteins was considered as the synaptic membrane compartment. All the pellets were dissolved in TE buffer (100 mM Tris–HCl, 10 mM EDTA) with 1% SDS. The samples were sonicated, boiled for 5 min and stored at −80 °C until use. Protein concentration was determined by the bicinchoninic acid method (Pierce). The purity of synaptic membranes was verified by Western blots as described (Jiang et al., 2011).
Western blotting from synaptic membrane proteins
For Western blot analysis, an equal amount of synaptic membrane protein (5 μg) from P7 cortex was applied to 4–12% Bis–Tris SDS polyacrylamide gel electrophoresis and transferred to polyvinyl difluoride membrane as described above. The blots were probed with the following primary antibodies overnight at 4 °C: Fyn (1:1000; Santa Cruz), phospho-Y416 (1:500; Cell Signaling), phospho-Y1472 NR2B (1:500; Cell Signaling), phospho-Y1252 NR2B (1:800; PhosphoSolutions, Aurora, CO), phospho-Y1336 (1:500; PhosphoSolutions) and NR2B (1:1000; BD). Appropriate secondary horseradish peroxidase-conjugated antibodies (1:2000, Santa Cruz) were used, and signal was visualized with enhanced chemiluminescence (Amersham). Image J software was used to measure the mean optical densities (OD) and areas of protein signal on radiographic film after scanning.
Immunoprecipitation (IP)
IP experiments were performed to measure tyrosine phosphorylation of NR2A and NR2B, and the specific Fyn activity. Cortical tissue from sham-operated and the ipsilateral side of HI-injured animals was homogenized in RIPA buffer. An equal amount of protein (250 μg) was diluted with RIPA buffer and pre-cleared by incubation with Protein G-agarose (Invitrogen) for 30 min at 4 °C. For NMDAR IPs, cell lysates were incubated with 4 μg of subunit antibodies (goat polyclonal NR2A or NR2B antibody; Santa Cruz) or 4 μg of normal goat IgG (Santa Cruz) as a negative control and Protein G PLUS-agarose (Santa Cruz) overnight at 4 °C. For Fyn IP, cell lysates were incubated with 15 μg of Fyn-conjugated agarose (Santa Cruz) or 15 μg of mouse IgG-conjugated agarose (Santa Cruz) as a negative control at 4 °C overnight. After centrifugation, IPs were washed 3× with RIPA buffer, then boiled with 30 μl of LDS sample buffer (Invitrogen). Eluted immune complexes were loaded onto a 4–12% Bis–Tris SDS polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membrane. Membranes from NR2A and NR2B IPs were incubated with mouse 4G10 anti-phosphotyrosine (anti-pY) antibody (1:800; Millipore), then stripped and reprobed with NR2A (1:1000; Cell Signaling) or NR2B (1:1000; BD Transduction Laboratories) antibodies. NR2A or NR2B tyrosine phosphorylation was expressed as the OD ratio of phosphotyrosine (pY) to NR2A or NR2B. Membranes of Fyn IPs were incubated with phospho-Src (pY416; 1:500; Cell Signaling) antibody, which is the active form of SFKs. The membranes were then stripped and reprobed with Fyn (1:1000; Santa Cruz) antibody. Specific Fyn activity was expressed as the OD ratio of pY416 to Fyn.
Statistical analysis
Data are presented as median and interquartile range for brain injury score using Prism 4 nonparametric tests for analysis of variance (Kruskal–Wallis test). Contingency tables were used to determine mortality differences. Data of optical densities of immunoblots are presented as mean ± SD and were evaluated statistically using SAS Wilcoxon–Mann–Whitney test. Differences were considered significant at p<0.05.
Results
Fyn is overexpressed in the developing cortex in Fyn OE mice
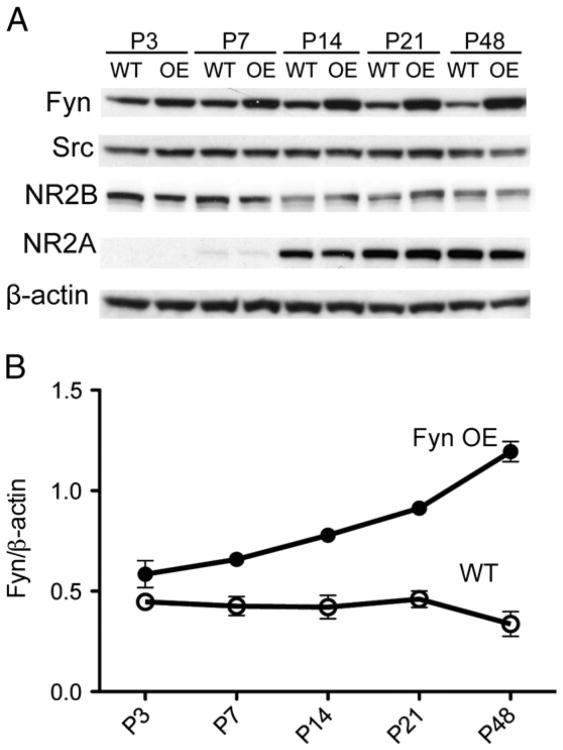
We determined the expression of Fyn, Src and NMDAR subunits in WT and Fyn OE mice at different stages of development. We made cortical lysates from WT and Fyn OE mice at different postnatal ages (P3, P7, P14, P21, P48). Fyn protein expression increased with age in the OE mice (Fig. 1) because the time course of the Fyn transgene expression corresponded to that of CaMKIIα (Kojima et al., 1997). There were no changes in Src, NR2A or NR2B protein levels in Fyn OE brains (Fig. 1A). Importantly, Fyn is overexpressed approximately 2-fold relative to WT mice at P7, the age at which we performed hypoxia–ischemia (Fig. 1B).
Fig. 1.
Fyn overexpression does not lead to compensatory changes in Src or NMDAR protein expression. A) Western blotting was performed on cortical lysates for Fyn, c-Src, NR2B, NR2A, and β-actin at P3, P7, P14, P21, and P48. B) Fyn developmental expression was normalized to β-actin. Representative data for n=2 experiments.
Neuronal Fyn overexpression worsens brain injury and increases mortality after neonatal HI
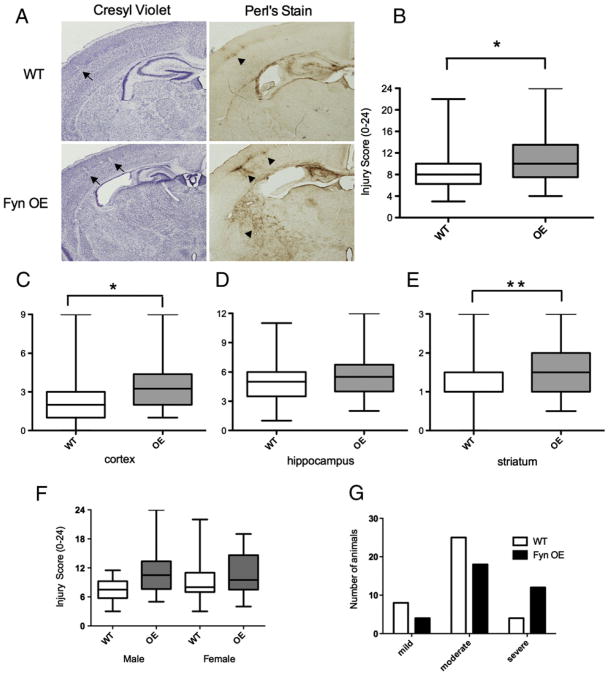
We examined the effect of neuronal Fyn overexpression on the degree of brain injury and mortality after HI. The brains of Fyn OE had more severe injury than the WT controls [median=10, range 7.5–13.5 in OE (n=34); median=8, range 6.5–10 in WT (n=37), WT vs. OE p=0.0141, Figs. 2A, B and Table 1]. The cortex and striatum showed a statistically significant increase in injury in Fyn OE mice compared to WT mice (cortex p=0.0139, striatum p=0.0077, Figs. 2C, E). Additionally, we found that Fyn OE mice had a 4-fold higher mortality than WT (Fyn OE 23.26% vs. WT 5.26%, p=0.0229, Table 1). All deaths occurred during hypoxia. The distribution of injury scores indicated that more Fyn OE brains had relatively severe injury (scores ≥12) than the WT (Fig. 2G, Table 1). There were no gender differences in mortality or brain injury in WT or Fyn OE mice after HI (Fig. 2F).
Fig. 2.
Fyn overexpression increases brain injury in the cortex and striatum following neonatal HI. A modified Vannucci procedure was performed on WT (n=37) and Fyn OE mice (n=34) at P7. A) Animals were perfused at P12, brains were sectioned and stained with Cresyl violet (morphology) and Perl's stain (iron deposition). Arrows indicate patches of cell loss in Cresyl violet stained sections. Arrowheads show iron accumulation in similar injured areas in Perl-stained adjacent sections. B) Composite injury score. Regional injury scores in the C) cortex, D) hippocampus, and E) striatum. Gender differences are shown in F. The distribution of brain injury scores is presented in G. Data are represented by box and whisker plots: the median is represented by the central horizontal line, 25th and 75th percentiles by the space within the box, and the range by the vertical lines extending from the box. Brain injury score was analyzed using nonparametric tests for analysis of variance (Kruskal–Wallis test). *p<0.05, **p<0.01.
Table 1.
Fyn OE mice have increased mortality and more severe brain injury due to HI. Mortality occurred during hypoxia. Brain sections were scored for injury with Cresyl violet (morphology) and Perl's stain (iron deposition). Injury was considered mild (≤5), moderate (6–12) and severe (≥12). Contingency tables were used for mortality differences. Brain injury score was analyzed using nonparametric tests for analysis of variance (Kruskal–Wallis test). p<0.05 was considered significant.
| Genotype | Animal number | Mortality (%) | Injury score | Distribution of injury scores | |||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Median | Range | ≤5 | 6–12 | ≥12 | |||
| WT | 37 | 5.26 | 8 | 6.5–10 | 8 | 25 | 4 |
| OE | 34 | 23.26 | 10 | 7.5–13.5 | 4 | 18 | 12 |
WT vs. OE: p=0.0229 (mortality), p=0.014 (injury score).
Fyn OE mice have elevated Fyn expression and activity after HI
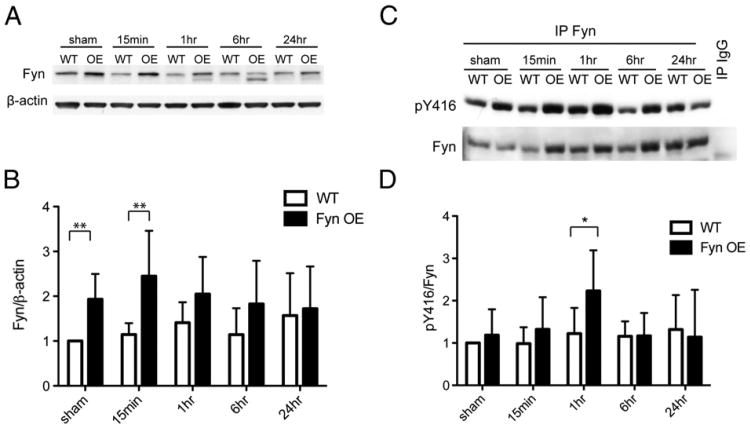
Next, we examined the expression and activity of SFKs after neonatal HI. In both WT and Fyn OE brains, Fyn protein expression did not change in response to HI in the whole cell cortical lysates or in the synaptic membranes (Figs. 3A, 5A). However, synaptic SFK activity increased in response to HI and peaked at 1 h after HI in both genotypes (Figs. 5A, B). Fyn OE mice had significantly higher Fyn protein expression compared to WT in sham-operated animals and at 15 min after HI (sham WT vs. OE p=0.003, 15 min WT vs. OE p=0.004, Figs. 3A, B). Fyn activity was higher in OE brain than WT 1 h after injury (1 h WT vs. OE p=0.037, Figs. 3C, D).
Fig. 3.
Fyn OE mice have elevated Fyn protein expression and activity than the WT mice. A) Western blotting using anti-Fyn, and β-actin antibodies was carried out on cortical lysates from sham and HI animals at the time points shown. B) Fyn protein levels were normalized to β-actin and then to WT sham. C) Fyn was immunoprecipitated from cortical lysates and blotted for anti-pY416 antibody, then stripped and reprobed with anti-Fyn antibody. D) Fyn activity is expressed as an OD ratio of pY416 to Fyn. Data was normalized to WT sham values. Representative data for n=6 experiments. *p<0.05, **p<0.01.
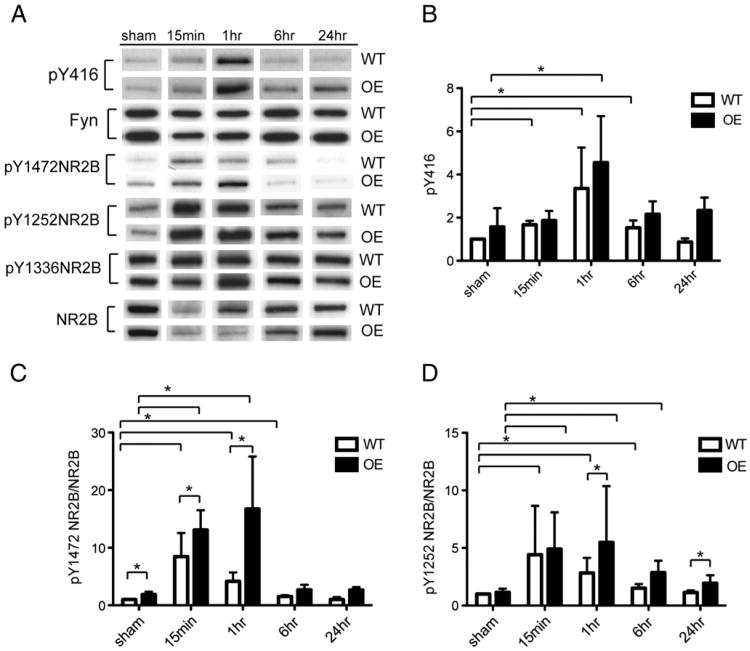
Fig. 5.
pY1472 NR2B and pY1252 NR2B are elevated in synaptic membranes in Fyn OE mice in response to HI. A) Western blotting using anti-pY416, Fyn, pY1472NR2B, pY1252NR2B, pY1336NR2B and NR2B was carried out on synaptic membrane fractions from sham and HI animals at the time points shown. B) pY416 was normalized to WT sham values in synaptic membranes. C, D) pY1472NR2B and pY1252NR2B were normalized to synaptic NR2B and then to WT sham values. Representative data for n=3 experiments. Graphs indicate mean ± SD. Data was analyzed using SAS Wilcoxon–Mann–Whitney test. *p<0.05.
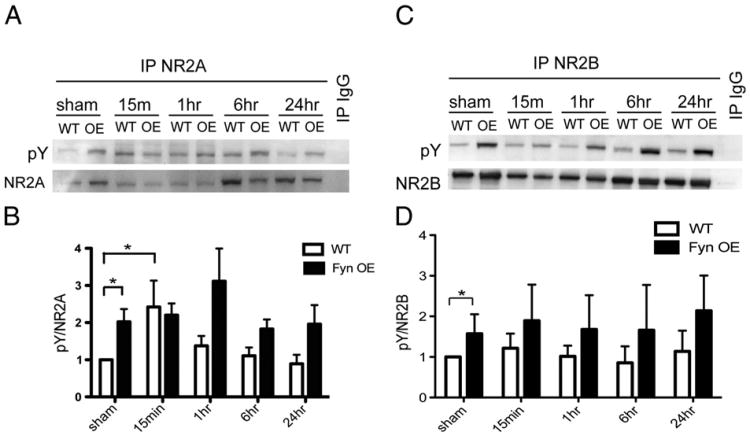
Fyn OE mice have sustained phosphorylation of NR2A and NR2B after HI
To determine if Fyn overexpression affects tyrosine phosphorylation of NR2A and NR2B, we immunoprecipitated NMDAR subunits and performed western blotting for phosphorylated tyrosine (pY). In sham-operated cortical lysates, Fyn OE had higher NR2A and NR2B tyrosine phosphorylation than WT (Figs. 4A–D). In WT animals, NR2A tyrosine phosphorylation peaked at 15 min after HI and declined thereafter (Figs. 4A, B), while in Fyn OE brains, it remained at higher levels than the WT through 24 h. NR2B tyrosine phosphorylation was higher in Fyn OE animals relative to WT at all time points examined (Figs. 4C, D).
Fig. 4.
Fyn-mediated tyrosine phosphorylation of NR2A and NR2B following neonatal HI. NR2A (A) or NR2B (C) was immunoprecipitated from sham and HI-injured cortical lysates and blotted with anti-phosphotyrosine (pY). The membranes were stripped and re-probed with NR2A (A) or NR2B (B) antibody. Tyrosine phosphorylation of NR2A or NR2B was expressed as the OD ratio of pY to NR2A (B) or to NR2B (D). Data was normalized to WT sham values. Representative data for n=6 experiments. *p<0.05.
Fyn-mediated tyrosine phosphorylation of NR2B
Next, we examined NR2B tyrosine phosphorylation sites regulated by Fyn (Nakazawa et al., 2001; Prybylowski et al., 2005; Wu et al., 2007). We measured the protein expression of NR2B phosphorylated at tyrosine 1472, 1336 and 1252 in synaptic membranes. We found significant increases in pY1472, and pY1252 for up to 6 h after injury in WT mice (Figs. 5A, C, D). Fyn OE had significantly more pY1472 in synaptic fractions in sham-operated animals, 15 min and 1 h after injury (Figs. 5A, C). pY1252 NR2B was higher in Fyn OE compared to WT at 1 h and 24 h (Figs. 5A, D). We did not observe changes and differences in pY1336NR2B expression in the synapses after HI, nor the 115 kDa calpain cleavage fragment generated by pY1336 NR2B (data not shown) (Wu et al., 2007).
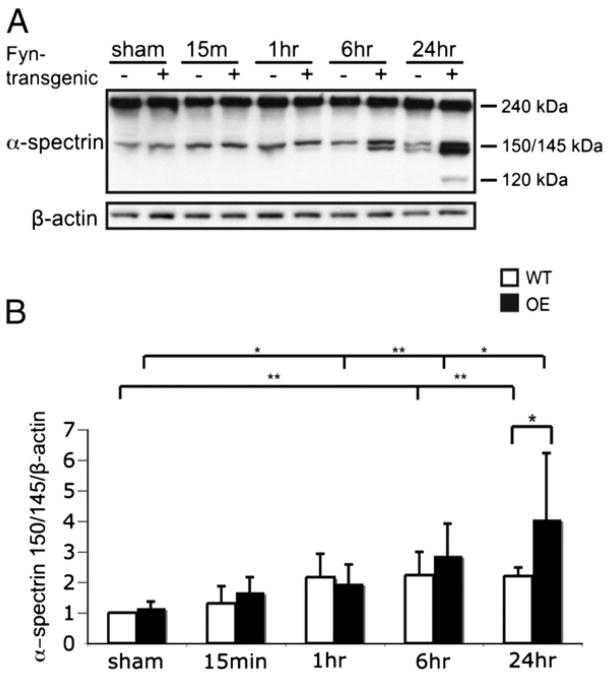
Fyn OE mice have elevated calpain activity after HI
We examined the activity of calcium-activated proteases calpain and caspase implicated in necrotic and apoptotic cell death (Nath et al., 1996). Calpain cleavage of α-spectrin produces a 150 and 145 kDa fragment, also known as spectrin breakdown product (SBDP) 150 and SBDP145, which is evident in cells undergoing necrosis and apoptosis (Nath et al., 1996; Wang, 2000). Caspase cleavage of α-spectrin produces a 120 kDa fragment (SBDP120) which is present in apoptotic cells (Nath et al., 1996, 1998). WT mice had elevated SBDP150/1456 h and 24 h after injury (Figs. 6A, B). Fyn OE mice had elevated SBDP150/145 1 h, 6 h, and 24 h after injury, with significantly higher SBDP150/145 at 24 h compared to WT mice (Figs. 6A, B). There were no differences in SBDP120 at the time points investigated (data not shown).
Fig. 6.
Fyn OE mice have elevated calpain activity in response to neonatal HI. A) Western blotting was carried out for α-spectrin and β-actin in sham animals and after HI. B) Expression of α-spectrin 150/145 kDa cleavage product was normalized to β-actin and then to WT sham values. Representative data for n=6 experiments. *p<0.05, **p<0.01.
Discussion
We find that neuronal Fyn overexpression leads to increased mortality and brain injury in response to neonatal HI. Fyn overexpression is associated with elevated and sustained NR2A and NR2B tyrosine phosphorylation. There is also enhanced NR2B phosphorylation at Y1472 and Y1252 in synaptic membranes in Fyn OE brain. These early changes in NMDAR tyrosine phosphorylation correlate with elevated calpain activity at 24 h following HI. Taken together, our results suggest that Fyn is involved in HI brain injury and Fyn mediated-NMDAR tyrosine phosphorylation may influence neuronal cell death after neonatal HI.
Our results may underestimate the effect of neuronal Fyn overexpression due to the high mortality in Fyn OE mice following HI. The mice died during the 40 min of hypoxia, rather than at any point of reperfusion. It is unclear why Fyn overexpression leads to acute death, however previous studies indicate that mice overexpressing constitutively active Fyn were prone to premature death due to seizures and also had higher seizure activity upon pharmacological or electrical stimulation (Kojima et al., 1998). It is possible that mice with high levels of Fyn kinase activity are more susceptible to seizures and mortality during HI. A novel video EEG methodology has been established recently to monitor and quantify seizures in the Vannucci neonatal HI model (Cuaycong et al., 2011), it would be helpful to use this tool to study seizure responses during HI in Fyn OE mice.
SFKs are activated in the synaptic membranes following neonatal HI despite the fact that the protein levels of Fyn did not change, consistent with our previous findings (Jiang et al., 2008). At baseline (in sham animals), Fyn protein expression was higher in Fyn OE mice, which correlates with elevated NR2A and NR2B tyrosine phosphorylation and NR2B phosphorylation at tyrosine (Y) 1472. This is in line with the findings in the adult Fyn overexpressing mice (Kojima et al., 1998). In response to HI injury, NR2A tyrosine phosphorylation peaked at 15 min in WT brains and declined thereafter, while in Fyn OE mice, it remained at the levels higher than the WT brain. Similarly, the total tyrosine phosphorylation of NR2B remained elevated in the Fyn OE than in the WT mice, although the differences were not statistically significant. These results suggest that Fyn leads to phosphorylation of both NR2A and NR2B during HI. One study found that phosphorylation of the NR2B C-terminal domain (CTD) by SFKs leads to a more open conformation of the CTD in vitro (Choi et al., 2011). Surprisingly, we did not see association of Fyn with NR2A or NR2B at the time points examined (data not shown), Fyn may transiently associate with the NMDAR and cause prolonged phosphorylation by increasing the accessibility of the CTD to other kinases. Additionally, SFK activity is elevated in synaptic membranes and could lead to elevated NMDAR phosphorylation by activating another tyrosine kinase in the PSD.
In this study, we provide the first description of three NR2B phosphorylation sites regulated by Fyn in response to neonatal HI. In WT animals, we found increased phosphorylation of tyrosine (pY) 1472 and 1252 NR2B, but not pY1336 NR2B, for up to 6 h after HI, coinciding with elevated Src kinase activity in the synapse. Consistent with a previous report, pY1472 is increased up to 1 h after neonatal HI in rats (Gurd et al., 1999). Neuronal Fyn overexpression further enhanced NR2B phosphorylation at this site. Y1472 is associated with surface expression of NR1/NR2B and synaptic enrichment of the receptor (Goebel-Goody et al., 2009; Nakazawa et al., 2006; Prybylowski et al., 2005). In neonatal HI, pY1472 may contribute to excitotoxic cell death by maintaining synaptic expression of the NMDAR and increasing NMDAR responses to glutamate. In addition, pY1472 may alter the protein composition of the NMDAR complexes after HI and affect downstream signaling pathways leading to cell death/surviving (Nakazawa et al., 2006).
Wu et al. (2007) found that phosphorylation of Y1336 by Fyn promotes calpain cleavage of NR2B in an in vitro glutamate toxicity model. We did not observe the calpain cleavage fragment generated by pY1336 after HI. pY1336 is also reported to mediate the interaction of NR2B with phosphatidylinositol 3-kinase (PI3K), which is increased after transient ischemia in the adult rats (Hisatsune et al., 1999; Takagi et al., 2003; Waxman and Lynch, 2005). Our results showed that pY1336 NR2B is not affected by Fyn overexpression. In fact, Fyn preferentially phosphorylates NR2B at Y1472 and Y1252 in the neonatal cortex, since pY1472 NR2B and pY1252 NR2B are absent in Fyn knock out brain (data not shown). Additionally, pY1472NR2B and pY1252NR2B were up-regulated in synaptic membranes in Fyn OE mice where SFK activity was also increased. pY1336 NR2B is the main NR2B form localized at extrasynaptic membranes at P7 (Jiang et al., 2011) suggesting that it may have different, or maybe opposite, functional role from the other phosphorylation site. These three sites may also coordinate with each other or with sites targeted by other kinase/phosphatases to regulate NMDAR trafficking and localization. Further biochemical studies are required to determine the functional consequences of Fyn-mediated NR2B phosphorylation at specific sites following neonatal HI.
Although Fyn has been implicated in the apoptosis pathway (Atkinson et al., 1996; Du, et al., 2012), there was no increase in caspase-3 cleavage or caspase activity as assessed by α-spectrin cleavage in Fyn OE mice relative to WT. Our data suggests that Fyn increases calpain activity which functions in necrotic and apoptotic cell death. Calpain activity may be elevated in Fyn OE mice due to increased calcium signaling via NMDAR-dependent and independent pathways (Wang, 2000). Calpain is activated downstream of NR2B in the hippocampus during traumatic mechanical injury (DeRidder et al., 2006). Fyn may lead to increased calpain activity by upregulating NR2B receptor activity via tyrosine phosphorylation. Changes in calpain activity are indicative of calcium dysregulation and can also occur independent of calcium flux from the NMDAR (Vanderklish and Bahr, 2000).
In the brain, Fyn has many substrates and is involved in complex signal transduction pathways in a variety of cell types and at different developmental stages. Besides enhancing NMDAR function, Fyn also regulates AMPA receptors (Fox et al., 2007; Hayashi and Huganir, 2004; Rong et al., 2001) and GABAergic synaptic neurotransmission (Boehm et al., 2004; Jurd et al., 2010), balancing neuronal excitation/inhibition. Multiple factors could be responsible for its contribution to neuronal cell death after neonatal HI. Further investigation is needed to determine whether Fyn-mediated NMDAR tyrosine phosphorylation is directly linked to brain injury following neonatal HI.
In conclusion, we report that neuronal Fyn overexpression is associated with increased brain injury and mortality, enhanced NR2A and NR2B tyrosine phosphorylation, as well as elevated calpain activity following neonatal HI. Our results implicate Fyn and Fyn-mediated NR2B phosphorylation in the pathogenesis of HI brain injury. NMDAR and Src family kinases are critical for brain development and also mediate pro-survival signaling, therefore, it's important to selectively uncouple these molecules from harmful consequences while preserving their beneficial machinery.
Acknowledgments
This work was funded by NINDS F31 NS073145 to Renatta Knox; NINDS R21 NS059613 to Xiangning Jiang and NINDS RO1 NS33997 to Donna Ferriero.
References
- Atkinson EA, et al. A physical interaction between the cell death protein Fas and the tyrosine kinase p59fynT. J Biol Chem. 1996;271:5968–5971. doi: 10.1074/jbc.271.11.5968. [DOI] [PubMed] [Google Scholar]
- Boehm SL, et al. Deletion of the fyn-kinase gene alters sensitivity to GABAergic drugs: dependence on beta2/beta3 GABAA receptor subunits. J Pharmacol Exp Ther. 2004;309:1154–1159. doi: 10.1124/jpet.103.064444. [DOI] [PubMed] [Google Scholar]
- Choi DW. Ionic dependence of glutamate neurotoxicity. J Neurosci. 1987;7:369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi UB, et al. Effect of Src kinase phosphorylation on disordered C-terminal domain of N-methyl-d-aspartic acid (NMDA) receptor subunit GluN2B protein. J Biol Chem. 2011;286:29904–29912. doi: 10.1074/jbc.M111.258897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuaycong M, et al. A novel approach to the study of hypoxia–ischemia-induced clinical and subclinical seizures in the neonatal rat. Dev Neurosci. 2011;33:241–250. doi: 10.1159/000331646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRidder MN, et al. Traumatic mechanical injury to the hippocampus in vitro causes regional caspase-3 and calpain activation that is influenced by NMDA receptor subunit composition. Neurobiol Dis. 2006;22:165–176. doi: 10.1016/j.nbd.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Du CP, et al. Fyn kinases play a critical role in neuronal apoptosis induced by oxygen and glucose deprivation or amyloid-β peptide treatment. CNS Neurosci Ther. 2012;18:754–761. doi: 10.1111/j.1755-5949.2012.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- Ferriero DM, et al. Selective destruction of nitric oxide synthase neurons with quisqualate reduces damage after hypoxia–ischemia in the neonatal rat. Pediatr Res. 1995;38:912–918. doi: 10.1203/00006450-199512000-00014. [DOI] [PubMed] [Google Scholar]
- Fox CJ, et al. Tyrosine phosphorylation of the GluR2 subunit is required for long-term depression of synaptic efficacy in young animals in vivo. Hippocampus. 2007;17:600–605. doi: 10.1002/hipo.20302. [DOI] [PubMed] [Google Scholar]
- Goebel-Goody SM, et al. Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience. 2009;158:1446–1459. doi: 10.1016/j.neuroscience.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Gurd JW, et al. Hypoxia–ischemia in perinatal rat brain induces the formation of a low molecular weight isoform of striatal enriched tyrosine phosphatase (STEP) J Neurochem. 1999;73:1990–1994. [PubMed] [Google Scholar]
- Hayashi T, Huganir RL. Tyrosine phosphorylation and regulation of the AMPA receptor by SRC family tyrosine kinases. J Neurosci. 2004;24:6152–6160. doi: 10.1523/JNEUROSCI.0799-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisatsune C, et al. Phosphorylation-dependent interaction of the N-methyl-d-aspartate receptor epsilon 2 subunit with phosphatidylinositol 3-kinase. Genes Cells. 1999;4:657–666. doi: 10.1046/j.1365-2443.1999.00287.x. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Jiang X, et al. Neonatal hypoxia–ischemia differentially upregulates MAGUKs and associated proteins in PSD-93-deficient mouse brain. Stroke. 2003;34:2958–2963. doi: 10.1161/01.STR.0000102560.78524.9D. [DOI] [PubMed] [Google Scholar]
- Jiang X, et al. Activated Src kinases interact with the N-methyl-d-aspartate receptor after neonatal brain ischemia. Ann Neurol. 2008;63:632–641. doi: 10.1002/ana.21365. [DOI] [PubMed] [Google Scholar]
- Jiang X, et al. Developmental localization of NMDA receptors, Src and MAP kinases in mouse brain. Neurosci Lett. 2011;503:215–219. doi: 10.1016/j.neulet.2011.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurd R, et al. Fyn kinase contributes to tyrosine phosphorylation of the GABA(A) receptor gamma2 subunit. Mol Cell Neurosci. 2010;44:129–134. doi: 10.1016/j.mcn.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhr G, Seeburg PH. Subtype-specific regulation of recombinant NMDA receptor-channels by protein tyrosine kinases of the src family. J Physiol (Lond) 1996;492:445–452. doi: 10.1113/jphysiol.1996.sp021320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima N, et al. Rescuing impairment of long-term potentiation in fyn-deficient mice by introducing Fyn transgene. Proc Natl Acad Sci U S A. 1997;29:4761–4765. doi: 10.1073/pnas.94.9.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima N, et al. Higher seizure susceptibility and enhanced tyrosine phosphorylation of N-methyl-d-aspartate receptor subunit 2B in fyn transgenic mice. Learn Mem. 1998;5:429–445. [PMC free article] [PubMed] [Google Scholar]
- Lu YF, et al. Enhanced synaptic transmission and reduced threshold for LTP induction in fyn-transgenic mice. Eur J Neurosci. 1999;11:75–82. doi: 10.1046/j.1460-9568.1999.00407.x. [DOI] [PubMed] [Google Scholar]
- McDonald JW, et al. MK-801 protects the neonatal brain from hypoxic–ischemic damage. Eur J Pharmacol. 1987;140:359–361. doi: 10.1016/0014-2999(87)90295-0. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, et al. Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-d-aspartate receptor. J Biol Chem. 2001;276:693–699. doi: 10.1074/jbc.M008085200. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, et al. NR2B tyrosine phosphorylation modulates fear learning as well as amygdaloid synaptic plasticity. EMBO J. 2006;25:2867–2877. doi: 10.1038/sj.emboj.7601156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath R, et al. Non-erythroid alpha-spectrin breakdown by calpain and interleukin 1 beta-converting-enzyme-like protease(s) in apoptotic cells: contributory roles of both protease families in neuronal apoptosis. Biochem J. 1996;319:683–690. doi: 10.1042/bj3190683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath R, et al. Evidence for activation of caspase-3-like protease in excitotoxin- and hypoxia/hypoglycemia-injured neurons. J Neurochem. 1998;71:186–195. doi: 10.1046/j.1471-4159.1998.71010186.x. [DOI] [PubMed] [Google Scholar]
- Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164:719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- Olney JW, et al. Cytotoxic effects of acidic and sulphur containing amino acids on the infant mouse central nervous system. Exp Brain Res. 1971;14:61–76. doi: 10.1007/BF00234911. [DOI] [PubMed] [Google Scholar]
- Prybylowski K, et al. The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron. 2005;47:845–857. doi: 10.1016/j.neuron.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JE, et al. The influence of immaturity on hypoxic–ischemic brain damage in the rat. Ann Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- Roche KW, et al. Molecular determinants of NMDA receptor internalization. Nat Neurosci. 2001;4:794–802. doi: 10.1038/90498. [DOI] [PubMed] [Google Scholar]
- Rong Y, et al. Tyrosine phosphorylation of ionotropic glutamate receptors by Fyn or Src differentially modulates their susceptibility to calpain and enhances their binding to spectrin and PSD-95. J Neurochem. 2001;79:382–390. doi: 10.1046/j.1471-4159.2001.00565.x. [DOI] [PubMed] [Google Scholar]
- Sheldon RA, et al. Strain-related brain injury in neonatal mice subjected to hypoxia–ischemia. Brain Res. 1998;810:114–122. doi: 10.1016/s0006-8993(98)00892-0. [DOI] [PubMed] [Google Scholar]
- Takagi N, et al. The effect of transient global ischemia on the interaction of Src and Fyn with the N-methyl-D-aspartate receptor and postsynaptic densities: possible involvement of Src homology 2 domains. J Cereb Blood Flow Metab. 1999;19:880–888. doi: 10.1097/00004647-199908000-00007. [DOI] [PubMed] [Google Scholar]
- Takagi N, et al. Transient ischemia enhances tyrosine phosphorylation and binding of the NMDA receptor to the Src homology 2 domain of phosphatidylinositol 3-kinase in the rat hippocampus. J Neurochem. 2003;84:67–76. doi: 10.1046/j.1471-4159.2003.01500.x. [DOI] [PubMed] [Google Scholar]
- Vanderklish PW, Bahr BA. The pathogenic activation of calpain: a marker and mediator of cellular toxicity and disease states. Int J Exp Pathol. 2000;81:323–339. doi: 10.1111/j.1365-2613.2000.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vexler ZS, Ferriero DM. Molecular and biochemical mechanisms of perinatal brain injury. Semin Neonatol. 2001;6:99–108. doi: 10.1053/siny.2001.0041. [DOI] [PubMed] [Google Scholar]
- Wang K. Calpain and caspase: can you tell the difference? Trends Neurosci. 2000;23:20–26. doi: 10.1016/s0166-2236(99)01536-2. [DOI] [PubMed] [Google Scholar]
- Waxman EA, Lynch DR. N-methyl-d-aspartate receptor subtype mediated bidirectional control of p38 mitogen-activated protein kinase. J Biol Chem. 2005;280:29322–29333. doi: 10.1074/jbc.M502080200. [DOI] [PubMed] [Google Scholar]
- Wu H, et al. Fyn-mediated phosphorylation of NR2B Tyr-1336 controls calpain-mediated NR2B cleavage in neurons and heterologous systems. J Biol Chem. 2007;282:20075–20087. doi: 10.1074/jbc.M700624200. [DOI] [PMC free article] [PubMed] [Google Scholar]