Abstract
Objective
To determine common and distinctive brain activation patterns associated with encoding and recognition of face-name associations and identify the neural structures with BOLD amplitude differences specific to binding and memory consolidation processes.
Methods
Five healthy adult participants viewed face-name pairs during the encoding phase and completed the multiple choice recognition memory task after a brief delay. BOLD response amplitudes in specific regions of interest and whole brain activation maps were analyzed.
Results
Common activations were observed in encoding and recognition memory tasks in several ROI encompassing the medial temporal and occipital regions. Higher amplitudes occurred in right fusiform gyrus and right hippocampus during encoding. In contrast, higher BOLD response amplitudes were detected in the lingual gyrus bilaterally during recognition memory. Encoding activated distributed prefrontal and temporal cortical regions bilaterally that spanned attentional, executive, language, and memory systems. Recognition memory recruited convergence zones in the left prefrontal cortex and the parietal-occipital-temporal region bilaterally where multimodal visual association, language, memory and decision-making systems interact.
Conclusions
The higher right fusiform gyrus and right hippocampus activation during encoding suggests a potentially specific binding pathway. The increased lingual gyrus BOLD response during recognition memory may indicate a neural substrate for memory consolidation and long-term knowledge. Average activation maps revealed task-specific differences in areas of the prefrontal, temporal, and occipital-parietal-temporal cortices. Findings suggest that lesions in fairly widespread cerebral regions may potentially disrupt specific binding or memory consolidation processes.
Keywords: fMRI, encoding, memory retrieval, face name processing
Introduction
Since reliable fMRI methods have been established, brain activations associated with memory and cognitive processes have been investigated in order to identify both the anatomical and physiological substrate for these complex adaptive behaviors. Contemporary fMRI research is focused in part on undertaking a more in depth investigation of brain-behavior relationships than may be possible with clinical lesion studies. This can include contrasts according to age, gender, and multiple task parameters as well as localized anatomical and networking analyses (Miezin et al., 2000). In this study, we utilized fMRI to investigate brain activation patterns and BOLD response differences of anatomical regions specific to associative learning and memory of an essential set of stimuli for everyday functioning, namely face- name pairs. Such memory complaints are common in clinical conditions of traumatic brain injury, Alzheimer's disease, and major depression. Face- name learning and memory are also important areas of research for understanding social cognition (Haxby et al., 2000), aging-associated cognitive changes, and face-specific processing disorders such as prospoagnosia (Marotta et al., 2001). However, the neural substrate for face-name learning is minimally understood.
In extant literature, several studies have focused on brain activations related to viewing faces only (Allison et al., 1994, Clarke et al., 1997), face processing vs. non-face objects (Haxby et al., 1991, Chao et al., 1999, Duchaine and Nakayama, 2005), faces associated with emotions (Sprengelmeyer et al., 1998, Blair et al., 1999, Kilts et al., 2003), scrambled or inverted faces (Clark et al., 1998, Collishaw and Hole, 2000), and brain activations during viewing of famous and/or familiar faces (Leube et al., 2003; Leveroni et al., 2000, Bernard et al., 2004). A smaller set of studies have investigated learning of face-name pairs with widely different cognitive, electrophysiological and brain imaging methods (Joassin et al., 2004; Kirwan & Stark, 2004, Naveh-Benjamin et al., 2004, Gimenez et al., 2005; Groninger, 2006; Tsukiura et al., 2006), but none have specifically analyzed functional brain activations and BOLD response amplitudes during both encoding and recognition of face-name pairs across multiple anatomical areas (i.e., medial temporal lobe and visual cortex. Although much research has contributed to the study of the brain's response to faces and to face-name pairing, there is still considerable ambiguity related to the neural substrate for such learning and memory. To our knowledge, there have been no region of interest (ROI) studies focused on specific neural structures in the medial temporal lobe and occipital lobe thought to mediate encoding and consolidation of face-name pairs, not fMRI studies to evaluate the BOLD response peak amplitude parameter, allowing comparison of ROI's and their level of involvement in each task. We were thus interested in analyzing face and name pairing involved in an encoding as well as retrieval task by using fMRI and comparing structures level of involvement in each task.
Based upon the clinical and basic neuroscience literatures regarding anatomical regions involved in learning and memory, we chose eight regions of interest that we suspected would be involved in the associative face-name encoding and retrieval tasks. These regions span visual association cortex, medial temporal lobe, and the prefrontal cortex, and include: 1) anterior fusiform, 2) posterior fusiform, 3) hippocampus, 4) lingual gyrus, 5) occiptital middle gyrus, 6) temporal inferior gyrus, 7) occipital inferior gyrus, and the 8) dorsolateral prefrontal cortex (PFC). Within each ROI, we were interested in analyzing the BOLD response peak amplitude and comparing the response differences between tasks as well as between ROI structures. Such knowledge may be important for understanding the structures involved in associative learning and memory as well as the similarity and distinctiveness of their roles in each task.
Based on previous findings (Andreasen et al., 1996, Eslinger et al., 1996, Verstichel, 2001, Henson et al., 2003, Kirwan and Stark, 2004) we predicted that of the eight ROI's that we chose, the structures of greatest interest in these tasks would be the posterior fusiform, the prefrontal cortex, and the hippocampus. The posterior fusiform gyrus, particularly on the right side (Kim, Andreasen et al. 1999, (Hoffman and Haxby, 2000, Takahashi and Kawamura, 2002) is very popular in the study of the brain's response to human faces. It is widely known as the “face area” of the brain. The hallmark symptom of unfortunate disease, known as prospoaganosia, is the inability to recognize faces and occurs due to damage to this face processing area (Bodamer, 1947). Because of the fusiform gyrus' evident role in face processing, one would suspect that the BOLD response to faces in this region would then be similar for both the tasks considering both tasks involve faces. Previous findings support that this structure is indeed involved in both tasks, but at different levels (Zeineh et al., 2003). Thus, we expected to find a higher BOLD response peak amplitude in the encoding task because of the novelty of the faces and the perceptual component involved and less activation in retrieval due to familiarity and other processes taking place in other structures (Rhodes and McLean, 1990, George et al., 1999). Recent studies also indicate this structure to be activated as an “expertise” effect or processing due to encoding of a unique individual (Gauthier et al., 2000). From this information, we predicted that there would be a significant BOLD response in the posterior fusiform (higher on the right side) in the encoding task vs. the retrieval task.
The hippocampus was the second major region of interest of study. Despite,the popular thought that the hippocampus is tied to memory recognition, recent studies (Clarke et al., 1997, Eichenbaum, 1997, Guo et al., 2005) show that it is more involved in making memories and associations, or as Cohen, Ryan et al. (Cohen et al., 1999) describes the hippocampus as a mediator of “memory binding.” In this study, participants were required to associate or “bind” a face with a name. We predicted that the hippocampus would be more highly involved in the encoding task and to a higher extent on the right side, as suggested by Kirwan and Stark (2004).
Lastly, we sought to analyze was the dorsolateral PFC. Many studies suggest that the PFC is highly involved in memory retrieval (Buckner et al., 1999, McDermott et al., 1999, Rugg et al., 1999, Konishi et al., 2000), particularly on the left side (Cansino et al., 2002). While the left PFC appears to be biased toward language processing (Puce et al., 1996, Cohen et al., 2000), it has also been implicated in the encoding of faces and names (Haxby et al., 1994, Summerfield et al., 2006). Combining these findings, we predicted that the left PFC would be have a higher BOLD response during encoding because of the name/word component as well as the highly associative demands of face-name pairing.
Materials and Methods
Study Participants
The fMRI protocol was conducted on 5 healthy, volunteer participants 20-50 years of age (4 male, 1 female). All subjects had at least a high school education.
fMRI Study Procedures
Preparation and Positioning
The present study was carried out on a 3.0 tesla Philips MRI scanner (Philips Medical Systems, the Netherlands). Subjects laid flat in a head restrainer which minimized the amount of motion as well as provided proper positioning and comfort. We used a boxcar paradigm, consisting of alternating intervals of baseline and cognitive activation. During scanning, subjects responded to visual stimulation by pressing the buttons on a fiber optic button box.
Image Acquisition
Data was acquired by the MRI scanner. A T1 –weighted three-dimensional image (3-D TFE) was acquired for anatomical structure with time of repetition (TR)/ echo time (TE)/ flip angle (FA)=8.05ms/ 3.7ms/ 8°, FOV=256 × 150 mm3, acquisition matrix=256 × 256×150, and a SENSE factor =2. Echo planar imaging (EPI) was used for data acquisition with TR=3600ms, TE=30ms, FA=90°, 28 axial slices, slick thickness=2.5 mm, FOV=210 × 210 mm3, acquisition matrix=80 × 80, reconstruction resolution=128 × 128, and SENSE factor=2 . Two tasks were administered in each imaging session. For each session, 64 images were acquired during 8 blocks of stimulation and baseline. Each subject underwent two runs.
Encoding and Memory Recognition Paradigms
Black-and-white faces with hair removed and names displayed below were viewed through a reflection mirror in front of the eyes of the subject. Images were presented using E-Prime software (Psychology Software Tools, Inc, Pittsburg, PA, USA). The first task was an encoding task where the subjects viewed 16 faces paired to an uncommon first name (8 males, 8 females). Each face-name pair was presented for 3.6s. After 4 face-name pairs were presented, the baseline, which was a screen with a plus, would be displayed for 14.4s. During the second task, which alternated with each 4 face-name encoding block, the subjects would view a face with a choice of four names. The subjects were required to recognize the face-name pair and press the corresponding button (one of four) on the control device. Each face and choice of four names was presented for 3.6 seconds. There were a total of 16 face (each with four names) stimuli, divided into 4 blocks. After each recognition block, there was a 14.4 s baseline. The total scan duration was 230.7s.
Data Analysis
The fMRI data collected during the tasks was processed with SPM2 software (Wellcome Department of Cognitive Neurology, London, UK) implemented in Matlab (Mathworks, Inc.). Functional images were re-aligned to remove any minor movements. The anatomical high resolution T1-weighted images were co-registered with fMRI images and spatially normalized to the Montreal Neurological Institute brain template. The time-course images were normalized using the same normalization parameters and then smoothed with a 6×6×8 mm3 Gaussian smoothing kernel. A statistic parametric map (SPM) was generated for encoding and retrieval tasks by fitting the stimulation paradigm to the functional data which was convolved with a hemodynamic response function. Activation from the baseline paradigm was subtracted from the stimulation paradigms, allowing us to see activation specific to encoding and retrieval processes. For group analysis, average activation maps were generated (one-sample t- test, p<.001). Group activation maps that subtracted the opposite task activation were generated by a paired t-test (p<.001). For BOLD response amplitude measurements (units measured in signal change percentage), each subject's time course was averaged to give a hemodynamic curve from which the height could be recorded. T-tests (two-sided) were used to compare encoding and retrieving stimulation within several regions of interests.
Results
Subjects performed with an average accuracy of 41.41% (SD±12.02%) in the retrieval of face-name pairs. The average response time for the tasks was 1828.91ms (SD±390.07ms).
Activation maps for the encoding and memory recognition tasks were analyzed with summary of activated clusters in Table 1. Across the 2 tasks, there were common, prominent activations detected in primary visual cortex and multiple secondary visual areas including extensive portions of Brodmann's areas 18 and 19 bilaterally (mainly in the inferior infracalcarine regions) but with notable extension to occipital-temporal regions (areas 37 and 20/21) as well as occipital-parietal and cuneus regions. There were also common hippocampal activations bilaterallyfor the 2 tasks. When encoding and memory recognition conditions were contrasted, more specific distinctions were observed (see Figure 1). Results indicated that during encoding , hippocampal regions were activated bilaterally, whereas during memory recognition, the most prominent activations occurred in the visual association areas.
Table 1.
Summary of Significant Activations Identified during Associative Encoding and Memory Recognition Tasks
| Brodmann area | Talairich coordinates | voxels (k) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Anatomical Region | BA | x | y | z | Encoding | Retrieval |
| Calcarine R | 17/18 | 12 | -88 | 8 | 498 | 492 |
| Fusiform L | 19/37 | -42 | -42 | -20 | 353 | 182 |
| Fusiform R | 19/37 | 41 | -43 | -20 | 325 | 311 |
| Occipital Mid L | 18/19 | -32 | -80 | 5 | 306 | 196 |
| Occiptal Mid R | 18/19 | 37 | -80 | -1 | 277 | 241 |
| Occipital Inf R | 17/18 | 38 | -88 | -5 | 260 | 116 |
| Occipital Inf L | 17/18 | -32 | -88 | -6 | 251 | 163 |
| Temporal Inf L | 20/21 | -53 | -57 | -12 | 250 | 154 |
| Lingual R | 18 | 19 | -63 | -11 | 240 | 404 |
| Calcarine L | 17/18 | -6 | -88 | -6 | 207 | 296 |
| Lingual L | 18 | -16 | -56 | -7 | 190 | 299 |
| Temporal Inf R | 20/21 | 50 | -52 | -18 | 188 | 74 |
| Occipital Sup R | 19 | 28 | -71 | 40 | 165 | 127 |
| Cuneus R | 18/19 | 11 | -87 | 20 | 109 | 158 |
| Occipital Sup L | 19 | -20 | -84 | 23 | 107 | 127 |
| Cuneus L | 18/19 | -9 | -86 | 24 | 88 | 129 |
| Anterior fusi R | 20 | 30 | -6 | -35 | 23 | 17 |
| Anterior fusi L | 20 | -30 | -4 | -35 | 10 | 13 |
| Hippocampus L | 28/35 | -31 | -18 | -16 | 15 | 10 |
| Hippocampus R | 28/35 | 22 | -13 | -16 | 18 | 21 |
| PFC R | 47 | -42 | 34 | 25 | 14 | 18 |
| PFC L | 47 | -50 | 20 | 21 | 12 | 14 |
Note: For the regions included under the encoding and retrieval regions heading, a one-sample t-test was used and an uncorrected p<.001. For the structures under the encoding regions only and retrieval regions only headings, a paired t-test was used and an uncorrected p<0.001.
Figure 1.
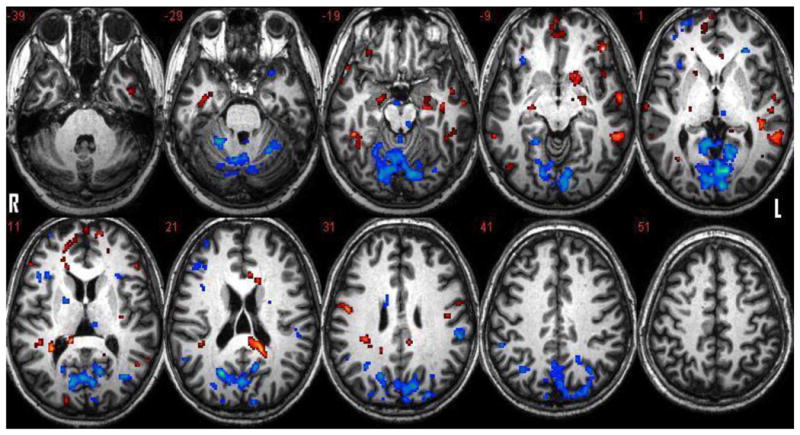
Activation clusters associated with encoding and memory recognition tasks. Red represents activations specific to encoding in contrast to memory recognition. Blue represents activations specific to memory recognition in contrast to encoding. (Level of axial slice noted by z coordinate). P =.005 with a 10 voxel threshold.
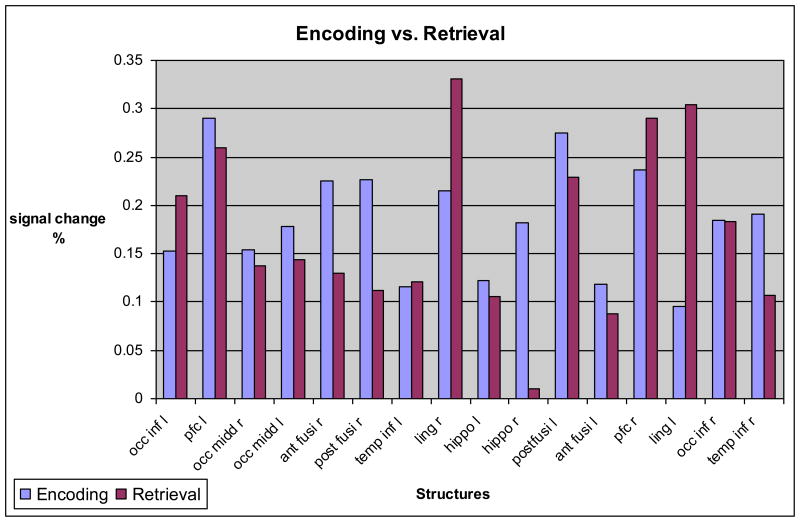
Location of the eight specific ROI are shown in Figure 2. We measured the BOLD response amplitude response for each task in each ROI and then compared the amplitude difference between encoding and memory recognition tasks in each structure. The results are shown in Figure 3. Specifically, in the encoding task, both the right posterior fusiform [E (encoding) A (amplitude) = 0.226, R (recognition) A (amplitude) = 0.112; p=0.027], and the right hippocampus (EA = 0.182, RA = 0.011, p.= 0.013) generated significantly higher BOLD response amplitudes than in the memory recognition task. The right anterior fusiform (EA = 0.225, RA = 0.130, p = 0.073) approached significance in respect to higher BOLD response amplitude during encoding vs. memory recognition. In the memory recognition task, the right lingual gyrus BOLD response (RA = 0.331, EA = 0.215, p = 0.07) also approached significance while the left lingual gyrus (RA =.304, EA = .095, p = 0.003) was significantly higher in comparison to the encoding task. We did not detect significant differences in BOLD response between encoding and recognition tasks in the remaining ROIs.
Figure 2.
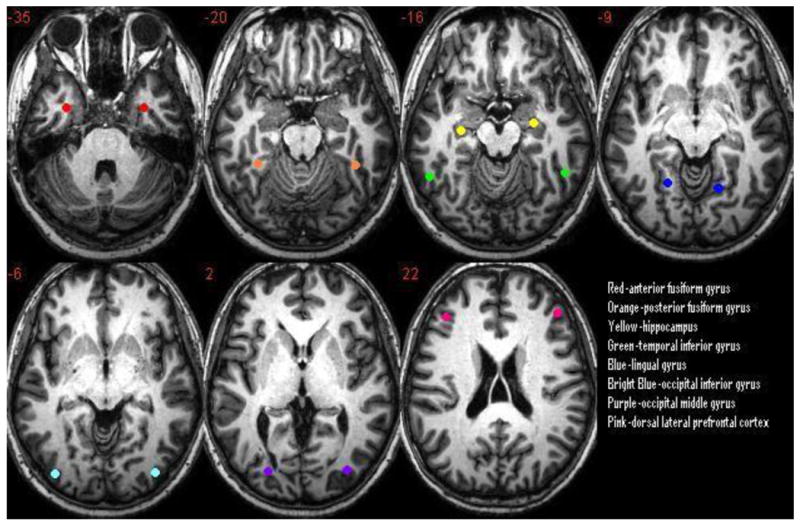
The eight regions of interest chosen for comparison of the BOLD response between encoding and memory recognition tasks.
Figure 3.
Structures that had a significant or an approaching significance difference in BOLD response between encoding and retrieval tasks. P<.001 used for posterior fusiform right (post fusi r), and lingual gyrus right and left (ling r and l). P<.001 used for anterior fusiform right (anti fusi r), and hippocampus right (hippo r).
With regard to large scale cerebral activation patterns for encoding and memory recognition, we were interested in identifying general trends in activation differences between the tasks. During encoding, there were much broader right frontal-temporal activations, whereas memory recognition recruited a broad expanse of posterior temporal-occipital-parietal cortices bilaterally and left prefrontal activity (see Figure 4 for 3-D representations).
Figure 4.
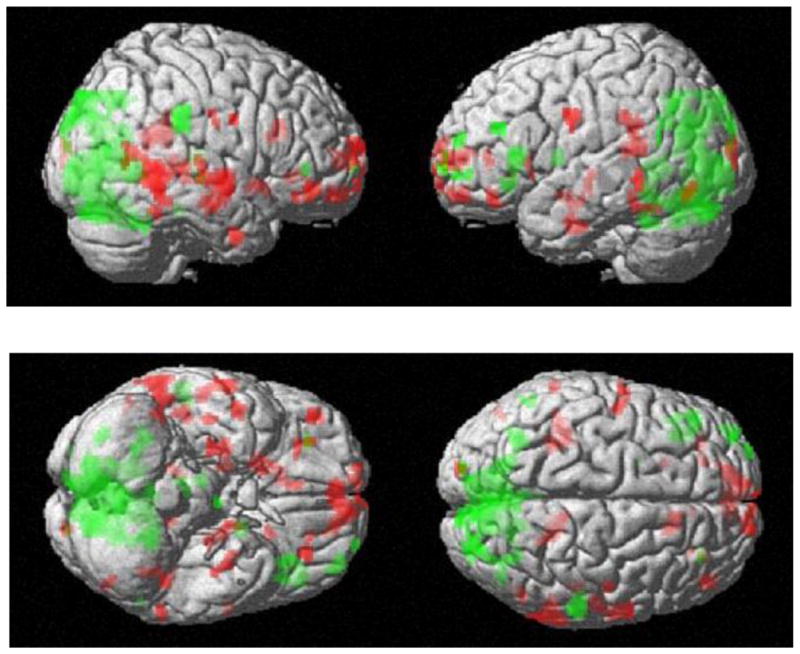
Average activation maps overlaid on a 3D- image of the brain. Red represents encoding activations. Green represents memory recognition activation. (p=.005 with a threshold of 10 voxels).
Discussion
Findings support the conclusion that there are clear and consistent brain activation differences related to associative encoding and memory recognition processes, suggesting somewhat distinct neural substrates. Associative encoding of face-name information aligned with significantly increased BOLD response amplitudes in the right posterior fusiform gyrus and right hippocampus in healthy adults. In contrast, BOLD response amplitudes were increased in the lingual gyrus during subsequent memory recognition. These findings suggest that the right hippocampus may be more involved in the information binding or associative mechanisms of face-name encoding, consistent with the observations of Kirwan and Stark (2004) as well as Cohen (1999), but also add an important extension to the neural substrate. That is, increased BOLD response amplitude was concurrently detected in the right posterior fusiform gyrus, a known face-processing area, suggesting that encoding may also depend upon linkages of the hippocampus with cortical processing areas. The right anterior fusiform region showed a trend toward a difference between tasks with a higher BOLD response during encoding, though we are uncertain whether this was due to its proximity to the hippocampal region and the networking of the neural structures involved in encoding.
Contrast of memory recognition to encoding identified a unique increase in the BOLD response amplitude in the lingual gyrus, which as a visual association area may be critical to long-term representation of acquired knowledge. Contrary to our predictions, the BOLD response amplitudes in the PFC were not significantly different between tasks or hemispheres, although the PFC had the highest overall BOLD response amplitude in both tasks compared to the other regions of interests, suggesting an important physiological role in associative processing.
We know that visual information at the cortical level is first processed through the primary visual cortex and then depending on its characteristics may engage either the dorsal/”where” and/or ventral “what” specialized streams. In this case of face-name stimuli, feed-forward pathways from inferior occipital cortex extend to more rostral occipital, temporal and parietal structures (Eacott and Gaffan, 1992; Shen et al., 1999; Haxby et al., 1999, Ishai et al., 1999). Our results support the role of these feed-forward pathways in face-name associative learning and recognition and suggest that pathophysiology from a variety of causes in these regions (e.g., stroke, anoxia, trauma, neoplasm) may have clinically significant disruptive effects on such learning and memory.
With regard to the neural substrate mediating associative face-name encoding, we propose that the right anterior and posterior fusiform regions together with the right hippocampus are key neural resources. Because of the attentional, associative and working memory demands of the task, the PFC is also likely to play a key role during learning (Kanwisher et al., 1997). The PFC is linked closely with the hippocampal region through strong interconnections (Grady et al., 1995, Sperling et al., 2003, Bernard et al., 2004) and likely contributes to how disparate information can be bound in sufficient fashion to be available for consolidation and long-term storage in association cortices (Squire et al., 1992).
Derived from our activation maps, we also found that structures specific for encoding included the superior and middle temporal gyri and the posterior cingulate gyrus in the left hemisphere. The middle temporal gyrus has been implicated in processing names in relation to faces (e.g. (Gorno-Tempini et al., 1998) and the superior temporal gyrus has been linked to social perception of facial characteristics, such as eye gaze and lip movement (Puce et al., 1998). The posterior cingulate gyrus has significance in that it seems to be a connecting pathway between anterior structures and the hippocampus (Goldman-Rakic et al., 1984).
With regard to the neural substrate related to recognition memory for face-name processing, although face stimuli must be processed by the “face structures” such as the fusiform, there is significantly less of a BOLD response than in the encoding task, suggesting that retrieval uses a different network. We detected significantly less activation in the right hippocampus as well, suggesting again that this may not as essential a part of the recognition network for face-name pairs. Our results, however, showed a significantly higher BOLD response amplitude in the bilateral lingual gyrus during recognition, indicating that this structure may mediate more of an important role than previously thought. In recognition, increased activity of the PFC may signal the initiation of a top-down cascade of regulatory processing that works to retrieve episodic visual associations such as face-name pairs from long-term memory (Haxby et al., 1996, Tomita et al., 1999, Joassin et al., 2004, Ranganath et al., 2004).
Recognition memory studies concerning face-name information have not been given as much attention as encoding studies, and therefore do not have overwhelming evidence supporting a specified network, although the medial frontal gyri, the inferior parietal lobe, supramarginal gyrus, and inferior frontal gyrus in the left hemisphere have been raised as possibilities (Gorno-Tempini et al., 1998, Campanella et al., 2001, Leube et al., 2003). We propose that the parietal lobe activations detected from the average activation maps may be of interest. These regions seem to act as convergence zones that are accessible to many cortical association areas including the PFC, and that can provide multimodal resources important for recognition and episodic retrieval (Yonelinas et al. 2005, Culham and Kanwisher 2001, Vincent 2007, Wagner et al. 2005). Taken together, the findings suggest that there are both key regions of processing in the hippocampal and visual association cortices that are necessary for associative encoding and recognition memory of face-name information, as well as processing resources provided by regions of the PFC, temporal and parietal cortices that are vital to effective learning and memory retention. With such a large-scale network, pathophysiology in the form of trauma, cerebrovascular disease, neurodegeneration, and neoplasm can cause symptoms that range from being clinically disabling (from damage to key structures) to milder deficits (from damage to resources areas) that can be managed with compensatory learning and memory strategies.
Abbreviations
- fMRI
functional MRI
- r
right
- l
left
- post fusi
posterior fusiform gyrus
- ant fusi
anterior fusiform gyrus
- hippo
hippocampus
- temp inf
temporal inferior gyrus
- lingual
lingual gyrus
- occ inf
occipital inferior gyrus
- occ midd
occipital middle gyrus
- PFC
dorsal lateral prefrontal cortex
References
- Allison T, Ginter H, McCarthy G, Nobre AC, Puce A, Luby M, Spencer DD. Face recognition in human extrastriate cortex. J Neurophysiol. 1994;71:821–825. doi: 10.1152/jn.1994.71.2.821. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O'Leary DS, Arndt S, Cizadlo T, Hurtig R, Rezai K, Watkins GL, Ponto LB, Hichwa RD. Neural substrates of facial recognition. J Neuropsychiatry Clin Neurosci. 1996;8:139–146. doi: 10.1176/jnp.8.2.139. [DOI] [PubMed] [Google Scholar]
- Bernard FA, Bullmore ET, Graham KS, Thompson SA, Hodges JR, Fletcher PC. The hippocampal region is involved in successful recognition of both remote and recent famous faces. Neuroimage. 2004;22:1704–1714. doi: 10.1016/j.neuroimage.2004.03.036. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122(Pt 5):883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Bodamer J. Die Prospo-agnosie. Arch Psychiatr and Nervenkr. 1947;179:6–53. doi: 10.1007/BF00352849. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Kelley WM, Petersen SE. Frontal cortex contributes to human memory formation. Nat Neurosci. 1999;2:311–314. doi: 10.1038/7221. [DOI] [PubMed] [Google Scholar]
- Campanella S, Joassin F, Rossion B, De Volder A, Bruyer R, Crommelinck M. Association of the distinct visual representations of faces and names: a PET activation study. Neuroimage. 2001;14:873–882. doi: 10.1006/nimg.2001.0877. [DOI] [PubMed] [Google Scholar]
- Cansino S, Maquet P, Dolan RJ, Rugg MD. Brain activity underlying encoding and retrieval of source memory. Cereb Cortex. 2002;12:1048–1056. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nat Neurosci. 1999;2:913–919. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- Clark VP, Maisog JM, Haxby JV. fMRI study of face perception and memory using random stimulus sequences. J Neurophysiol. 1998;79:3257–3265. doi: 10.1152/jn.1998.79.6.3257. [DOI] [PubMed] [Google Scholar]
- Clarke S, Lindemann A, Maeder P, Borruat FX, Assal G. Face recognition and postero-inferior hemispheric lesions. Neuropsychologia. 1997;35:1555–1563. doi: 10.1016/s0028-3932(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff MA, Michel F. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123(Pt 2):291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, Nash C. Hippocampal system and declarative (relational) memory: summarizing the data from functional neuroimaging studies. Hippocampus. 1999;9:83–98. doi: 10.1002/(SICI)1098-1063(1999)9:1<83::AID-HIPO9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Collishaw SM, Hole GJ. Featural and configurational processes in the recognition of faces of different familiarity. Perception. 2000;29:893–909. doi: 10.1068/p2949. [DOI] [PubMed] [Google Scholar]
- Culham J, Kanwisher N. Neuroimaging of cognitive functions in human parietal cortex. Current Opinion in Neurobiology. 2001;11:157–163. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- Dubois S, Rossion B, Schiltz C, Bodart JM, Michel C, Bruyer R, Crommelinck M. Effect of familiarity on the processing of human faces. Neuroimage. 1999;9:278–289. doi: 10.1006/nimg.1998.0409. [DOI] [PubMed] [Google Scholar]
- Duchaine B, Nakayama K. Dissociations of face and object recognition in developmental prosopagnosia. J Cogn Neurosci. 2005;17:249–261. doi: 10.1162/0898929053124857. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Gaffan D. Inferotemporal-frontal Disconnection: The Uncinate Fascicle and Visual Associative Learning in Monkeys. Eur J Neurosci. 1992;4:1320–1332. doi: 10.1111/j.1460-9568.1992.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. How does the brain organize memories? Science. 1997;277:330–332. doi: 10.1126/science.277.5324.330. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Easton A, Grattan LM, Van Hoesen GW. Distinctive forms of partial retrograde amnesia after asymmetric temporal lobe lesions: possible role of the occipitotemporal gyri in memory. Cereb Cortex. 1996;6:530–539. doi: 10.1093/cercor/6.3.530. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Moylan J, Skudlarski P, Gore JC, Anderson AW. The fusiform “face area” is part of a network that processes faces at the individual level. J Cogn Neurosci. 2000;12:495–504. doi: 10.1162/089892900562165. [DOI] [PubMed] [Google Scholar]
- George N, Dolan RJ, Fink GR, Baylis GC, Russell C, Driver J. Contrast polarity and face recognition in the human fusiform gyrus. Nat Neurosci. 1999;2:574–580. doi: 10.1038/9230. [DOI] [PubMed] [Google Scholar]
- Gimenez M, Junque C, Vendrell P, Caldu X, Narberhaus A, Bargallo N, Falcon C, Botet F, Mercader JM. Hippocampal functional magnetic resonance imaging during a face-name learning task in adolescents with antecedents of prematurity. Neuroimage. 2005;25:561–569. doi: 10.1016/j.neuroimage.2004.10.046. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12:719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Price CJ, Josephs O, Vandenberghe R, Cappa SF, Kapur N, Frackowiak RS. The neural systems sustaining face and proper-name processing. Brain. 1998;121(Pt 11):2103–2118. doi: 10.1093/brain/121.11.2103. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, Pietrini P, Schapiro MB, Haxby JV. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995;269:218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- Groninger L. Variables influencing memory over long periods of time for recently learned face-name pairs. Am J Psychol. 2006;119:175–191. [PubMed] [Google Scholar]
- Guo C, Voss JL, Paller KA. Electrophysiological correlates of forming memories for faces, names, and face-name associations. Brain Res Cogn Brain Res. 2005;22:153–164. doi: 10.1016/j.cogbrainres.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Grady CL, Horwitz B, Ungerleider LG, Mishkin M, Carson RE, Herscovitch P, Schapiro MB, Rapoport SI. Dissociation of object and spatial visual processing pathways in human extrastriate cortex. Proc Natl Acad Sci U S A. 1991;88:1621–1625. doi: 10.1073/pnas.88.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Horwitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL. The functional organization of human extrastriate cortex: a PET-rCBF study of selective attention to faces and locations. J Neurosci. 1994;14:6336–6353. doi: 10.1523/JNEUROSCI.14-11-06336.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Ungerleider LG, Clark VP, Schouten JL, Hoffman EA, Martin A. The effect of face inversion on activity in human neural systems for face and object perception. Neuron. 1999;22:189–199. doi: 10.1016/s0896-6273(00)80690-x. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Ungerleider LG, Horwitz B, Maisog JM, Rapoport SI, Grady CL. Face encoding and recognition in the human brain. Proc Natl Acad Sci U S A. 1996;93:922–927. doi: 10.1073/pnas.93.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Goshen-Gottstein Y, Ganel T, Otten LJ, Quayle A, Rugg MD. Electrophysiological and haemodynamic correlates of face perception, recognition and priming. Cereb Cortex. 2003;13:793–805. doi: 10.1093/cercor/13.7.793. [DOI] [PubMed] [Google Scholar]
- Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nat Neurosci. 2000;3:80–84. doi: 10.1038/71152. [DOI] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Martin A, Schouten JL, Haxby JV. Distributed representation of objects in the human ventral visual pathway. Proc Natl Acad Sci U S A. 1999;96:9379–9384. doi: 10.1073/pnas.96.16.9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joassin F, Campanella S, Debatisse D, Guerit JM, Bruyer R, Crommelinck M. The electrophysiological correlates sustaining the retrieval of face-name associations: an ERP study. Psychophysiology. 2004;41:625–635. doi: 10.1111/j.1469-8986.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Egan G, Gideon DA, Ely TD, Hoffman JM. Dissociable neural pathways are involved in the recognition of emotion in static and dynamic facial expressions. Neuroimage. 2003;18:156–168. doi: 10.1006/nimg.2002.1323. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Stark CE. Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus. 2004;14:919–930. doi: 10.1002/hipo.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Wheeler ME, Donaldson DI, Buckner RL. Neural correlates of episodic retrieval success. Neuroimage. 2000;12:276–286. doi: 10.1006/nimg.2000.0614. [DOI] [PubMed] [Google Scholar]
- Kuskowski MA, Pardo JV. The role of the fusiform gyrus in successful encoding of face stimuli. Neuroimage. 1999;9:599–610. doi: 10.1006/nimg.1999.0442. [DOI] [PubMed] [Google Scholar]
- Leube DT, Erb M, Grodd W, Bartels M, Kircher TT. Successful episodic memory retrieval of newly learned faces activates a left fronto-parietal network. Cogn Brain Res. 2003;18:97–101. doi: 10.1016/j.cogbrainres.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Leveroni CL, Seidenberg M, Mayer AR, Mead LA, Binder JR, Rao SM. Neural systems underlying the recognition of familiar and newly learned faces. J Neurosci. 2000;20:878–886. doi: 10.1523/JNEUROSCI.20-02-00878.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marotta JJ, Genovese CR, Behrmann M. A functional MRI study of face recognition in patients with prospoagnosia. Neuroreport. 12(8):1581–1587. doi: 10.1097/00001756-200106130-00014. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Buckner RL, Petersen SE, Kelley WM, Sanders AL. Set- and code-specific activation in frontal cortex: an fMRI study of encoding and retrieval of faces and words. J Cogn Neurosci. 1999;11:631–640. doi: 10.1162/089892999563698. [DOI] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Guez J, Kilb A, Reedy S. The associative memory deficit of older adults: further support using face-name associations. Psychol Aging. 2004;19:541–546. doi: 10.1037/0882-7974.19.3.541. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Asgari M, Gore JC, McCarthy G. Differential sensitivity of human visual cortex to faces, letterstrings, and textures: a functional magnetic resonance imaging study. J Neurosci. 1996;16:5205–5215. doi: 10.1523/JNEUROSCI.16-16-05205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A, Allison T, Bentin S, Gore JC, McCarthy G. Temporal cortex activation in humans viewing eye and mouth movements. J Neurosci. 1998;18:2188–2199. doi: 10.1523/JNEUROSCI.18-06-02188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Cohen MX, Dam C, D'Esposito M. Inferior temporal, prefrontal, and hippocampal contributions to visual working memory maintenance and associative memory retrieval. J Neurosci. 2004;24:3917–3925. doi: 10.1523/JNEUROSCI.5053-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes G, McLean IG. Distinctiveness and expertise effects with homogeneous stimuli: towards a model of configural coding. Perception. 1990;19:773–794. doi: 10.1068/p190773. [DOI] [PubMed] [Google Scholar]
- Rossion B, Caldara R, Seghier M, Schuller AM, Lazeyras F, Mayer E. A network of occipito-temporal face-sensitive areas besides the right middle fusiform gyrus is necessary for normal face processing. Brain. 2003;126:2381–2395. doi: 10.1093/brain/awg241. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Fletcher PC, Chua PM, Dolan RJ. The role of the prefrontal cortex in recognition memory and memory for source: an fMRI study. Neuroimage. 1999;10:520–529. doi: 10.1006/nimg.1999.0488. [DOI] [PubMed] [Google Scholar]
- Shen L, Hu X, Yacoub E, Ugurbil K. Neural correlates of visual form and visual spatial processing. Hum Brain Mapp. 1999;8:60–71. doi: 10.1002/(SICI)1097-0193(1999)8:1<60::AID-HBM5>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL, Albert M. Putting names to faces: successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage. 2003;20:1400–1410. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H. Neural structures associated with recognition of facial expressions of basic emotions. Proc Biol Sci. 1998;265:1927–1931. doi: 10.1098/rspb.1998.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Ojemann JG, Miezin FM, Petersen SE, Videen TO, Raichle ME. Activation of the hippocampus in normal humans: a functional anatomical study of memory. Proc Natl Acad Sci U S A. 1992;89:1837–1841. doi: 10.1073/pnas.89.5.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield C, Greene M, Wager T, Egner T, Hirsch J, Mangels J. Neocortical connectivity during episodic memory formation. PLoS Biol. 2006;4:e128. doi: 10.1371/journal.pbio.0040128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Kawamura M. Pure topographical disorientation--the anatomical basis of landmark agnosia. Cortex. 2002;38:717–725. doi: 10.1016/s0010-9452(08)70039-x. [DOI] [PubMed] [Google Scholar]
- Tomita H, Ohbayashi M, Nakahara K, Hasegawa I, Miyashita Y. Top-down signal from prefrontal cortex in executive control of memory retrieval. Nature. 1999;401:699–703. doi: 10.1038/44372. [DOI] [PubMed] [Google Scholar]
- Tsukiura T, Mochizuki-Kawai H, Fujii T. Dissociable roles of the bilateral anterior temporal lobe in face-name associations: an event-related fMRI study. Neuroimage. 2006;30:617–626. doi: 10.1016/j.neuroimage.2005.09.043. [DOI] [PubMed] [Google Scholar]
- Verstichel P. [Impaired recognition of faces: implicit recognition, feeling of familiarity, role of each hemisphere] Bull Acad Natl Med. 2001;185:537–549. discussion 550-533. [PubMed] [Google Scholar]
- Wagner A, Shannon B, Kahn I, Buckner R. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Yonelinas A, Otten L, Shaw K, Rugg M. Separating the brain regions involved in recollection and familiarity in recognition memory. Journal of Neuroscience. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299:577–580. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]