Abstract
As Parkinson’s disease appears to be a multifactoral disorder, the use of animal models to investigate combined effects of genetic and environmental risk factors are of great importance especially in the context of aging which is the single major risk factor for the disorder. Here, we assessed the combined effects of neonatal iron feeding and environmental paraquat exposure on age-related nigrostriatal degeneration in transgenic mice expressing the A53T familial mutant form of human α-synuclein within these neurons. We report here that A53T α-synuclein mice exhibit greater susceptibility to paraquat. Increased oral intake of iron in the neonatal period leads to a progressive age-related enhancement of dopaminergic neurodegeneration associated with paraquat neurotoxicity. Furthermore, neurodegeneration associated with these combined genetic and environmental risk factors could be attenuated by systemic treatment with the bioavailable antioxidant compound EUK-189. These data suggest that environmental factors previously identified as contributors to neurodegeneration associated with sporadic Parkinson’s disease may also be candidates for observed variations in symptoms and disease progression in monogenic forms and that this may mechanistically involve increased levels of oxidatively-induced post-translational nitration of α-synuclein.
Keywords: α-synuclein, dopaminergic neurons, iron, oxidative, paraquat, parkinsonism
α-Synuclein is highly enriched in pre-synaptic terminals particularly in the neocortex, hippocampus, striatum, thalamus, and cerebellum. Three missense mutations (A53T, A30P, and E46K) in the α-synuclein gene have been linked to autosomal dominant, early-onset forms of Parkinson’s disease (PD) along with duplications and triplications of the normal gene (Polymeropoulos et al. 1997; Kruger et al. 1998; Zarranz et al. 2004). α-Synuclein protein is present at high levels in Lewy bodies, intracytoplasmic inclusions that are characteristic of both sporadic and inherited forms of PD (Spillantini et al. 1997). Postmortem studies suggest that temporal patterns of α-synuclein-containing Lewy body accumulation within various brain regions track with disease progression and could contribute to both motor and non-motor features of the disorder (Braak et al. 2003). Recent gene-wide association studies have identified common gene polymorphisms in the α-synuclein gene as potential susceptibility factors for idiopathic PD (Simon-Sanchez et al. 2009). Epidemiological evidence from an extensive twin study indicates that idiopathic PD in individuals with an age of onset after the age of 50 is not explainable by strict genetic heritability (Tanner et al. 1999; Wirdefeldt et al. 2004; Fung et al. 2006). This along with variations in incidence of PD by geographic region suggests that sporadic late onset PD may be because of environmental factors perhaps in combination with genetic susceptibility (Lanska 1997; Liou et al. 1997). Indeed, pesticides, such as fumigants, fungicides, herbicides, insecticides, and rodenticides, represent one of the primary classes of environmental agents associated with PD (Dick 2006; Hatcher et al. 2008). Exposure to either agricultural chemicals or metals have both widely been postulated as potential environmental risk factors for the disease and this is supported by extensive epidemiological evidence demonstrating increased PD incidence in persons living in a rural environment, drinking well water, and experiencing occupational exposure to such agents (Hertzman et al. 1990; Jimenez-Jimenez et al. 1992; Semchuk et al. 1992; Hubble et al. 1993; Liou et al. 1997; Gorell et al. 1998; Stephenson 2000). The widely used herbicide 1,1′-dimethyl-4,4′-bipyridium (paraquat, PQ) has previously been shown not only to cross the blood-brain-barrier through the neutral amino acid transporter (McCormack and Di Monte 2003) but also to selectively damage the nigrostriatal dopaminergic system in mice (McCormack et al. 2002; Peng et al. 2004, 2005). Elevated substantia nigra (SN) iron levels have been reported to be associated with sporadic PD (Sofic et al. 1991; Riederer et al. 1992; Jellinger et al. 1993; Griffiths et al. 1999). The high concentration of iron within the SN may act to catalyze the conversion of hydrogen peroxide produced during breakdown of dopamine to highly reactive hydroxyl radicals resulting in increased oxidative damage in the region (Jellinger et al. 1993). Significantly, high levels of iron have been also found within Lewy bodies (Gaeta and Hider 2005). Previous data from our laboratory suggests that neonatal iron exposure may increase risk for the disorder and that this is exacerbated in the presence of PQ exposure likely via an oxidative event and with age (Kaur et al. 2007; Peng et al. 2007, 2009).
The potential role of variations in environmental exposures combined with genetic susceptibility factors in PD remains elusive, especially in the context of aging which is the single major risk factor for the disease. As PD is likely to be a multifactoral disorder, the use of in vivo models to explore the additive or synergistic effects of combined risk factors especially in the context of aging are likely to be of great importance in understanding disease etiology. This may apply not only to sporadic disease but also observed variations in symptoms and disease progression in monogenic forms of the disorder. To explore the effects of environmental risk factors on familial genetic mutations associated with the disease, we investigated the combined effects of neonatal iron feeding and environmental paraquat-induced parkinsonian symptoms in transgenic mice expressing mutant human A53T α-synuclein within dopaminergic neurons and the possible mechanisms involved in subsequent neurodegeneration.
Materials and methods
Materials
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), 1,1′-dimethyl-4,4′-bipyridium dichloride (paraquat), and carbonyl iron were from Sigma (St. Louis, MO, USA). Media and sera were obtained from Invitrogen, Carlsbad, CA, USA. Rabbit anti-3-nitrotyrosine polyclonal antibody was purchased from Molecular Probes (Eugene, OR, USA). Rabbit and sheep anti-tyrosine hydroxylase polyclonal antibodies were obtained from Chemicon (Temecula, CA, USA). Mouse anti-nitro-α/β-synuclein (Tyr39) monoclonal antibody was purchased from Millipore (Billerica, MA, USA). Osmotic minipumps (Alzet 2004) were from Alza Scientific Products (Mountain View, CA, USA). The salen manganese complex EUK-189 was a gift from Proteome Systems, Inc. (Woburn, MA, USA).
Cell culture
Generation of the stable A53T mutant α-synuclein expressing dopaminergic cell line 1RB3AN27 (N27) has been described elsewhere (Vali et al. 2008). Cells were grown in RPMI 1640 medium supplemented with 10% fetal calf serum (Invitrogen), 100 units/mL penicillin, and 100 µg/mL streptomycin. Cell viability was determined by MTT incorporation (Peng et al. 2004, 2005). DNA fragmentation was examined by terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeled (TUNEL) analysis with an in situ cell death detection kit (Roche Molecular Biochemicals, Indianapolis, IN, USA) according to the manufacturer’s instructions (Peng et al. 2004, 2005). 4′,6-Diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA, USA) was used to counter-stain nuclei. Control experiments were performed in which the primary antibody was omitted. No staining was observed under these conditions. Stained cells were counted in 10 randomly chosen microscopic fields (at least 500 cells). Data were expressed as the mean ± SEM of the percentage of total cells that showed positive staining. To evaluate the effect of the salen manganese complex EUK-189 on cell death, the compound was added 1 h prior to paraquat combined with iron.
Primary mesencephalic cultures
Primary mesencephalic cell cultures were prepared from gestation day 15 mouse embryos as described previously (Peng et al. 2004, 2005). Briefly, dissociated cells were seeded at 7 × 105 cells per well onto poly-d-lysine-coated 24-well culture plates. Cultures were maintained at 37°C in a humidified atmosphere containing 95% air and 5% carbon dioxide, in Neurobasal medium (Life Technologies, Gaithersburg, MD, USA) containing 2% B27 supplement, 2 mM glutamate, 100 units/mL penicillin, and 100 µg/mL streptomycin. After 4 days, one-half of the medium was replaced with fresh medium. Cells were grown an additional 3 days and then treated with 30 µM paraquat with or without 8 µM FeCl2 for 24 h. To evaluate the effect of the salen manganese complex EUK-189 on cell death, the compound was added 1 h prior to paraquat combined with iron.
Immunocytochemistry
Cultures were fixed with paraformaldehyde in phosphate-buffered saline and permeabilized with 0.3% Triton X-100 in phosphate-buffered saline as described previously (Peng et al. 2004, 2005). Briefly, cells were then incubated with rabbit polyclonal anti-tyrosine hydroxylase (TH) (1 : 100) in phosphate-buffered saline (PBS) containing 10% normal goat serum and 0.3% Triton X-100 overnight at 4°C. Cells were washed with PBS and incubated with fluorochrome-conjugated secondary antibody (Jackson Immuno-Research, West Grove, PA, USA; 1 : 200) for 2 h at 25°C. Nuclei were counter-stained with 4′,6-diamidino-2-phenylindole (Vector). Control experiments were performed in which the primary antibody was omitted. No staining was observed under these conditions. The number of tyrosine TH-positive neurons in mesencephalic cultures was determined as described previously (Peng et al. 2004, 2005).
Drug administration
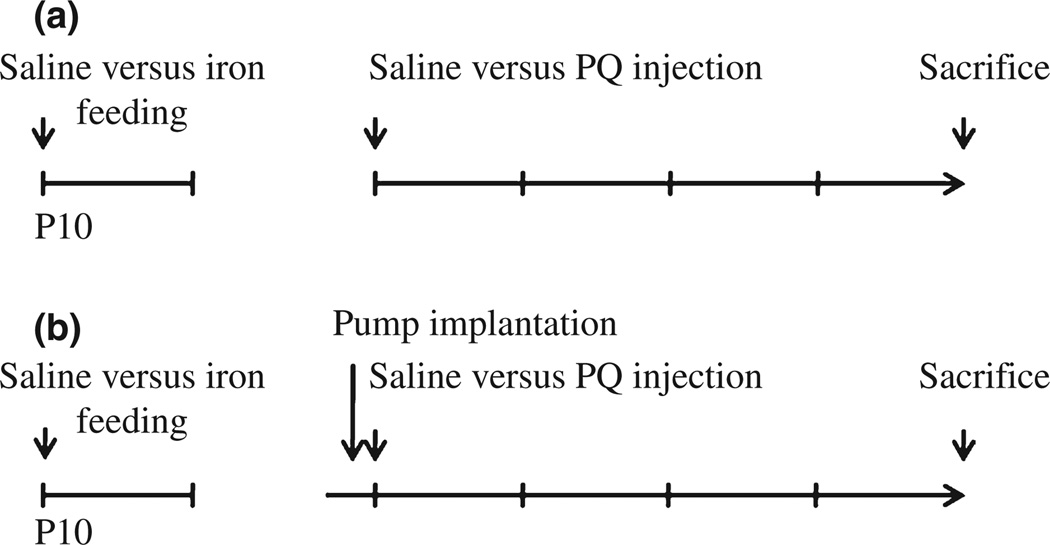
Generation of vector and human mutant α-synuclein A53T transgenic mice has been described elsewhere (Matsuoka et al. 2001). Carbonyl iron was administered to male mice from postnatal days 10 to 17 via oral gavage at a dosage of 120 mg/kg. Control animals were fed equal volumes of sterile saline (Kaur et al. 2007; Peng et al. 2007). Mice were aged to 2, 12, and 23 months of age and were intraperitoneally injected with saline or paraquat twice per week for 3 weeks. The dose of paraquat was 10, 9, and 8 mg/kg for 2-, 12-, and 23-month-old mice, respectively, because of the higher reported incidence of drug-induced death at later ages. Animals were killed at day 7 after final administration (Fig. 1a). Administration of the salen manganese complex EUK-189 was performed as previously described (Zhang et al. 2004; Peng et al. 2005, 2007). Briefly, mice were anesthetized with 4% isoflurane in 70% N2O/30% O2 and subcutaneously implanted with an osmotic minipump containing either 5% mannitol (as vehicle control) or 15 mM EUK-189 (dissolved in 5% mannitol). Pumps delivered EUK-189 at a rate of 0.25 µL/h for a 28-day period (Fig. 1b). Experimental protocols were in accordance with the National Institutes of Health Guidelines for Use of Live Animals and were approved by the Animal Care and Use Committee at the Buck Institute of Age Research.
Fig. 1.
Schematic of experimental paradigms. (a) Saline or carbonyl iron was administered to mice from postnatal days 10 to 17 via oral gavage. Mice were aged to 2, 12, and 23 months of age and were intraperitoneally injected with saline or paraquat twice per week for 3 weeks. Animals were killed at day 7 after final administration. (b) Pumps were implanted 3 days prior to paraquat treatment.
TH staining and stereology
Mice were fixed by perfusion as previously described (Peng et al. 2004). Cryostat-cut sections were taken through the entire midbrain. TH-positive neurons were immuno-labeled by incubating the tissue sections successively with a rabbit polyclonal anti-TH antibody (1 : 500) and biotinylated horse anti-rabbit IgG (1 : 200, Vector Laboratories) and following the staining procedure outlined by the manufacturers of the Vectastain ABC kit (Vector Laboratories) in combination with 3,3′-diaminobenzidine (DAB) reagents. TH-positive neuronal counts were performed by using a computer-assisted image analysis system consisting of a Zeiss Axioplan2 photomicroscope equipped with a Neurolucida stereo investigator (MicroBrightField, Williston, VT, USA). TH-positive neurons were counted in the SNpc of every third section throughout the entire extent of the substantia nigra pars compacta (SNpc). The total number of TH-positive neurons in the substantia nigra pars compacta was counted from four to five mice per group by using the optical fractionator method, an unbiased stereological technique of cell counting (West 1999) as previously described (Peng et al. 2004).
Quantitative analysis of double labeled neurons in SNpc
The accuracy of counting of double-labeled neurons was determined by analysis of systematically sampled candidate neurons from saline- or paraquat-treated brains. Sections were fixed with 4% paraformaldehyde in PBS, and incubated in blocking buffer (2% horse serum/0.2% Triton X-100/0.1% bovine serum albumin in PBS) for 1 h at 25°C as described previously (Peng et al. 2004, 2007). Primary antibodies used in this study were as following: sheep polyclonal anti-TH (1 : 500), rabbit polyclonal anti-3-nitrotyrosine (1 : 1000), and mouse monoclonal anti-nitro-α-synuclein (Tyr39) (1 : 200). Primary antibodies were added in blocking buffer and incubated with sections at 4°C overnight. The secondary antibodies were Alexa Fluro 488- or 594-conjugated donkey anti-sheep, anti-rabbit, or anti-mouse IgG (Molecular Probes; 1 : 200). Nuclei were counter-stained with DAPI using proLong Gold anti-fade reagent (Molecular Probes) and fluorescence signals were detected with an LSM 510 NLO Confocal Scanning System mounted on an Axiovert 200 inverted microscope (Carl Zeiss Ltd., Thornwood, NY, USA) equipped with a two-photon Chameleon laser (Coherent Inc., Santa Clara, CA, USA). Three-color images were scanned using Argon, 543 HeNe, and Chameleon (750–780 nm for DAPI) lasers. IMARIS (Bitplane AG, Saint Paul, MN, USA) imaging software was used for three-dimensional image reconstruction. Images were acquired using LSM 510 Imaging Software (Carl Zeiss Ltd). The specificity of each label was first verified using single-channel scans that were then merged into multiple-channel views. Neurons were considered double-labeled if co-labeling with relevant morphology was seen throughout the extent of the nucleus for nuclear markers or if a cytoplasmic marker surrounds a nuclear marker when viewed in x-y cross-section as well as in x-z and y-z cross-sections produced by orthogonal reconstructions from z-stacks taken at 400× magnification. TH single-labeled neurons and neurons double labeled for TH and 3-nitrotyrosine or nitro-α-synuclein (Tyr39) were recorded in three 50-µm sections per animal. Controls included omitting or pre-absorbing primary or omitting secondary antibodies.
Statistical analysis
All data are expressed as mean ± SEM for the number (n) of independent experiments performed. Differences among the means for all experiments described were analyzed using two-way analysis of variance. Newman–Keuls post-hoc analysis was employed when differences were observed by analysis of variance testing (p < 0.05).
Results
Paraquat neurotoxicity is exacerbated by iron and A53T mutant α-synuclein expression
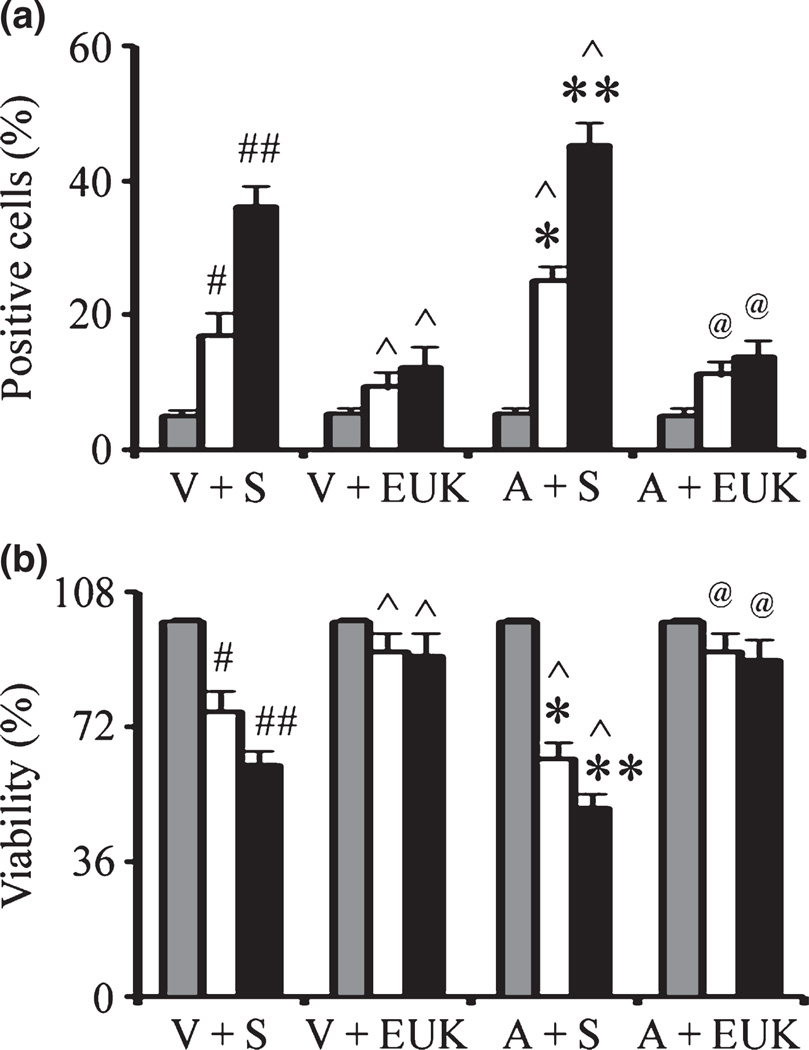
In the current study, we have utilized the stable rat dopaminergic cell line 1RB3AN27 (N27) expressing either empty vector or human mutant A53T α-synuclein. This cell line has been previously used by our laboratory to study the effects of α-synuclein expression in the absence and presence of proteasome inhibition on GSH metabolism as a model for PD (Vali et al. 2008). To study the potential neurotoxic synergism between paraquat and redox-active iron in relation to mutant familial α-synuclein expression, we treated cells with combined paraquat and FeCl2 and measured apoptotic DNA fragmentation by transferase-mediated dUTP nick end-labeled staining. As previously shown (Peng et al. 2007), although exposure to 80 µM FeCl2 was non-toxic on its own, FeCl2 at this concentration significantly increased vulnerability to neuronal toxicity induced by paraquat (Fig. 2a). This coincided with decreased cell viability via measurement of MTT reduction capacity. (Fig. 2b) as previously observed in cells treated with paraquat combination with FeCl2 (Peng et al. 2007). Significantly for the purposes of these current studies, expression of the familial A53T mutant of α-synuclein resulted in synergistic affects on N27 cell death (Fig. 2).
Fig. 2.
Mutant α-synuclein over-expression enhances the affects of combined iron-paraquat-induced apoptosis in a dopaminergic cell line. α-Synuclein transfected N27 cells were treated with 350 µM paraquat and 80 µM FeCl2 for 24 h. Cells were treated with EUK-189 1 h prior to the addition of FeCl2 and paraquat. (a) TUNEL-positive cells were measured at 24 h. (b) Cell viability was measured via the MTT assay and expressed as a percentage of viability in control at 24 h. Gray bars, saline; white bars, paraquat alone; and black bars, paraquat plus FeCl2. Mean ± SEM, n = 5. #p < 0.01, significantly different from vector plus saline plus saline; ##p < 0.05, significantly different from vector plus saline plus paraquat; ^p < 0.05, significantly different from matched vector plus saline; *p < 0.05, significantly different from A53T plus saline plus saline; **p < 0.05, significantly different from A53T plus saline plus paraquat; and @p < 0.01, significantly different from matched A53T plus saline. V + S, vector plus saline; V + EUK, vector plus EUK-189; A + S, A53T plus saline; A + E, A53T plus EUK-189.
We previously demonstrated that the salen manganese complexes (EUKs), synthetic superoxide dismutase and catalase mimetics, can reduce neonatal iron and paraquat-induced dopaminergic neuronal apoptosis (Peng et al. 2007, 2009). We next investigated whether EUK-189 was protective against exacerbated paraquat-induced neurotoxicity in the presence of FeCl2 and familial A53T α-synuclein expression. Cells were treated with the EUK-189 (40 µM) beginning 1 h before paraquat and/or FeCl2 co-treatment. As previously shown (Peng et al. 2007), pre-treatment with EUK-189 was efficacious in preventing additional apoptosis generated by iron treatment of paraquat-exposed cells (Fig. 2). It also protected cells against combined iron and A53T α-synuclein exacerbated paraquat-mediated neuronal toxicity (Fig. 2). EUK-189 alone at the concentrations used was neither stimulatory nor inhibitory in terms of neuronal survival (data not shown).
Effects of A53T α-synuclein expression on combined paraquat-iron neurotoxicity in primary mesencephalic cultures
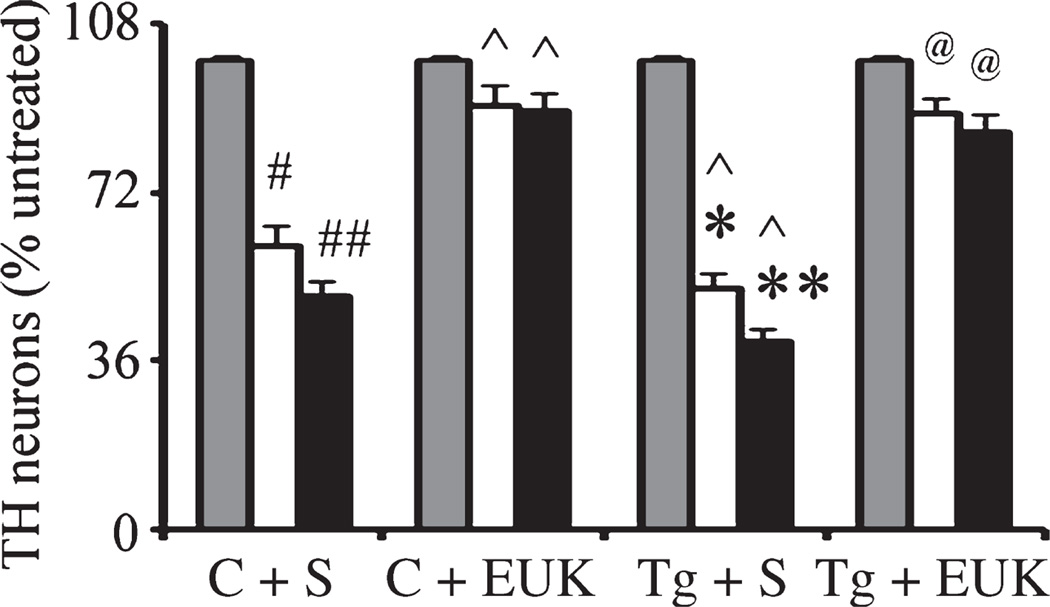
N27 is a transformed cell line that may have survival mechanisms that are absent in normal primary dopaminergic neurons. Therefore, to investigate further the effects of A53T α-synuclein expression on combined paraquat-iron neurotoxicity in midbrain dopaminergic neurons, we generated primary mesencephalic cultures from familial A53T α-synuclein expressing transgenic and wildtype littermate control mice. We counted the number of tyrosine hydroxylase (TH)-positive neurons in primary mesencephalic cultures via immunofluorescence with antibodies specific for TH coupled with 4′,6-diamidino-2-phenylindole staining. As previously shown (Peng et al. 2007), paraquat alone or combined paraquat and iron significantly induces TH-positive neuron loss. Furthermore, A53T α-synuclein expression resulted in synergistic affects on TH-positive neuronal loss (Fig. 3). Pre-treatment of cultures with EUK-189 protected cells from additional neuronal death induced by paraquat in combination with FeCl2 in the presence of A53T α-synuclein expression (Fig. 3).
Fig. 3.
Mutant α-synuclein over-expression enhances combined iron-paraquat-induced dopaminergic neuron death in primary mesencephalic cultures. Cultures from control or A53T over-expression mice were treated with 30 µM paraquat without or with 8 µM FeCl2. Cultures were treated with EUK-189 1 h prior to the addition of paraquat and FeCl2. TH-positive neuron counts in mesencephalic cultures 24 h after paraquat without or with FeCl2 treatment. Gray bars, saline; white bars, paraquat alone; and black bars, paraquat plus FeCl2. Mean ± SEM, n = 4. #p < 0.01, significantly different from control plus saline plus saline; ## p < 0.05, significantly different from control plus saline plus paraquat; ^p < 0.05, significantly different from matched control plus saline; *p < 0.01, significantly different from A53T plus saline plus saline; **p < 0.05, significantly different from A53T plus saline plus paraquat; and @p < 0.01, significantly different from matched A53T plus saline. C + S, control plus saline; C + EUK, control plus EUK-189; Tg + S, transgenic A53T plus saline; Tg + E, transgenic A53T plus EUK-189.
Synergistic effects of familial A53T α-synuclein expression on combined paraquat/neonatal iron-mediated dopaminergic neurodegeneration in vivo increases in an age-dependent manner
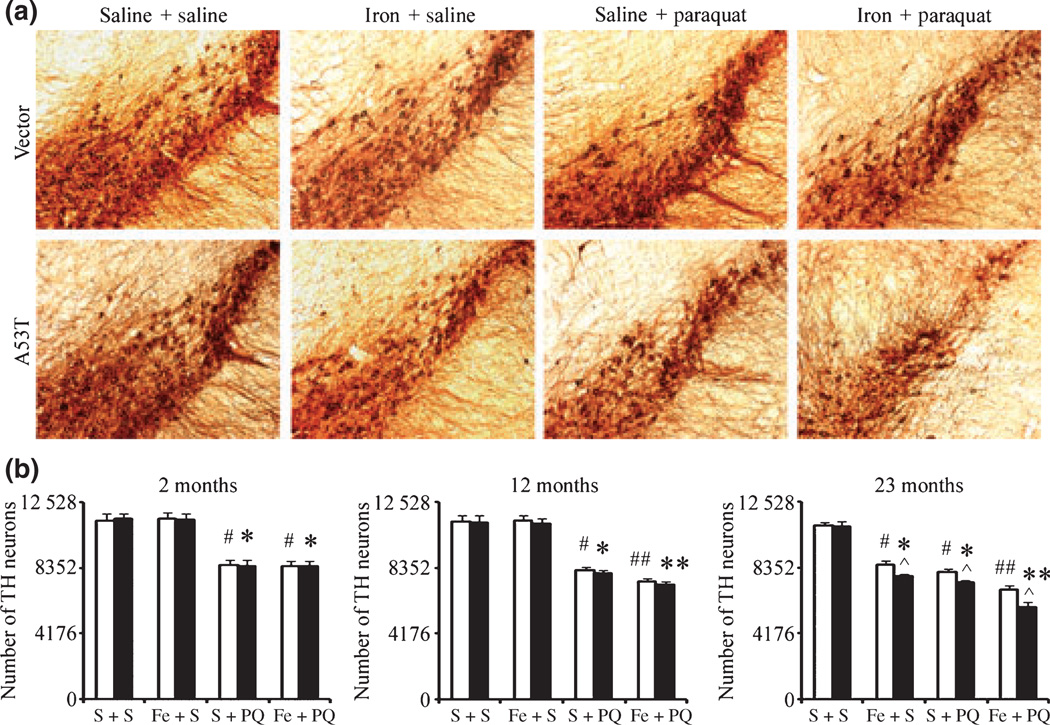
We previously demonstrated that neonatal iron feeding results in a progressive age-related exacerbation of dopaminergic neurodegeneration in the SNpc elicited by MPTP or paraquat administration (Kaur et al. 2007; Peng et al. 2007). To further assess whether presence of the familial A53T mutation acts to further exacerbate paraquat/iron-induced nigral dopaminergic neuronal death, pups were fed iron daily from postnatal days 10 to 17 as previously described (Kaur et al. 2007; Peng et al. 2007). They were housed with their mothers until weaning at week three and aged to either 2, 12, or 23 months of age. At the end of each time period, a subset of iron vs. vehicle-fed mice were intraperitoneally injected with saline or paraquat as previously described (Thiruchelvam et al. 2003; Peng et al. 2007) and killed at day 7. No signs of acute systemic toxicity were observed in the iron-fed pups at the dosages used in this study (Kaur et al. 2007; Peng et al. 2007). No changes in body weights were observed in the mice at the dosage of paraquat used in the experiments (data not shown) as previously shown by ourselves and others (Thiruchelvam et al. 2003; Peng et al. 2007). As previously shown (Peng et al. 2007), paraquat exposure reduced dopaminergic neurons in all three age groups to the same extent in wild-type and A53T α-synuclein expressing transgenic mice, suggesting that paraquat toxicity is age-independent (Fig. 4). By 12 months of age, paraquat-induced loss in dopaminergic cell numbers was exacerbated in those animals that had previously received elevated oral iron during the neonatal period and this effect was even more pronounced by 23 months of age (Fig. 4) demonstrating that older iron-fed animals are more susceptible to exposure to the herbicide paraquat than younger iron-fed animals and that paraquat administration accelerates SNpc dopaminergic neuron loss in these animals as a consequence of early iron exposure. Furthermore, the loss of dopaminergic neurons was more prominent in A53T α-synuclein expressing mice at 23 months of age than in wild-type littermate controls (Fig. 4). This is to our knowledge the first study performed assessing the impact of combined environmental risk factors and familial genetic mutations with age on this parameter.
Fig. 4.
Effects of combined environmental and genetic factors on dopaminergic neurodegeneration with increasing age. (a) Photomicrographs of SNpc TH-immunostained sections from 23-month-old control versus A53T transgenic mice treated with either paraquat or iron alone or in combination. Original magnification, ×10. (b) Quantitative stereological analysis of the number of TH-stained profiles from 2-month-old, 12-month-old, and 23-month-old mice. Mean ± SEM, the number of animal per group is 4 or 5. White bars, control; black bars, A53T over-expression. #p < 0.01, significantly different from control plus saline plus saline group;*p < 0.01, significantly different from A53T plus saline plus saline group; ##p < 0.05, significantly different from control plus saline plus paraquat group;**p < 0.05, significantly different from A53T plus saline plus paraquat group; and ^p < 0.05, significantly different from matched control group. S + S, saline plus saline; Fe + S, iron plus saline; S + PQ, saline plus paraquat; Fe + PQ, iron plus paraquat.
Combined environmental risk factors and genetic mutation results in increased indices of oxidative stress in the SNpc
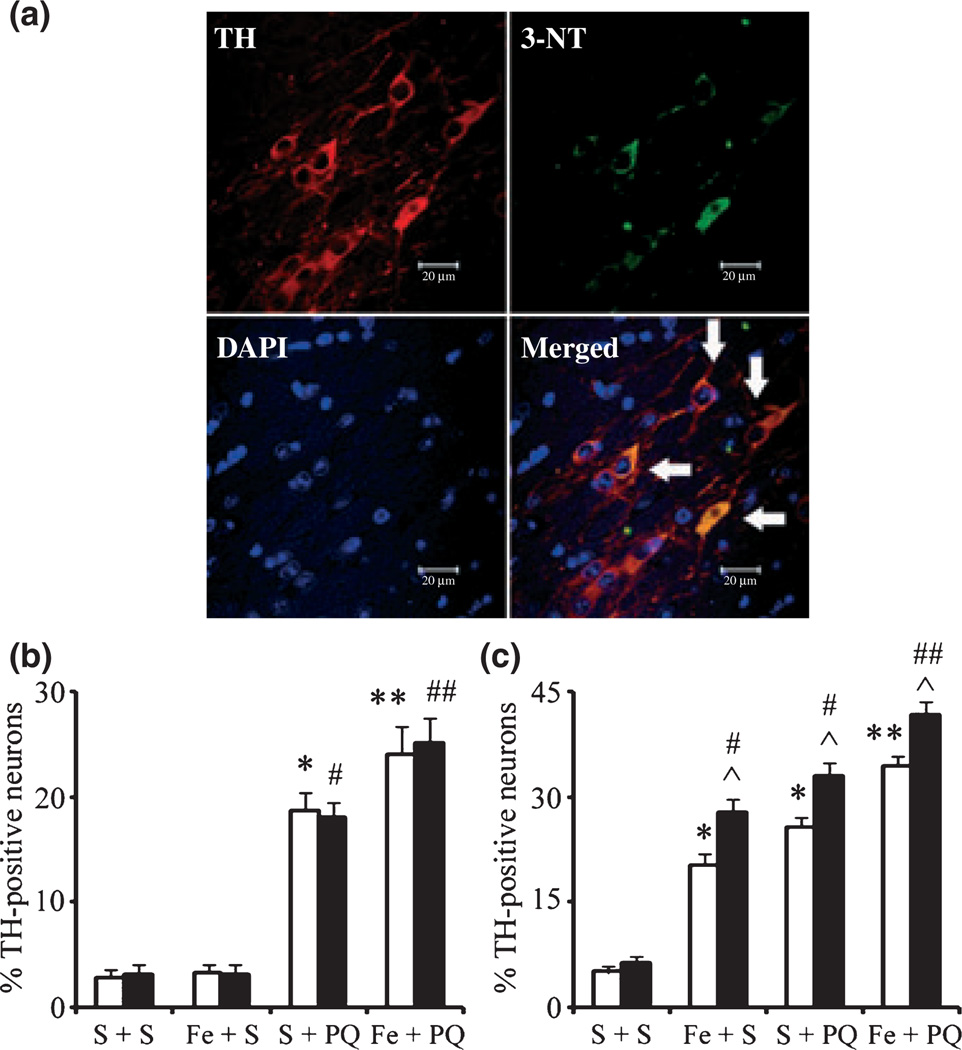
Systemic administration of paraquat alone or paraquat combined with iron was previously reported to increase levels of 3-nitrotyrosine (3-NT) oxidation in dopaminergic neurons of the SNpc (McCormack et al. 2005; Peng et al. 2007). In order to determine whether additional loss of SNpc neurons elicited by combined environmental risk factors and genetic mutation is because of increased levels of oxidative stress, we performed immunofluorescent double-labeling experiments using TH as a dopaminergic marker and 3-NT as a biomarker for protein oxidation. Neonatal iron alone does not result in increased levels of oxidative damage within dopaminergic SNpc neurons at 2 months of age, but by 23 months of age there is a significant increase in this parameter (Fig. 5), although we previously demonstrated that this is not associated with an increase in dopaminergic cell loss in this brain region (Kaur et al. 2007; Peng et al. 2007). Also, as previously demonstrated, paraquat administration at 2 months was found to result in increases in 3-NT levels (McCormack et al. 2005) that are further increased by 23 months of age and are exacerbated in the presence of neonatal iron exposure (Fig. 5). Significantly, the levels of protein oxidation were more prominent in A53T α-synuclein expressing mice than wild-type littermate controls at 23 months of age (Fig. 5).
Fig. 5.
3-Nitrotyrosine immunopositive SNpc cell counts. (a) An example of localization (arrows) of 3-nitrotyrosine-immunopositive staining (green) within dopaminergic neurons (red). 4′,6-Diamidino-2-phenylindole (blue) was used to counter-stain nuclei. Scale bar, 20 µm. Quantitative analysis of double labeling for TH with 3-nitrotyrosine in the SNpc of 2-month-old (b) and 23-month-old (c) mice. White bars, control; black bars, A53T over-expression. Mean ± SEM, the number of animal per group is 4 or 5. *p < 0.01, significantly different from control plus saline plus saline group; **p < 0.05, significantly different from control plus saline plus paraquat group; #p < 0.01, significantly different from A53T plus saline plus saline group; ##p < 0.05, significantly different from A53T plus saline plus paraquat group; and ^p < 0.05, significantly different from matched control group. S + S, saline plus saline; Fe + S, iron plus saline; S + PQ, saline plus paraquat; Fe + PQ, iron plus paraquat.
Dopaminergic neuronal death can be reduced by systemic EUK-189 administration
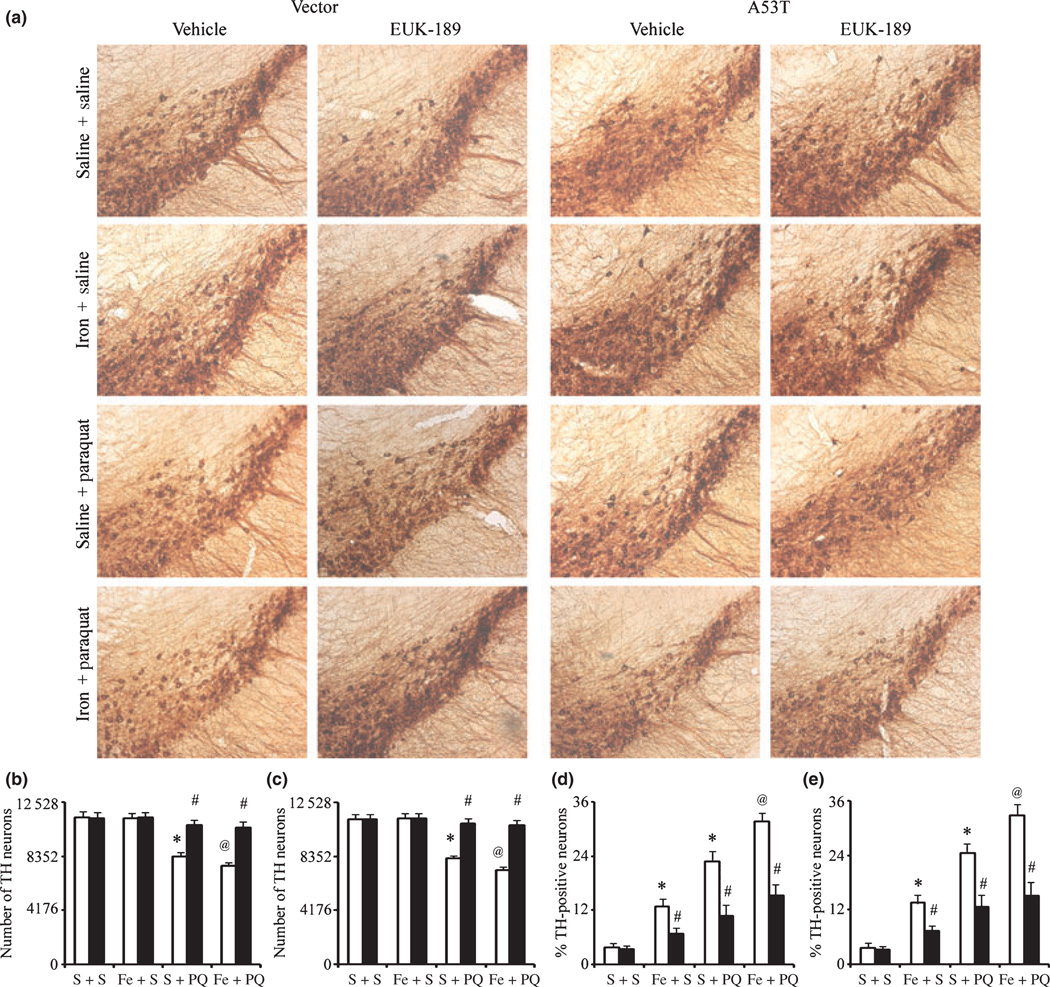
To investigate whether increased dopaminergic neuronal death following paraquat administration in the 12-month-old iron-fed wild-type or A53T α-synuclein expressing mice versus those treated with paraquat alone could be attenuated by antioxidant treatment, we implanted mice with osmotic minipumps containing either 5% mannitol (as vehicle control) or 15 mM EUK-189 3 days prior to paraquat treatment (Zhang et al. 2004; Peng et al. 2005, 2007). As previously shown (Kaur et al. 2007; Peng et al. 2007), at this age, neonatal iron exposure alone does not result in dopaminergic SN cell loss (Figs 4 and 6). In comparison with unlesioned controls, administration of EUK-189 attenuated the additional loss of nigral dopamine neurons elicited by neonatal iron feeding versus paraquat treatment alone (Fig. 6a–c). Furthermore, pre-treatment with EUK-189 was found to attenuate the increases in 3-NT induced not only by either agent alone but also as a consequence of combined iron-paraquat exposure where an increase in dopaminergic SNpc cell loss occurs (Fig. 6d and e).
Fig. 6.
Administration of EUK-189 attenuates the exacerbation of combined neonatal iron-paraquat-induced dopaminergic neuronal death at 12 months of age in the A53T expressing mice. (a) Photomicrographs of SNpc TH-immunostained sections. Original magnification, ×10. Quantitative stereological analysis of the number of TH-stained profiles from control (b) and A53T over-expression (c) mice. Quantitative analysis of double labeling for TH with 3-nitrotyrosine in the SNpc of control (d) and A53T over-expression (e) mice. White bars, vehicle and black bars, EUK-189-treated. Mean ± SEM, the number of animal per group is 3 or 4. *p < 0.01, significantly different from saline plus saline plus vehicle group; @p < 0.05, significantly different from saline plus paraquat plus vehicle group; #p < 0.01, significantly different from matched vehicle group. S + S, saline plus saline; Fe + S, iron plus saline; S + PQ, saline plus paraquat; Fe + PQ, iron plus paraquat.
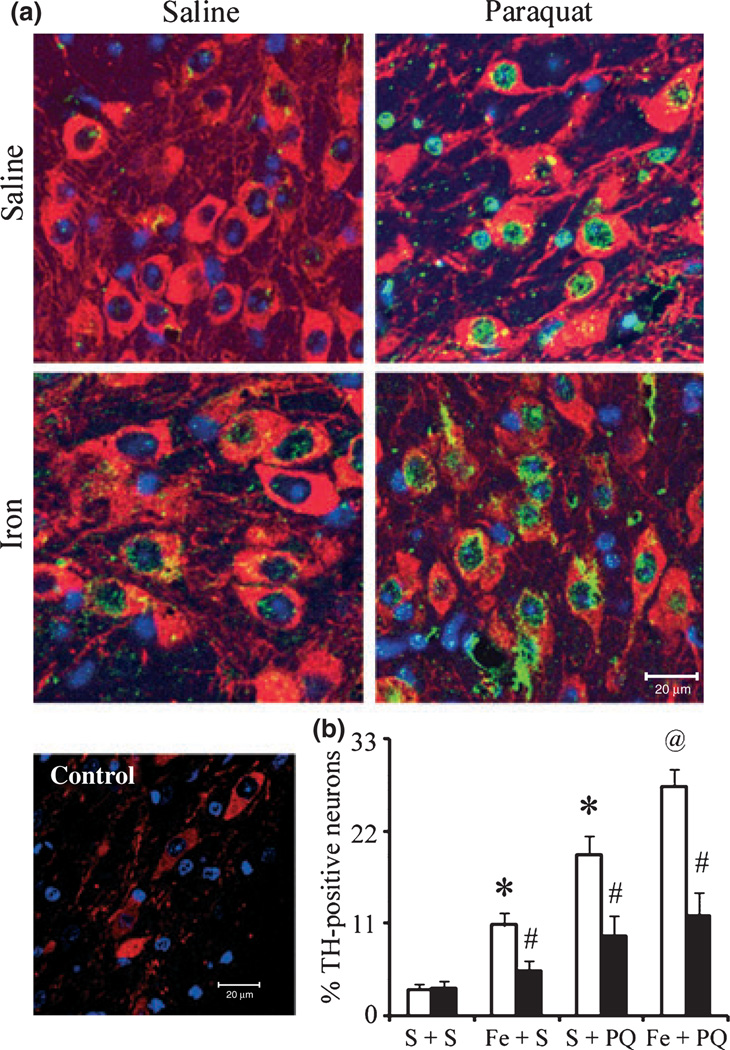
Iron and paraquat exacerbate α-synuclein nitration in the presence of dopaminergic neurodegeneration
Systemic administration of paraquat alone or paraquat combined with maneb was previously reported to increase levels of nitrated α-synuclein in neurons of A53T-expressing transgenics (Norris et al. 2007). Therefore, in order to determine whether additional loss of SNpc neurons elicited by combined iron-paraquat exposure is because of increased levels of α-synuclein nitration, we performed immunofluorescent double-labeling experiments combining TH staining with that for nitrated α-synuclein. Mouse monoclonal anti-nitro-α-synuclein (Tyr39) antibody at the concentration used was lack of nitro-synuclein staining in controls (Fig. 7a). Double-labeling demonstrated that nitrated α-synuclein immunoreactivity is weak in nigral dopaminergic neurons in midbrain tissues isolated from untreated mouse brains (Fig. 7). In contrast, in tissues from mice treated with either agent alone and as a consequence of combined iron-paraquat, co-localization of nitrated α-synuclein has found to be elevated in the TH-positive SNpc neurons (Fig. 7). Moreover, EUK-189 administration appeared to reduce the amount of co-localization of nitrated α-synuclein within these neurons induced by either paraquat alone or in combination with iron (Fig. 7). Taken together, our data demonstrate that EUK-189 attenuates both the exacerbation in paraquat-elicited α-synuclein nitration induced by neonatal iron feeding as well as age-related increases in nigrostriatal dopaminergic cell death.
Fig. 7.
Administration of EUK-189 prevents α-synuclein nitration in the SNpc. (a) Nitrated α-synuclein-immunopositive staining (green) within dopaminergic neurons (red). 4′,6-Diamidino-2-phenylindole (blue) was used to counter-stain nuclei. Scale bar, 20 µm. (b) Quantitative analysis of double labeling for TH with nitrated α-synuclein in the SNpc of 12-month-old A53T expressing transgenics. White bars, vehicle and black bars, EUK-189-treated. Mean ± SEM, the number of animal per group is 3 or 4. *p < 0.001, significantly different from saline plus saline plus vehicle group; @p < 0.05, significantly different from saline plus paraquat plus vehicle group; #p < 0.001, significantly different from matched vehicle group. S + S, saline plus saline; Fe + S, iron plus saline; S + PQ, saline plus paraquat; Fe + PQ, iron plus paraquat.
Discussion
Epidemiological evidence from an extensive twin study and genome-wide single nucleotide polymorphism analyses of a population cohort of parkinsonian patients versus neurologically normal controls strongly suggests that the common idiopathic form of the disease is not because of strict genetic heritability (Tanner et al. 1999; Wirdefeldt et al. 2004; Fung et al. 2006). This along with geographic variations in PD incidence suggests that sporadic PD is most likely because of environmental factors perhaps in combination with genetic susceptibilities. Conversely, variations in symptoms and disease progression in familial forms of the disorder may be impacted by both environmental exposures and by aging itself. In this study, we explored the impact of increased oral iron administration during the neonatal period on nigrostriatal neurodegeneration associated with the herbicide paraquat in transgenic mice expressing the familial A53T mutant form of α-synuclein. Here, we demonstrate that older mutant α-synuclein transgenic animals are more susceptible to the combined effects of these environmental risk factors than wildtype animals suggesting that environmental factors in the context of aging can influence presentation of neuropathology associated with this monogenic mutation.
Oxidative stress has been suggested to be a key pathological process in PD neuropathology. While oxidative stress can occur throughout brain, the nigral environment appears to be more sensitive to its affects. Oxidative stress markers have been observed to be increased in the SN in many PD studies. Oxidative biomarkers shown to be elevated in the parkinsonian midbrain include 8-hydroxyguanosine (Zhang et al. 1999), 4-hydroxy-2-nonenal (Yoritaka et al. 1996), and protein carbonyls (Alam et al. 1997). Paraquat has been demonstrated to mediate dopaminergic cell death via oxidative stress-mediated events (Peng et al. 2004, 2005, 2007). Superoxide anion radicals are generates by paraquat through both redox cycling via reaction with molecular oxygen and electron transfer reactions with NADH-dependent oxireductases (Bus and Gibson 1984; Clejan and Cederbaum 1989; Burkitt et al. 1993). Superoxide dismutase/catalase mimetics (Peng et al. 2005, 2007) and superoxide dismutase over-expression (Patel et al. 1996) are capable of inhibiting the actions of paraquat, suggesting that superoxide is a key mediator in the herbicide’s neurotoxicity. Superoxide itself is relatively inert, however, covalent bonding of superoxide radical to nitroxide results in formation of peroxynitrite which under physiological conditions is very reactive and can oxidize proteins, nucleic acids, and membrane phospholipids.
Like paraquat exposure, oxidant-induced iron signaling and the subsequent occurrence of free radical-mediated oxidative damage are increasingly understood to play highly significant roles in neurodegeneration (Drechsel and Patel 2008). Changes in iron homeostasis which increase the labile iron pool potentially promote neuronal toxicity by catalyzing conversion of a less reactive inert species (H2O2) to the highly reactive hydroxyl radical. The oxidation state of iron has been found to change from ferrous to ferric ion in SNpc dopaminergic neurons during PD progression (Yoshida et al. 2001) and moreover redox-available iron has been detected in Lewy bodies within SNpc of postmortem parkinsonian brains (Castellani et al. 2000). This alteration in redox state has been postulated to be because of iron’s capacity to catalyze oxidative reactions. Aging also plays an important role in this process. Reported age-related alterations in brain iron homeostasis may contribute to basal iron elevation compared to young animals (de Lima et al. 2007). In keeping with previous studies (Kaur et al. 2007; Peng et al. 2007), elevation in basal iron levels achieved via neonatal iron feeding in the adult brain have been found to result in a significant age-related increases in oxidative damage within dopaminergic SNpc neurons. The additive age-related effects of neonatal iron and paraquat at 12 months of age may be because of combined increases in oxidative damage. The increase in oxidative damage by 12 months of age in this current study was found to correlate with an elevation in paraquat-mediated dopaminergic neuronal death in animals that received oral iron neonatally versus those that did not. Although iron alone itself did not result in an age-related cell loss, there was an increase in paraquat-mediated dopaminergic neuronal death at 2 vs. 12 months of age. The increase in paraquat-induced dopaminergic neuronal death in the presence of previous neonatal iron feeding was found to be preventable by systemic administration of EUK-189 which could remove free radicals that would normally interact with elevated SNpc iron levels.
Mutations in α-synuclein in familial PD have suggested that this particular gene is involved in the molecular pathogenesis of parkinsonism (Polymeropoulos et al. 1997; Kruger et al. 1998; Zarranz et al. 2004). Transgenic mouse models over-expressing mutant forms of α-synuclein show an increased vulnerability of SNpc neurons to neurotoxins such as MPTP and 6-hydroxydopamine (Fleming et al. 2005). Conversely, α-synuclein-knockout mice have been found to be resistance to MPTP (Dauer et al. 2002) and other mitochondrial toxins including malonate and 3-nitropropionic acid (Klivenyi et al. 2006). α-Synuclein post-translational modifications include nitration, glycosylation, and phosphorylation are likely to influence α-synuclein aggregation and subsequent neurotoxicity. Exposure of α-synuclein-expressing cells to oxidative or nitrative agents increases α-synuclein fibril formation (Hodara et al. 2004) perhaps by promoting the formation of high order oligomers which can perforate vesicular membranes including those associated with dopamine storage synaptic vesicles, resulting in leakage of dopamine into the cytoplasm (Lotharius and Brundin 2002). Increased levels of nitratively modified α-synuclein have also been reported in Lewy bodies in the postmortem PD midbrain (Giasson et al. 2000). Recently, we reported that levels of nitration of α-synuclein were increased specifically at Tyr39 in an inducible transgenic cellular model of PD in which oxidative stress levels were increased via up-regulation of monoamine oxidase B, possibly as a consequence of increased nitration of α-synuclein production. These affects were abrogated by the addition of the selective monoamine oxidase B inhibitor deprenyl (Danielson et al. 2009). Expression of the α-synuclein A53T mutant in the current study was found to have increased paraquat and paraquat-iron sensitivity suggesting a link between oxidative/nitrative-mediated alterations of α-synuclein and dopaminergic neuronal death in PD. Our data from this current study may also explain previous findings linking paraquat to increased α-synuclein aggregation (Manning-Bog et al. 2002).
EUKs are known to catalytically eliminate both superoxide and hydrogen peroxide (Baudry et al. 1993; Gonzalez et al. 1995; Baker et al. 1998). These compounds have been shown to cross the blood-brain-barrier and be neuroprotective in several animal models including those for ischemia (Baker et al. 1998), epilepsy (Rong et al. 1999), amyotrophic lateral sclerosis (Jung et al. 2001), age-related learning deficits (Liu et al. 2003), and PD (Peng et al. 2005, 2007). The EUK-189 compound used in this study is more lipophilic than earlier generations of salen-manganese complexes thus improving intracellular and intra-organellular delivery (Melov et al. 2001). The present findings support a pre-eminent role for oxidative stress in neurodegeneration and impairment of dopaminergic function in an experimental model of PD which combines environmental exposures with genetic factors known to separately be involved in the disease and suggest that further epidemiological analyses in which the relationship between environmental and genetic parameters in human PD incidence is more thoroughly explored are warranted.
Acknowledgements
This work was supported by National Institutes of Health Grant U54 ES12077 (JKA).
Abbreviations used
- 3-NT
3-nitrotyrosine
- DAPI
4′ 6-diamidino-2-phenylindole
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- PBS
phosphate-buffered saline
- PD
Parkinson’s disease
- SN
substantia nigra
- TH
tyrosine hydroxylase
References
- Alam ZI, Daniel SE, Lees AJ, Marsden DC, Jenner P, Halliwell B. A generalised increase in protein carbonyls in the brain in Parkinson’s but not incidental Lewy body disease. J. Neurochem. 1997;69:1326–1329. doi: 10.1046/j.1471-4159.1997.69031326.x. [DOI] [PubMed] [Google Scholar]
- Baker K, Marcus CB, Huffman K, et al. Synthetic combined superoxide dismutase/catalase mimetics are protective as a delayed treatment in a rat stroke model: a key role for reactive oxygen species in ischemic brain injury. J. Pharmacol. Exp. Ther. 1998;284:215–221. [PubMed] [Google Scholar]
- Baudry M, Etienne S, Bruce A, Palucki M, Jacobsen E, Malfroy B. Salen-manganese complexes are superoxide dismutasemimics. Biochem. Biophys. Res. Commun. 1993;192:964–968. doi: 10.1006/bbrc.1993.1509. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Burkitt MJ, Kadiiska MB, Hanna PM, Jordan SJ, Mason RP. Electron spin resonance spin-trapping investigation into the effects of paraquat and desferrioxamine on hydroxyl radical generation during acute iron poisoning. Mol. Pharmacol. 1993;43:257–263. [PubMed] [Google Scholar]
- Bus JS, Gibson JE. Paraquat: model for oxidant-initiated toxicity. Environ. Health Perspect. 1984;55:37–46. doi: 10.1289/ehp.845537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani RJ, Siedlak SL, Perry G, Smith MA. Sequestration of iron by Lewy bodies in Parkinson’s disease. Acta Neuropathol. (Berl) 2000;100:111–114. doi: 10.1007/s004010050001. [DOI] [PubMed] [Google Scholar]
- Clejan L, Cederbaum AI. Synergistic interactions between NADPH-cytochrome P-450 reductase, paraquat, and iron in the generation of active oxygen radicals. Biochem. Pharmacol. 1989;38:1779–1786. doi: 10.1016/0006-2952(89)90412-7. [DOI] [PubMed] [Google Scholar]
- Danielson SR, Held JM, Schilling B, Oo M, Gibson BW, Andersen JK. Preferentially increased nitration of alpha-synuclein at tyrosine-39 in a cellular oxidative model of Parkinson’s disease. Anal. Chem. 2009;81:7823–7828. doi: 10.1021/ac901176t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Kholodilov N, Vila M, et al. Resistance of alpha-synuclein null mice to the parkinsonian neurotoxin MPTP. Proc. Natl Acad. Sci. USA. 2002;99:14524–14529. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick FD. Parkinson’s disease and pesticide exposures. Br. Med. Bull. 2006;79–80:219–231. doi: 10.1093/bmb/ldl018. [DOI] [PubMed] [Google Scholar]
- Drechsel DA, Patel M. Role of reactive oxygen species in the neurotoxicity of environmental agents implicated in Parkinson’s disease. Free Radic. Biol. Med. 2008;44:1873–1886. doi: 10.1016/j.freeradbiomed.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Fernagut PO, Chesselet MF. Genetic mouse models of parkinsonism: strengths and limitations. NeuroRx. 2005;2:495–503. doi: 10.1602/neurorx.2.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung HC, Chen CM, Hardy J, Singleton AB, Lee-Chen GJ, Wu YR. Analysis of the PINK1 gene in a cohort of patients with sporadic early-onset parkinsonism in Taiwan. Neurosci. Lett. 2006;394:33–36. doi: 10.1016/j.neulet.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Gaeta A, Hider RC. The crucial role of metal ions in neurodegeneration: the basis for a promising therapeutic strategy. Br. J. Pharmacol. 2005;146:1041–1059. doi: 10.1038/sj.bjp.0706416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- Gonzalez PK, Zhuang J, Doctrow SR, et al. EUK-8, a synthetic superoxide dismutase and catalase mimetic, ameliorates acute lung injury in endotoxemic swine. J. Pharmacol. Exp. Ther. 1995;275:798–806. [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Richardson RJ. The risk of Parkinson’s disease with exposure to pesticides, farming, well water, and rural living. Neurology. 1998;50:1346–1350. doi: 10.1212/wnl.50.5.1346. [DOI] [PubMed] [Google Scholar]
- Griffiths PD, Dobson BR, Jones GR, Clarke DT. Iron in the basal ganglia in Parkinson’s disease. An in vitro study using extended X-ray absorption fine structure and cryo-electron microscopy. Brain. 1999;122:667–673. doi: 10.1093/brain/122.4.667. [DOI] [PubMed] [Google Scholar]
- Hatcher JM, Pennell KD, Miller GW. Parkinson’s disease and pesticides: a toxicological perspective. Trends Pharmacol. Sci. 2008;29:322–329. doi: 10.1016/j.tips.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzman C, Wiens M, Bowering D, Snow B, Calne D. Parkinson’s disease: a case-control study of occupational and environmental risk factors. Am. J. Ind. Med. 1990;17:349–355. doi: 10.1002/ajim.4700170307. [DOI] [PubMed] [Google Scholar]
- Hodara R, Norris EH, Giasson BI, Mishizen-Eberz AJ, Lynch DR, Lee VM, Ischiropoulos H. Functional consequences of alpha-synuclein tyrosine nitration: diminished binding to lipid vesicles and increased fibril formation. J. Biol. Chem. 2004;279:47746–47753. doi: 10.1074/jbc.M408906200. [DOI] [PubMed] [Google Scholar]
- Hubble JP, Cao T, Hassanein RE, Neuberger JS, Koller WC. Risk factors for Parkinson’s disease. Neurology. 1993;43:1693–1697. doi: 10.1212/wnl.43.9.1693. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Kienzl E, Rumpelmaier G, Paulus W, Riederer P, Stachelberger H, Youdim MB, Ben-Shachar D. Iron and ferritin in substantia nigra in Parkinson’s disease. Adv. Neurol. 1993;60:267–272. [PubMed] [Google Scholar]
- Jimenez-Jimenez FJ, Mateo D, Gimenez-Roldan S. Exposure to well water and pesticides in Parkinson’s disease: a case-control study in the Madrid area. Mov. Disord. 1992;7:149–152. doi: 10.1002/mds.870070209. [DOI] [PubMed] [Google Scholar]
- Jung C, Rong Y, Doctrow S, Baudry M, Malfroy B, Xu Z. Synthetic superoxide dismutase/catalase mimetics reduce oxidative stress and prolong survival in a mouse amyotrophic lateral sclerosis model. Neurosci. Lett. 2001;304:157–160. doi: 10.1016/s0304-3940(01)01784-0. [DOI] [PubMed] [Google Scholar]
- Kaur D, Peng J, Chinta SJ, Rajagopalan S, Di Monte DA, Cherny RA, Andersen JK. Increased murine neonatal iron intake results in Parkinson-like neurodegeneration with age. Neurobiol. Aging. 2007;28:907–913. doi: 10.1016/j.neurobiolaging.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Klivenyi P, Siwek D, Gardian G, Yang L, Starkov A, Cleren C, Ferrante RJ, Kowall NW, Abeliovich A, Beal MF. Mice lacking alpha-synuclein are resistant to mitochondrial toxins. Neurobiol. Dis. 2006;21:541–548. doi: 10.1016/j.nbd.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat. Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Lanska DJ. The geographic distribution of Parkinson’s disease mortality in the United States. J. Neurol. Sci. 1997;150:63–70. doi: 10.1016/s0022-510x(97)05371-9. [DOI] [PubMed] [Google Scholar]
- de Lima MN, Dias CP, Torres JP, et al. Reversion of age-related recognition memory impairment by iron chelation in rats. Neurobiol. Aging. 2007;6:6. doi: 10.1016/j.neurobiolaging.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Liou HH, Tsai MC, Chen CJ, Jeng JS, Chang YC, Chen SY, Chen RC. Environmental risk factors and Parkinson’s disease: a case-control study in Taiwan. Neurology. 1997;48:1583–1588. doi: 10.1212/wnl.48.6.1583. [DOI] [PubMed] [Google Scholar]
- Liu R, Liu IY, Bi X, Thompson RF, Doctrow SR, Malfroy B, Baudry M. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc. Natl Acad. Sci. USA. 2003;100:8526–8531. doi: 10.1073/pnas.1332809100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotharius J, Brundin P. Pathogenesis of Parkinson’s disease: dopamine, vesicles and alpha-synuclein. Nat. Rev. Neurosci. 2002;3:932–942. doi: 10.1038/nrn983. [DOI] [PubMed] [Google Scholar]
- Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Di Monte DA. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein. J. Biol. Chem. 2002;277:1641–1644. doi: 10.1074/jbc.C100560200. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Vila M, Lincoln S, et al. Lack of nigral pathology in transgenic mice expressing human alpha-synuclein driven by the tyrosine hydroxylase promoter. Neurobiol. Dis. 2001;8:535–539. doi: 10.1006/nbdi.2001.0392. [DOI] [PubMed] [Google Scholar]
- McCormack AL, Di Monte DA. Effects of L-dopa and other amino acids against paraquat-induced nigrostriatal degeneration. J. Neurochem. 2003;85:82–86. doi: 10.1046/j.1471-4159.2003.01621.x. [DOI] [PubMed] [Google Scholar]
- McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, Di Monte DA. Environmental risk factors and Parkinson’s disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol. Dis. 2002;10:119–127. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- McCormack AL, Atienza JG, Johnston LC, Andersen JK, Vu S, Di Monte DA. Role of oxidative stress in paraquat-induced dopaminergic cell degeneration. J. Neurochem. 2005;93:1030–1037. doi: 10.1111/j.1471-4159.2005.03088.x. [DOI] [PubMed] [Google Scholar]
- Melov S, Doctrow SR, Schneider JA, Haberson J, Patel M, Coskun PE, Huffman K, Wallace DC, Malfroy B. Lifespan extension and rescue of spongiform encephalopathy in superoxide dismutase 2 nullizygous mice treated with superoxide dismutase-catalase mimetics. J. Neurosci. 2001;21:8348–8353. doi: 10.1523/JNEUROSCI.21-21-08348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris EH, Uryu K, Leight S, Giasson BI, Trojanowski JQ, Lee VM. Pesticide exposure exacerbates alpha-synucleinopathy in an A53T transgenic mouse model. Am. J. Pathol. 2007;170:658–666. doi: 10.2353/ajpath.2007.060359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M, Day BJ, Crapo JD, Fridovich I, McNamara JO. Requirement for superoxide in excitotoxic cell death. Neuron. 1996;16:345–355. doi: 10.1016/s0896-6273(00)80052-5. [DOI] [PubMed] [Google Scholar]
- Peng J, Mao XO, Stevenson FF, Hsu M, Andersen JK. The herbicide paraquat induces dopaminergic nigral apoptosis through sustained activation of the JNK pathway. J. Biol. Chem. 2004;279:32626–32632. doi: 10.1074/jbc.M404596200. [DOI] [PubMed] [Google Scholar]
- Peng J, Stevenson FF, Doctrow SR, Andersen JK. Superoxide dismutase/catalase mimetics are neuroprotective against selective paraquat-mediated dopaminergic neuron death in the substantial nigra: implications for Parkinson disease. J. Biol. Chem. 2005;280:29194–29198. doi: 10.1074/jbc.M500984200. [DOI] [PubMed] [Google Scholar]
- Peng J, Peng L, Stevenson FF, Doctrow SR, Andersen JK. Iron and paraquat as synergistic environmental risk factors in sporadic Parkinson’s disease accelerate age-related neurodegeneration. J. Neurosci. 2007;27:6914–6922. doi: 10.1523/JNEUROSCI.1569-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Stevenson FF, Oo ML, Andersen JK. Iron-enhanced paraquat-mediated dopaminergic cell death due to increased oxidative stress as a consequence of microglial activation. Free Radic. Biol. Med. 2009;46:312–320. doi: 10.1016/j.freeradbiomed.2008.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Riederer P, Dirr A, Goetz M, Sofic E, Jellinger K, Youdim MB. Distribution of iron in different brain regions and subcellular compartments in Parkinson’s disease. Ann. Neurol. 1992;32:S101–S104. doi: 10.1002/ana.410320717. [DOI] [PubMed] [Google Scholar]
- Rong Y, Doctrow SR, Tocco G, Baudry M. EUK-134, a synthetic superoxide dismutase and catalase mimetic, prevents oxidative stress and attenuates kainate-induced neuropathology. Proc. Natl Acad. Sci. USA. 1999;96:9897–9902. doi: 10.1073/pnas.96.17.9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semchuk KM, Love EJ, Lee RG. Parkinson’s disease and exposure to agricultural work and pesticide chemicals. Neurology. 1992;42:1328–1335. doi: 10.1212/wnl.42.7.1328. [DOI] [PubMed] [Google Scholar]
- Simon-Sanchez J, Schulte C, Bras JM, et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat. Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofic E, Paulus W, Jellinger K, Riederer P, Youdim MB. Selective increase of iron in substantia nigra zona compacta of parkinsonian brains. J. Neurochem. 1991;56:978–982. doi: 10.1111/j.1471-4159.1991.tb02017.x. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Stephenson J. Exposure to home pesticides linked to Parkinson disease. JAMA. 2000;283:3055–3056. doi: 10.1001/jama.283.23.3055. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Ottman R, Goldman SM, Ellenberg J, Chan P, Mayeux R, Langston JW. Parkinson disease in twins: an etiologic study. JAMA. 1999;281:341–346. doi: 10.1001/jama.281.4.341. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M, McCormack A, Richfield EK, Baggs RB, Tank AW, Di Monte DA, Cory-Slechta DA. Age-related irreversible progressive nigrostriatal dopaminergic neurotoxicity in the paraquat and maneb model of the Parkinson’s disease phenotype. Eur. J. Neurosci. 2003;18:589–600. doi: 10.1046/j.1460-9568.2003.02781.x. [DOI] [PubMed] [Google Scholar]
- Vali S, Chinta SJ, Peng J, Sultana Z, Singh N, Sharma P, Sharada S, Andersen JK, Bharath MM. Insights into the effects of alpha-synuclein expression and proteasome inhibition on glutathione metabolism through a dynamic in silico model of Parkinson’s disease: validation by cell culture data. Free Radic. Biol. Med. 2008;45:1290–1301. doi: 10.1016/j.freeradbiomed.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- Wirdefeldt K, Gatz M, Schalling M, Pedersen NL. No evidence for heritability of Parkinson disease in Swedish twins. Neurology. 2004;63:305–311. doi: 10.1212/01.wnl.0000129841.30587.9d. [DOI] [PubMed] [Google Scholar]
- Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc. Natl Acad. Sci. USA. 1996;93:2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Ektessabi A, Fujisawa S. XANES spectroscopy of a single neuron from a patient with Parkinson’s disease. J. Synchrotron Radiat. 2001;8:998–1000. doi: 10.1107/s0909049500017726. [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- Zhang J, Perry G, Smith MA, Robertson D, Olson SJ, Graham DG, Montine TJ. Parkinson’s disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am. J. Pathol. 1999;154:1423–1429. doi: 10.1016/S0002-9440(10)65396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HJ, Doctrow SR, Xu L, Oberley LW, Beecher B, Morrison J, Oberley TD, Kregel KC. Redox modulation of the liver with chronic antioxidant enzyme mimetic treatment prevents age-related oxidative damage associated with environmental stress. FASEB J. 2004;18:1547–1549. doi: 10.1096/fj.04-1629fje. [DOI] [PubMed] [Google Scholar]