Abstract
Background
Mild cognitive impairment (MCI) is often a precursor to Alzheimer’s disease. Little research has examined the efficacy of cognitive rehabilitation in patients with MCI, and the relevant neural mechanisms have not been explored. We previously reported on a pilot study showing the behavioral efficacy of cognitive rehabilitation using mnemonic strategies for face-name associations in patients with MCI. Here we used functional magnetic resonance imaging (fMRI) to test whether there were training-specific changes in activation and connectivity within memory-related areas.
Methods
Six patients with amnestic, multi-domain MCI underwent pre- and post-training fMRI scans, during which they encoded 90 novel face-name pairs, and completed a 4-choice recognition memory test immediately after scanning. Patients were taught mnemonic strategies for half the face-name pairs during three intervening training sessions.
Results
Training-specific effects comprised significantly increased activation within a widespread cerebral cortical network involving medial frontal, parietal, and occipital regions, the left frontal operculum and angular gyrus, and regions in left lateral temporal cortex. Increased activation common to trained and untrained stimuli was found in a separate network involving inferior frontal, lateral parietal and occipital cortical regions. Effective connectivity analysis using multivariate, correlation-purged Granger causality analysis revealed generally increased connectivity after training, particularly involving the middle temporal gyrus and foci in the occipital cortex and the precuneus.
Conclusion
Our findings suggest that the effectiveness of explicit memory training in patients with MCI is associated with training-specific increases in activation and connectivity in a distributed neural system that includes areas involved in explicit memory.
Keywords: cognitive rehabilitation, mnemonic strategy, Alzheimer’s disease, aging, functional magnetic resonance imaging (fMRI), Granger causality analysis
Memory deficits are common consequences of neurologic injury and disease and can be ameliorated through cognitive rehabilitation[1]. Explicit memory training (EMT), which involves the conscious us of strategies, significantly improves learning and memory in healthy elderly[2, 3] and various patient populations[1]. Although some evidence supports the use of EMT in Alzheimer’s disease (AD)[4–6], consensus is lacking[7].
Mild cognitive impairment (MCI), characterized by cognitive deficits but intact daily functioning, is often a precursor to AD[8]. Therefore, MCI may allow a window of opportunity for interventions that prolong functional independence. However, patients with MCI[9, 10] and older adults with below-average memory functioning[11] demonstrated limited improvement following EMT. These studies taught patients multiple mnemonic strategies that may have been difficult for patients to select and use during post-training memory testing[10]. A single mnemonic strategy applicable to multiple types of information may be more beneficial for patients with cognitive deficits[12]. This is an especially important area of investigation given the rapidly growing elderly population and the continuum between normal aging, MCI, and AD.
Identifying the neural correlates of EMT may assist clinicians in selecting, developing, and employing patient-specific mnemonic techniques. Neurologically healthy (typically young) adults show improved memory test performance and increased activation within the middle and inferior frontal gyri[13–16] as well as the hippocampus and medial parietal cortex[17] after EMT. Thus, EMT may provide a mechanism through which patients can facilitate performance by recruiting distributed memory-related brain regions. To our knowledge, there have been no neuroimaging studies of EMT in patients with MCI or AD.
Assessing memory using face-name associations provides an ecologically valid measure that is dependent on the explicit memory system and is difficult for patients with MCI.[18–21] We previously reported the behavioral results of a pilot study in which patients with MCI demonstrated improved memory for face-name associations after EMT[22]. Further, we found that training-associated memory test improvement was positively correlated with memory for the mnemonic cues, but negatively correlated with the number of required training trials, suggesting that the behavioral improvement was attributable to EMT, rather than merely repeated exposure to the stimuli. We therefore hypothesized that EMT would lead to training-specific increases in activation and connectivity within explicit memory-related regions, such as those identified in previous EMT studies [13–17]. Alternatively, decreased activation in sensory and memory areas would be evident if training-related improvements were due to repeated stimulus exposure (repetition suppression) or perceptual priming[23–25].
Methods
Participants
Six right-handed, Caucasian patients were recruited from the Atlanta Veterans Affairs Medical Center and the Emory University Alzheimer’s Disease Research Center. They were among the eight patients in our previous behavioral report[22]. Each patient had been diagnosed with amnestic, multi-domain MCI according to Petersen’s criteria[8] at a consensus conference that included neurologists, neuropsychologists, and other key clinical staff. Individual demographic and neuropsychological data are shown in Table 1. Patients completed the Dementia Rating Scale-2 (DRS-2) after consenting to the study, in order to grossly re-assess cognitive functioning. Language was the most common non-memory domain affected (4 of 6 patients); executive dysfunction and attentional deficits were less commonly found. Although the nature and severity of these non-memory deficits varied across patients, it is important to note that every patient demonstrated training-related behavioral improvement (Table 1). Exclusion criteria included a history of other neurologic diseases (e.g. stroke, epilepsy, traumatic brain injury), psychiatric disorders (e.g. severe depression, bipolar disorder, schizophrenia), and current or past alcohol or drug abuse. The study was approved by the Institutional Review Board of Emory University and the R&D Committee of the Atlanta VAMC. All participants gave written informed consent.
Table 1.
Demographic, neuropsychological, and task-specific performance improvements for the 6 patients who took part in the study. All patients had been diagnosed with amnestic, multidomain MCI prior to taking part in the study; data for the affected non-memory cognitive domain(s) are shown. Some patients were categorized as having relative deficits in non-memory domains (below expectations for that individual but not markedly impaired compared to mean). The Dementia Rating Scale-2 (DRS-2) was administered at the start of the study to grossly re-assess cognitive functioning. Attn = Attention Index; I/P = Initiation/Perseveration Index; Const = Construction Index; Concept = Conceptualization Index; AEMSS = Age and Education Matched Scaled Score; GDS = Geriatric Depression Scale Score. Percent improvement on the trained and untrained lists was calculated by subtracting the pre-training performance from the post-training performance.
| Sex | Age | Educ | Amnestic plus |
Functioning in Non- Memory Domain(s) (in SD) |
DRS-2 Memory (SS) |
DRS-2 Attention (SS) |
DRS-2 I/P (SS) |
DRS-2 Construct (SS) |
DRS-2 Concept (SS) |
DRS-2 AEMSS |
GDS | Trained List Improvement (%) |
Untrained List Improvement (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | 73 | 15 | Language | −1.6 | 2 | 11 | 12 | 10 | 10 | 5.8 | 3 | 26.7 | 11.1 |
| M | 79 | 14 | Language | −1.2 | 5 | 12 | 3 | 10 | 8 | 3.9 | 1 | 46.7 | 6.7 |
| M | 72 | 16 | Attention (relative) | −0.8 | 6 | 11 | 12 | 10 | 13 | 10 | 0 | 68.9 | 20.0 |
| M | 63 | 16 | Language | −1.7 | 10 | 11 | 11 | 10 | 9 | 8.9 | 4 | 64.4 | 31.1 |
| F | 75 | 15 | Executive (relative) | −0.9 | 10 | 12 | 10 | 10 | 12 | 10.3 | 0 | 60.0 | 28.9 |
| M | 79 | 18 | Executive & Language (relative) |
−1.8 −0.7 |
4 | 10 | 5 | 10 | 9 | 3.8 | 2 | 44.4 | 0 |
| Mean (SD) | 73.5 (5.9) |
15.7 (1.4) |
6.2 (3.3) |
11.17 (.75) |
8.8 (3.9) |
10 (0) |
10.17 (1.9) |
7.1 (3.0) |
2 (1.6) |
51.85 (15.7) |
16.3 (12.5) |
Stimuli
As detailed earlier[22], a total of ninety-two faces were selected from a previously used set[19]. These faces were transformed to grayscale and randomly paired with a gender- and age-appropriate name (based on popularity by approximate birth decade). Ninety of these face-name pairs were divided into two lists of 45 that were matched for gender, race, and approximate age (by decade). The remaining two faces (one of each gender) served as control stimuli during fMRI scanning.
General procedures
The procedures have been described earlier[22] and are summarized here. Each patient completed a total of 5 sessions within a two-week period. During the first (pre-training) and fifth (post-training) sessions, patients underwent fMRI scanning as they encoded the face-name associations. Approximately 15 minutes after each scan was completed, patients took a four-choice recognition memory test (outside the scanner) involving all 92 face-name pairs. Stimuli were presented within the scanner using a laptop PC running Presentation software (Neurobehavioral Systems Inc., Albany, California). The post-scan memory test used a PC-based program specifically developed for this study. During sessions 2, 3, and 4, patients received EMT.
As detailed earlier[22], patients were trained using one of the two lists of 45 pairs, in counterbalanced order across patients. This list is hereafter referred to as the “trained” list, the other being the “untrained” list. Patients learned 15 face-name pairs during each training session. For each pair, patients were required to spontaneously recall the name on three consecutive trials, with a maximum of 10 trials to reach this criterion. Training used a modified Biographical Information Module from the Ecologically Oriented Neurorehabilitation of Memory (EON-Mem) program[12]. Although this program teaches patients to self-generate cues, we provided cues to standardize procedures across patients. For each face-name pair, patients were directed to a salient facial feature (visual cue) and given a nickname that often rhymed with the actual name (verbal cue) linking the feature to the name. Patients were instructed to associate the visual and verbal cues by creating mental images that exaggerated and emphasized the facial feature. On subsequent training trials, patients first recalled the visual cue, then the verbal cue, and finally the corresponding name.
fMRI procedures
Imaging parameters
MR scans were performed on a Siemens Trio 3T MRI scanner (Siemens Medical Solutions, Malvern, PA), using a 12-channel head coil. For blood oxygenation level-dependent (BOLD) contrast, T2*-weighted functional images were acquired using a single-shot, gradient-recalled, echo-planar imaging (EPI) sequence with the following parameters: repetition time (TR) 2000 msec, echo time (TE) 30 msec, field of view (FOV) 220 mm, flip angle (FA) 90°, 27 axial slices of 4 mm thickness, in-plane resolution 3.4×3.4 mm, and in-plane matrix 64×64. High-resolution anatomic images were acquired using a 3D MPRAGE sequence (TR 2300, TE 3.9 msec, inversion time 1100 msec, FA 8°) consisting of 176 sagittal slices of 1 mm thickness (FOV 256 mm, in-plane resolution 1×1 mm, in-plane matrix 256×256). Once the scanner achieved magnetic stabilization for each run, it triggered the computer running Presentation software in order to synchronize stimulation and scan acquisition.
fMRI paradigm
The same block design paradigm was used pre- and post-training, comprising alternating 20s rest and 30s active blocks, similar to previous studies of face-name encoding[20, 21]. There were three types of active blocks, comprising face-name pairs from (i) the trained list, (ii) the untrained list, or (iii) the two control stimuli repeated in alternation. During active blocks, 5 face-name pairs were shown for 5s each, with a 1s inter-stimulus interval. Patients were instructed to remember the name paired with each face; no responses were required since this could have distracted patients from employing the trained strategies. The MRI technologist (RFS) entered the room between functional runs to ensure that the patient was performing the task; no problems were reported by any patient. Memory performance was assessed on a post-scan test, as in other imaging studies of memory [20, 21]. Each block type occurred three times during each of three functional runs, so that the 45 pairs from the trained and untrained lists were each displayed once. Repeated stimuli were seen a total of 45 times.
Imaging data analysis
Image processing and analysis were performed using BrainVoyager QX v1.6.3 (Brain Innovation, Maastricht, The Netherlands). Functional runs were motion-corrected in real time using Siemens 3D-PACE (prospective acquisition motion correction). For each subject, the functional images were realigned to the first image of the series. Images were pre-processed using trilinear interpolation for motion correction, sinc interpolation for slice scan time correction, and high-pass temporal filtering to 3 cycles/run. They were then co-registered with anatomic images and transformed into Talairach space[26].
For group analysis, transformed data were spatially smoothed with an isotropic Gaussian kernel (full width half maximum = 4 mm) and baseline periods were normalized based on the percent signal change. A mask was created using all activated voxels within any condition relative to baseline (uncorrected p<0.01). Statistical analysis of group data used random-effects, general linear models (GLM) followed by pairwise contrasts and correction for multiple comparisons within the mask (corrected p<0.05) by imposing a cluster-volume threshold for contiguous voxels passing a voxel-wise significance threshold of p<0.05, using a 3D extension (implemented in Brain Voyager QX) of the 2D Monte Carlo simulation procedure[27]. (Unless otherwise noted, the Results section only refers to activations surviving such correction for multiple comparisons.) The foci of the resulting activations were located and localized with respect to 3D cortical anatomy using an MRI atlas[28]. These foci were based on activation maxima within a given anatomical region, determined by increasing the statistical threshold until just this center remained. A separate focus was identified if a cluster spanned more than one anatomical region or if multiple maxima existed within the same region. The corresponding slice was then identified within Duvernoy’s atlas [28] and the anatomical region attributed.
Activation maps were generated for three contrasts described below. For each activation site, regions of interest (ROIs) constrained to be no larger than 125 mm3 (5×5×5 mm cube) were created following the methods described in the preceding paragraph. The following contrasts were examined:
First, to compare our results with previous studies of face-name encoding, we combined the “trained” and “untrained” stimuli from the pre-training scan into a single group of “novel” stimuli (since training had yet to take place). Activation in response to these stimuli was compared to that for the repeated face-name pairs (Novel > Repeated) using a balanced contrast.
Second, we used the interaction between list-type (trained, untrained) and session (pre-training, post-training) to tease out training-specific effects while controlling for non-specific factors, including practice effects from scanning twice. This interaction identified regions whose activation increased or decreased between sessions, more for the trained list than the untrained list (Trained (Post > Pre) > Untrained (Post > Pre)). We refer to this interaction as “training-specific” changes.
Third, we assessed non-specific factors such as practice effects, and/or generalization of the EMT strategies, by analyzing the comparable task-by-session interaction for the untrained and repeated stimuli (Untrained (Post > Pre) > Repeated (Post > Pre)). We refer to this interaction as “non-specific” changes.
Effective connectivity analysis
We used Granger causality analysis (GCA), which relies on the principle of temporal precedence/lag between time series[29], to investigate effective connectivity (i.e. directional interactions between brain regions during task performance[30]). A multivariate implementation of GCA was used, as first applied to electrophysiological data[31] and subsequently by our group to fMRI data [32–38]. Since effective connectivity obtained from GCA is potentially contaminated by leakage of instantaneous correlation into the causal domain, we used a refined method termed correlation-purged Granger causality (CPGC) to filter out the zero-lag correlation leakage and obtain a purer estimate of directional interactions[36, 37]. Our focus was on training-specific cerebral cortical changes; thus, we included all cortical regions showing significant training-specific increases or decreases in activation. We selected the ROI with the highest t-max when multiple ROIs were identified within a given anatomical region. This resulted in a total of 19 ROIs that were entered into the CPGC analysis. This principled approach avoided arbitrary or subjective choices that are often pitfalls in connectivity analyses.
The time series data from the selected ROIs were averaged across voxels within each ROI, normalized separately for each run and subject by dividing by the grand mean for that run, and concatenated across all runs and subjects to form a single vector per ROI. Then, a modified vector autoregressive model (mVAR) was computed[36, 37]. From this model, a CPGC connectivity matrix was derived comprising path weights for all potential interactions between the selected ROIs. As noted previously [33–35, 37], interactions between any two ROIs that are mediated through another ROI in the matrix are filtered out by this approach, as are zero-lag correlations that may include task-induced correlations due to simultaneous onsets of activation as opposed to true functional connectivity.
Performing data-driven GCA (as in the present study) can require a large number of ROIs, which complicates interpretation. Therefore, we employed a network reduction method to eliminate redundancy in the network, as detailed earlier [34]. This was done by removing ROIs that did not significantly reduce the overall network connectivity when eliminated and repeating this process iteratively until no further ROIs could be removed without significantly impacting overall network connectivity. This is a principled method of network reduction that is data-driven rather than arbitrary.
Within this reduced network, we then examined connectivity specific to blocks for trained stimuli, separately for pre- and post-training scans. We calculated the statistical significance (p<0.05) of the path weights for interactions during presentation of trained stimuli by comparing them with those obtained from a surrogate null distribution derived by a Monte Carlo simulation technique with 10,000 repetitions, as detailed earlier [33]. As described previously [35], surrogate data were derived by transforming the original time series into the frequency domain, randomizing their phase so as to be uniformly distributed over (−π, π) and subsequently transforming the data back to the time domain using the randomized phase spectrum but retaining the original magnitude spectrum Note that a limitation of our GCA approach (and also other effective connectivity analyses) is that it can only provide connectivity information among the selected ROIs; interactions involving non-selected regions are not addressed.
Results
Behavioral results
A repeated-measures ANOVA revealed significant main effects of time (F1,5=42, p=.001), with significant post-training improvement in memory for both lists, and of list-type, with better performance for the trained relative to the untrained stimuli (F1,5=35, p=.002) (Figure 1). The time by list-type interaction (F1,5=54, p=.001) was also significant due to similar pre-training performances (t5=0.6, p=.5) but significantly better post-training performance on the trained than the untrained list (t5=9.5, p<.001).
Figure 1.
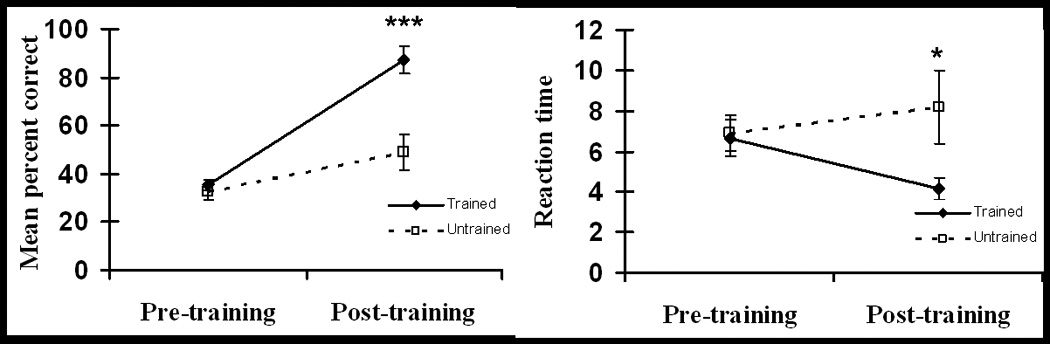
Analyses of reaction times (RTs) revealed no significant main effects (time: F1,5=0.5, p=.5; list-type: F1,5=5.4, p=.07) but a significant interaction (F1,5=7.2, p=.04), which resulted from similar pre-training RTs (t5=0.5, p=.6) but significantly faster post-training RTs for the trained than the untrained stimuli (t5=2.95, p=.05). In general, these changes in memory performance were similar to those in the larger group of eight subjects[22].
fMRI activation changes
Novel vs. repeated
In the pre-training session, encoding novel, relative to repeated face-name pairs, evoked widespread, bilateral activations within occipital cortex and the fusiform gyrus. Right fusiform activations were consistent with the location of the fusiform face area (FFA), as described previously[39]. Left fusiform activations were in posterior portions of the gyrus. In addition, activity was found in the left hippocampus (Hp) (Talairach x,y,z -20,-25,-5) and parahippocampal gyrus (PHG) (Talairach x,y,z -15,-27,-4), but did not survive correction for multiple comparisons. This pattern of activation is consistent with previous research investigating face-name associations in patients with MCI and AD[21, 40]. Greater activation for novel stimuli was also found in the left superior frontal gyrus and bilaterally along the central sulcus and posteroventral intraparietal sulcus (IPS) (V7 as designated by Swisher et al.[41]). Coordinates of the activations surviving correction for multiple comparisons are given in Table 2.
Table 2.
Activations for Novel > Repeated contrast (pre-training scan only). x,y,z: Talairach coordinates; t-max: peak t value; Abbreviations used in the Results section are provided in parentheses.
| Region | Side | x | y | z | t-max |
|---|---|---|---|---|---|
| Superior occipital gyrus (SOG) | R L |
27 13 |
−85 −91 |
7 14 |
11.1 5.2 |
| Inferior middle occipital gyrus (iMOG) | R L L |
36 −24 −47 |
−76 −88 −64 |
−5 −5 −8 |
6.3 14.5 5.7 |
| Intraoccipital sulcus | R | 30 | −82 | 13 | 10.9 |
| Lateral occipital sulcus | R L |
24 −27 |
−85 −91 |
−5 1 |
6.3 9.3 |
| Fusiform gyrus | |||||
| Middle (fusiform face area, FFA) | R R R |
26 33 34 |
−61 −64 −55 |
−11 −11 −12 |
7.5 11.1 5.2 |
| Posterior | L L |
−29 −17 |
−72 −91 |
−14 −9 |
5.3 7.6 |
| Intraparietal sulcus (IPS) (V7) | R L |
27 −27 |
−73 −82 |
22 25 |
25.2 10.8 |
| Postcentral gyrus | R | 41 | −19 | 61 | 5.7 |
| Central sulcus | R | 31 | −24 | 55 | 6 |
| Precentral gyrus | L | −47 | −9 | 52 | 10.7 |
| Superior frontal gyrus | L L |
−12 −12 |
44 36 |
44 52 |
4.3 9.7 |
Training-specific changes
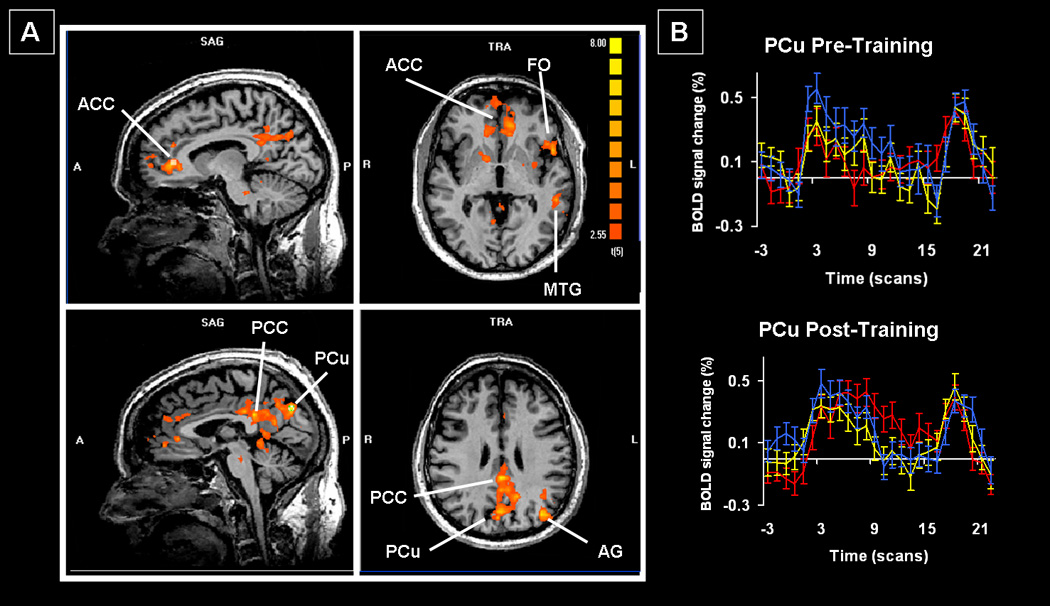
Training-specific increases in activation were found within a widespread network (Table 3; Figure 2). Many of these changes were within medial frontal (frontopolar gyrus, rostral anterior cingulate cortex (ACC)), medial parietal (posterior cingulate cortex (PCC) and precuneus (PCu)), and medial occipital cortex (infra- and supracalcarine, lingual gyrus(LG)). Increases were also found in more lateral regions, including the left frontal operculum (FO, in the vicinity of Broca’s area), around the left temporoparietal junction (angular gyrus; posterior superior temporal sulcus), and more inferiorly in left temporal cortex (middle and inferior temporal gyrus). Examination of the BOLD signal time courses confirmed that all these areas showed greater post-training increases in activation for trained compared to untrained stimuli. Several of these medial frontal and parietal regions and the lateral temporoparietal regions comprise what has become known as the default network[42]. Two regions, both in the middle occipital gyrus (Table 3) showed increases after training that were greater for the untrained than the trained list. Within the regions reported above, the supracalcarine activity was least consistent across subjects (3/6 patients) whereas all other cortical increases or decreases in activity following training were present in at least 4 of the 6 patients; the increases were evident in all 6 patients in the majority of regions.
Table 3.
Training-specific activations from interaction (Trained (Post > Pre) > Untrained (Post > Pre)). x,y,z: Talairach coordinates; t-max: peak t value.
| Region | Side | x | y | z | t-max |
|---|---|---|---|---|---|
| Activation increases greater for Trained than Untrained | |||||
| Lingual gyrus (LG) | R* | 7 | −55 | −1 | 3.9 |
| Infracalcarine | R* | 5 | −50 | 5 | 8.0 |
| Supracalcarine | R* R |
6 5 |
−76 −70 |
13 15 |
6.0 3.7 |
| Posterior precuneus (pPCu) | R* M/R L |
4 1 −3 |
−75 −68 −70 |
29 23 31 |
13.2 9.6 8.9 |
| Anterior precuneus (aPCu) | M/R* M/L L |
1 −2 −13 |
−57 −55 −54 |
31 19 26 |
9.1 5.7 7.2 |
| Posterior cingulate cortex (PCC) | M* L R |
0 −5 6 |
−37 −47 −40 |
25 27 41 |
10.6 5.5 3.8 |
| Angular gyrus (AG) | L* L |
−39 −44 |
−72 −59 |
28 32 |
11.3 5.1 |
| Posterior superior temporal sulcus (pSTS) | L* | −52 | −56 | 18 | 5.4 |
| Middle temporal gyrus (MTG) | L* | −50 | −34 | 2 | 8.0 |
| Inferior temporal sulcus (ITS) | L* | −62 | −22 | −3 | 4.9 |
| Inferior temporal gyrus (ITG) | L* | −48 | −40 | −7 | 6.3 |
| Mid-insular cortex | L L* |
−32 −27 |
−2 6 |
6 16 |
4.5 9.9 |
| Rostral anterior cingulate cortex (ACC) | R L* |
10 −7 |
34 36 |
2 4 |
4.1 9.6 |
| Anterior-medial frontal cortex | R* | 8 | 51 | 13 | 5.9 |
| (amFC) / frontopolar gyrus | R L* |
3 −11 |
56 54 |
4 9 |
4.5 8.0 |
| Frontal operculum (FO) | L* | −47 | 19 | −1 | 6.4 |
| Putamen | R L |
12 −25 |
1 5 |
6 3 |
8.9 4.0 |
| Superior cerebellum | L | −6 | −45 | −13 | 9.7 |
| Activation increases greater for Untrained than Trained | |||||
| Middle occipital gyrus | |||||
| Inferior (iMOG) | L* | −28 | −93 | −2 | −4.8 |
| Superior (sMOG) | L* | −36 | −80 | 7 | −4.7 |
denotes ROIs used in the Granger causality analysis. Abbreviations used in the Results and Discussion sections are provided in parentheses.
Figure 2.
Non-specific changes
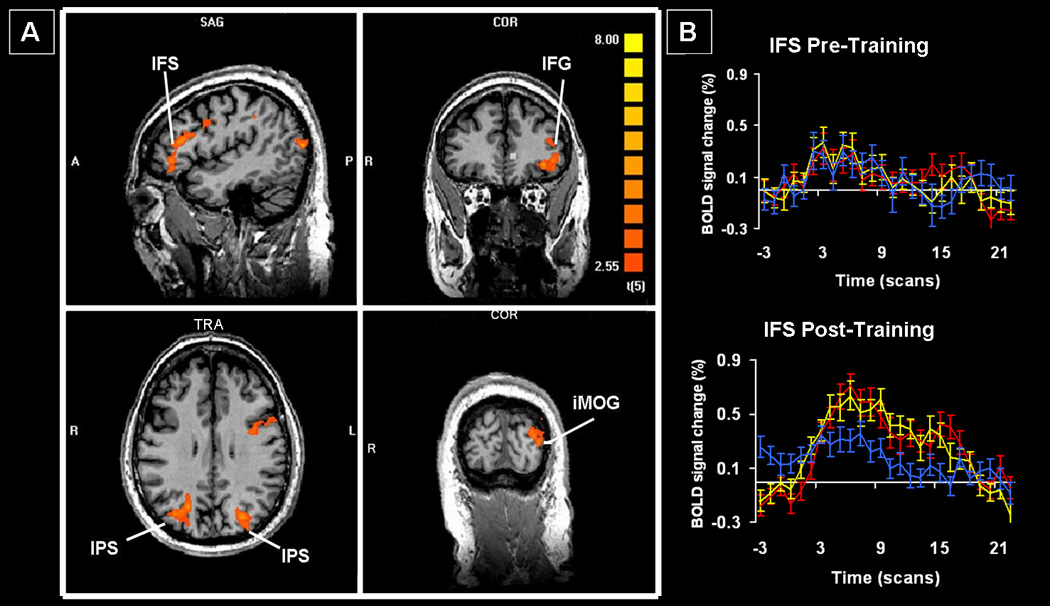
Non-specific changes included increased activation within the left occipital and inferior frontal cortex, and bilaterally in inferior parietal cortex (regions IPS1 and V7 by Swisher et al.[41]) (Table 3; Figure 3). The BOLD signal time courses were indistinguishable for the trained and untrained stimuli in most of these regions and were consistently greater than those for the repeated stimuli. There were no regions of significantly decreased activation. At least 5 of the 6 patients demonstrated activation in all the areas identified on this contrast.
Figure 3.
Effective connectivity changes
Thirteen of the 19 ROIs survived network reduction reduction; significant path weights during presentation of trained stimuli are shown in Figure 4 and Table 5. The number of significant paths increased from 7 during pre-training to 12 post-training. The MTG was a primary “driver” of activation in other areas during both scans; however, some of the targets changed to include two occipital (LG and iMOG) and two medial parietal regions (aPCu, pPCu) post-training, compared to a more distributed pattern of targets pre-training (one occipital, one medial parietal, one lateral temporal and one medial frontal). The sMOG and aPCu emerged as other significant drivers following EMT whereas these regions did not show any significant connectivity with other areas before training. (For abbreviations, see Tables 2–4).
Figure 4.
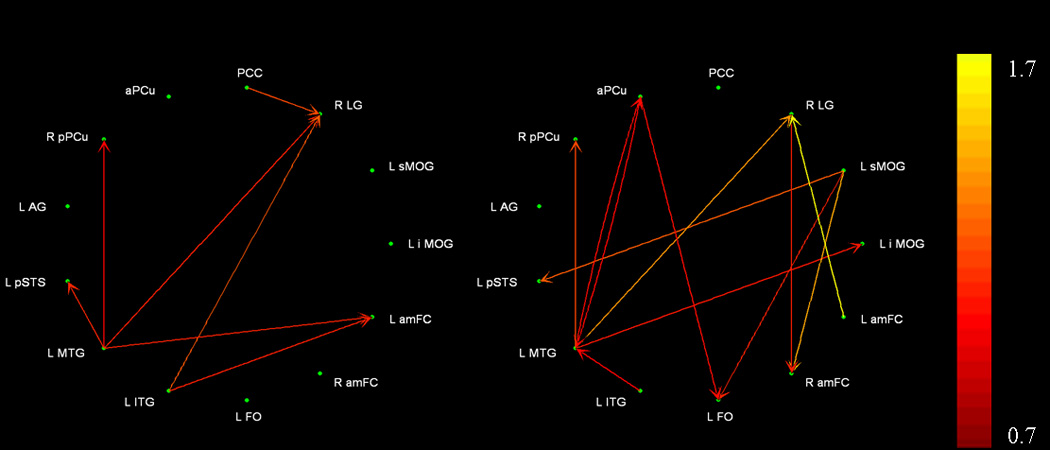
Table 5.
Significant path weights during encoding of trained stimuli. Outputs from each region are listed in columns whereas inputs into each region are listed in rows. Pre-training values are shown in the top table, post-training values are in the bottom table.
| Pre-Training | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L iMOG | L sMOG | R LG | PCC | aPCu | R pPCu | L AG | L pSTS | L MTG | L ITG | L FO | R amFC | L amFC | |
| L iMOG | |||||||||||||
| L sMOG | |||||||||||||
| R LG | 1.07 | 0.86 | 1.15 | ||||||||||
| PCC | |||||||||||||
| aPCu | |||||||||||||
| R pPCu | 0.74 | ||||||||||||
| L AG | |||||||||||||
| L pSTS | 0.94 | ||||||||||||
| L MTG | |||||||||||||
| L ITG | |||||||||||||
| L FO | |||||||||||||
| R amFC | |||||||||||||
| L amFC | 0. 88 | 0.91 | |||||||||||
| Post-Training | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L iMOG | L sMOG | R LG | PCC | aPCu | R pPCu | L AG | L pSTS | L MTG | L ITG | L FO | R amFC | L amFC | |
| L iMOG | 0.84 | ||||||||||||
| L sMOG | |||||||||||||
| R LG | 1.27 | 1.72 | |||||||||||
| PCC | |||||||||||||
| aPCu | 0.78 | ||||||||||||
| R pPCu | 1.02 | ||||||||||||
| L AG | |||||||||||||
| L pSTS | 1.10 | ||||||||||||
| L MTG | 0.78 | 0.75 | |||||||||||
| L ITG | |||||||||||||
| L FO | 0.85 | 0.80 | |||||||||||
| R amFC | 1.38 | 0.86 | |||||||||||
| L amFC | |||||||||||||
Table 4.
Non-specific activations from interaction (Untrained (Post > Pre) > Repeated (Post > Pre)). x,y,z: Talairach coordinates; t-max: peak t value. Abbreviations used in the Results section are provided in parentheses.
| Region | Side | x | y | z | t-max |
|---|---|---|---|---|---|
| Superior occipital gyrus (SOG) | L | −26 | −90 | 11 | 5.1 |
| Middle occipital gyrus | |||||
| Inferior (iMOG) | L | −30 | −88 | 6 | 4.7 |
| Superior (sMOG) | L | −39 | −80 | 16 | 5.8 |
| Supramarginal gyrus (SMG) | L | −48 | −34 | 37 | 8.4 |
| Angular Gyrus (AG) | R | 33 | −70 | 26 | 4.9 |
| Intraparietal sulcus (IPS) | |||||
| IPS1 | R L |
22 −31 |
−64 −72 |
32 37 |
6.9 4.1 |
| V7 | L L |
−25 −27 |
−73 −79 |
27 31 |
5.5 7.1 |
| Inferior frontal sulcus (IFS) | L L L |
−43 −39 −35 |
29 25 1 |
17 20 29 |
5.9 4.9 4.2 |
| Inferior frontal gyrus (IFG) | L | −40 | 37 | 1 | 4.9 |
| Orbitofrontal cortex | L | −32 | 34 | −3 | 6.4 |
Discussion
This pilot study demonstrated that significant behavioral improvement after EMT in patients with MCI is associated with training-specific increases in activity within medial parts of frontal, parietal, and occipital cortex as well as lateral areas around the temporoparietal junction and left frontal operculum. Additionally, training generally resulted in increased connectivity. A separate network involving lateral frontal and parietal regions was common to trained and untrained stimuli. Importantly, the pre-training pattern of activation was consistent with previous studies of face-name encoding[21, 40], showing that our imaging paradigm is comparable to previous research.
Behavioral Changes
As reported earlier[22], our results join only a few other studies indicating that EMT can be beneficial in patients with MCI[9] and early AD[4, 6]. Our focused intervention presumably reduced the confusion caused by using multiple mnemonic strategies[10] and allowed patients to implement the trained strategies. We reported earlier[22] that there were significant correlations between recall of names and of mnemonic cues, supporting the patients’ anecdotal reports that they were attempting to use the strategies during the post-training scan.
Training-specific fMRI changes
The most robust training-specific increases were within the medial frontal and parietal cortices and around the temporoparietal junction, which are part of the default network[42] and abnormal in MCI and AD[42, 43]. This network is posited to control self-directed, internally-based processes, including episodic memory[42, 44]; processes likely utilized by EMT strategies. Previous research has identified three primary regions through which the default network is posited to interact with other brain areas[42].
First, the PCC interacts with the medial temporal lobe memory system[42]. Dysfunction in this region may be at least partially responsible for the memory deficits in MCI and AD[45], consistent with the lack of PCC connectivity at either time point. The PCC and its neighbor, the PCu have extensive reciprocal connections[46]. Increased activity in these regions corresponds with better explicit memory functioning[45–47] while dysfunction is related to worse memory functioning[45] and increased rate of conversion from MCI to AD[48]. EMT results in increased activation in these regions in neurologically healthy subjects[13, 15, 17].The aPCu demonstrated increased connectivity post-training as it formed a reciprocal connection with the MTG and drove activation within the left FO. This may reflect the role of the PCu in mental imagery[46], an important mnemonic strategy used in EMT, or heightened personal relevance of information[46, 49]. Finally, the PCu is thought to mediate top-down control of attention during memory retrieval[50], as would be required when patients search for specific cues. The increased connectivity with the left FO could reflect the use of verbally-based cues. Thus, EMT appears to have provided a mechanism through which processes mediated by the PCC and PCu could be utilized.
The left temporoparietal junction is another region identified as containing key default network areas[42]. This region is posited to mediate the bottom-up capture of attentional resources by memory-relevant cues[50], which could occur as patients identify the salient stimulus features. The angular gyrus is important for language processing, which may explain the greater left hemisphere activation given our verbally laden strategies. However, the angular gyrus did not demonstrate significant connectivity whereas other language areas (FO and MTG) increased connectivity post-training. The MTG appeared especially important for the face-name association task, since it demonstrated the most connectivity at both time points and a similar MTG region was more active for familiar than novel face-name associations in patients with MCI [40]. A recent meta-analysis suggested the MTG and ITG are part of the semantic memory network and may play a particular role in supramodal processing and concept retrieval [51]. These findings suggest that our EMT procedures may have utilized semantically related memory areas. Future studies will be necessary to determine whether EMT bypasses or augments the dysfunctional episodic memory system in favor of the relatively preserved semantic network in patients with MCI [52, 53].
The medial frontal cortex (ACC, frontopolar cortex) is the third important default network region and may be responsible for coordinating multiple cognitive processes[42]; a role that accords with the frequency with which these areas show increased activation in functional neuroimaging studies[50]. Here, these regions exhibited significant post-training increases in activation and changes in connectivity, suggesting that EMT facilitated the coordination of multiple cognitive processes.
Non-specific fMRI changes
Significant post-training increases in activation were observed in lateral frontal and parietal cortical areas, with similar activation magnitudes for both trained and untrained stimuli. These changes may reflect practice effects given re-exposure to the same stimuli seen pre-training, or attempts to generalize the trained strategies to the untrained stimuli. The most notable changes were within the IFG and IFS. These areas have been implicated in the resolution of interference among conflicting stimulus attributes[54] and the rehearsal of internally based representations[55, 56]. Additionally, increased activation was found within the superior middle occipital gyrus (sMOG) and FFA; regions previously found more active after healthy individuals were trained to attend to specific facial features[57, 58]. Together, these findings tend to support our patients’ reported attempts to generalize trained strategies to the untrained stimuli, especially since practice effects typically result in decreased activation[18–20, 60–61]. Further work will be needed to definitively resolve the basis of these non-specific changes.
Conclusions and Implications
This pilot study provides the first evidence, to our knowledge, that the beneficial effect of focused EMT for patients with MCI results from the increased use of distributed neural networks mediating explicit memory functions (as hypothesized). Mere repetition of stimuli during training is unlikely to be solely responsible for the behavioral improvement since 1) we previously reported an inverse correlation between the number of training trials and subsequent memory test improvement[22] and 2) we primarily found increased activation following training as opposed to the well-established repetition suppression effect of the BOLD signal[23, 25, 59, 60] that persists in AD[24].
The level of effort required by a task could affect activation patterns. Although using EMT strategies is effortful, we do not think this can account for our findings since patients demonstrated significant improvements in both accuracy and RT, which suggests the memory test was easier post-training. EMT is, however, likely to be more difficult for MCI than healthy elderly. Ongoing studies by our group suggest that MCI patients demonstrate greater post-EMT increases in activation than healthy elderly individuals. However, it is currently unclear whether these changes represent compensatory activation or a relative restoration of normal activation that is associated with task performance.52
Despite our small sample size, the robust behavioral and neural changes are quite encouraging and should serve as the basis for future studies. We anticipate the importance of EMT for patients with MCI and AD will escalate once pharmacological agents that modify or arrest disease progression are developed, and that synergistic use of such agents and rehabilitative interventions[62] will become more prevalent.
Acknowledgements
This material is based upon work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, and Rehabilitation Research and Development Service through grants B4602H & B6366W to B.H., B3323K and B4954N to A.M. This work was also funded by the Atlanta VAMC RR&D Center of Excellence. Support to KS from the Atlanta VAMC and from National Institutes of Health (NIH) grant K24 EY017332 and to XH from Georgia Research Alliance and NIH grant R01EB002009 is also gratefully acknowledged. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government. Portions of this work were presented at the 2008 annual meetings of the International Neuropsychological Society, the American Academy of Clinical Neuropsychology, and the Society for Neuroscience. We wish to thank Dr. Felicia Goldstein for her assistance with patient recruitment.
Footnotes
Conflicts of Interest
Dr. Anthony Y. Stringer is author of the Ecologically Oriented Neurorehabilitation of Memory (EON-Mem) program and receives royalties from sales. No other author has any conflict of interest.
Author Contributions
Each author provided significant intellectual contribution to warrant authorship and declares that he/she has seen and approved the final version of this manuscript. Dr. Benjamin M. Hampstead had full access to all the data in the study; Dr. K. Sathian had final responsibility for the decision to submit for publication.
References
- 1.Cicerone KD, Dahlberg C, Malec JF, et al. Evidence-based cognitive rehabilitation: Update review of literature from 1998 through 2002. Arch Phys med Rehabil. 2005;86:1681–1692. doi: 10.1016/j.apmr.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Craik FIM, Winocur G, Palmer H, et al. Cognitive rehabilitation in the elderly: Effects on memory. J Int Neuropsychol Soc. 2007;13:132–142. doi: 10.1017/S1355617707070166. [DOI] [PubMed] [Google Scholar]
- 3.Wolinsky FD, Unverzagt FW, Smith DM, Jones R, Stoddard A, Tennstedt SL. The ACTIVE cognitive training trial and health-related quality of life: Protection that lasts for 5 years. J Gerontol A Biol Sci Med Sci. 2006;61:1324–1329. doi: 10.1093/gerona/61.12.1324. [DOI] [PubMed] [Google Scholar]
- 4.Acevedo A, Loewenstein DA. Nonpharmacological cognitive interventions in aging and dementia. J Geriatr Psychiatry Neurol. 2007;20:239–249. doi: 10.1177/0891988707308808. [DOI] [PubMed] [Google Scholar]
- 5.De Vreese LP, Neri M, Fioravanti M, Belloi L, Zanetti O. Memory rehabilitation in Alzheimer’s disease: A review of progress. Int J Geriatr Psychiatry. 2001;16:794–809. doi: 10.1002/gps.428. [DOI] [PubMed] [Google Scholar]
- 6.Loewenstein DA, Acevedo A, Czaja SJ, Duara R. Cognitive rehabilitation of mildly impaired Alzheimer’s disease patients on cholinesterase inhibitors. Am J Geriatr Psychiatry. 2004;12:395–402. doi: 10.1176/appi.ajgp.12.4.395. [DOI] [PubMed] [Google Scholar]
- 7.Clare L, Woods RT. Cognitive training and cognitive rehabilitation for people with early-stage Alzheimer’s disease: A review. Neuropsychol Rehabil. 2004;14:385–401. [Google Scholar]
- 8.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 9.Belleville S, Gilbert B, Fontaine F, Gagnon L, Menard E, Gauthier S. Improvement of episodic memory in persons with mild cognitive impairment and healthy older adults: Evidence from a cognitive intervention program. Dement Geriatr Cogn Disord. 2006;22:486–499. doi: 10.1159/000096316. [DOI] [PubMed] [Google Scholar]
- 10.Rapp S, Brenes G, Marsh AP. Memory enhancement training for older adults with mild cognitive impairment: a preliminary study. Aging Ment Health. 2002;6:5–11. doi: 10.1080/13607860120101077. [DOI] [PubMed] [Google Scholar]
- 11.Unverzagt FW, Kasten L, Johnson KE, et al. Effect of memory impairment on training outcomes in ACTIVE. J Int Neuropsychol Soc. 2007;13:953–960. doi: 10.1017/S1355617707071512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stringer AY. Ecologically-oriented Neurorehabilitation of Memory Therapist Manual. Los Angeles, CA: Western Psychological Services; 2007. [Google Scholar]
- 13.Kondo Y, Suzuki M, Mugikura S, et al. Changes in brain activation associated with use of a memory strategy: a functional MRI study. NeuroImage. 2005;24:1154–1163. doi: 10.1016/j.neuroimage.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 14.Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: Dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- 15.Miotto EC, Savage CR, Evans JJ, et al. Bilateral activation of the prefrontal cortex after strategic semantic cognitive training. Hum Brain Mapp. 2006;27:288–295. doi: 10.1002/hbm.20184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savage CR, Deckersbach T, Heckers S, et al. Prefrontal regions supporting spontaneous and directed application of verbal learning strategies - Evidence from PET. Brain. 2001;124:219–231. doi: 10.1093/brain/124.1.219. [DOI] [PubMed] [Google Scholar]
- 17.Nyberg L, Sandblom J, Jones S, et al. Neural correlates of training-related memory improvement in adulthood and aging. Proc Natl Acad Sci USA. 2003;100:13728–13733. doi: 10.1073/pnas.1735487100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chua EF, Schacter DL, Rand-Giovannetti E, Sperling RA. Evidence for a specific role of the anterior hippocampal region in successful associative encoding. Hippocampus. 2007;17:1071–1080. doi: 10.1002/hipo.20340. [DOI] [PubMed] [Google Scholar]
- 19.Kirwan CB, Stark CEL. Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus. 2004;14:919–930. doi: 10.1002/hipo.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sperling RA, Bates JF, Cocchiarella AJ, Schacter DL, Rosen BR, Albert MS. Encoding novel face-name associations: A functional MRI study. Hum Brain Mapp. 2001;14:129–139. doi: 10.1002/hbm.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sperling RA, Bates JF, Chua EF, et al. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74:44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hampstead BM, Sathian K, Moore AB, Nalisnick C, Stringer AY. Explicit memory training leads to improved memory for face-name pairs in patients with mild cognitive impairment: Results of a pilot investigation. J Int Neuropsychol Soc. 2008;14:883–889. doi: 10.1017/S1355617708081009. [DOI] [PubMed] [Google Scholar]
- 23.Henson RNA. Neuroimaging studies of priming. Prog Neurobiol. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- 24.Lustig C, Buckner RL. Preserved neural correlates of priming in old age and dementia. Neuron. 2004;42:865–875. doi: 10.1016/j.neuron.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Schacter DL, Dobbins IG, Schnyer DM. Specificity of priming: A cognitive neuroscience perspective. Nat Rev Neurosci. 2004;5:853–862. doi: 10.1038/nrn1534. [DOI] [PubMed] [Google Scholar]
- 26.Talairach JT, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York, NY: Thieme Medical Publishers; 1988. [Google Scholar]
- 27.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI) - Use of a cluster size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 28.Duvernoy HM. The Human Brain: Surface, blood supply, and three-dimensional sectional anatomy. New York, NY: Springer; 1999. [Google Scholar]
- 29.Granger CWJ. Investigating causal relations by econometric models and cross-spectral methods. Econometrica. 1969;37:424–438. [Google Scholar]
- 30.Buchel CF, Friston K. Extracting brain connectivity. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An introduction to methods. Oxford, UK: Oxford University Press; 2001. pp. 295–308. [Google Scholar]
- 31.Kus R, Kaminski M, Blinowska K. Determination of EEG activity propagation: pair-wise versus multichannel estimate. IEEE Trans Biomed Eng. 2004;51:1501–1510. doi: 10.1109/TBME.2004.827929. [DOI] [PubMed] [Google Scholar]
- 32.Deshpande G, Sathian K, Hu X. Effect of hemodynamic variability on Granger causality analysis of fMRI. NeuroImage. doi: 10.1016/j.neuroimage.2009.11.060. in press. PMID:20004248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stilla RF, Deshpande G, LaConte S, Hu X, Sathian K. Posteromedial parietal cortical activity and inputs predict tactile spatial acuity. J Neurosci. 2007;27:11091–11102. doi: 10.1523/JNEUROSCI.1808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deshpande G, Hu X, Stilla R, Sathian K. Effective connectivity during haptic perception: A study using Granger causality analysis of functional magnetic resonance imaging data. NeuroImage. 2008;40:1807–1814. doi: 10.1016/j.neuroimage.2008.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deshpande G, LaConte S, James G, Peltier S, Hu X. Multivariate Granger causality analysis of brain networks. Hum Brain Mapp. 2009;30:1361–1373. doi: 10.1002/hbm.20606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deshpande G, Sathian K, Hu X. Assessing and compensating for zero-lag correlation in time-lagged Granger causality analysis of fMRI. IEEE Trans Biomed Eng. doi: 10.1109/TBME.2009.2037808. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deshpande G, Hu X, Lacey S, Stilla R, Sathian K. Object familiarity modulates effective connectivity during haptic shape perception. NeuroImage. 2010;49:1991–2000. doi: 10.1016/j.neuroimage.2009.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stilla R, Hanna R, Hu X, Mariola E, Deshpande G, Sathian K. Neural processing underlying tactile microspatial discrimination in the blind: a functional magnetic resonance imaging study. J Vis. 2008;8:1–19. doi: 10.1167/8.10.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grill-Spector K, Sayres R, Ress D. High-resolution imaging reveals highly selective nonface clusters in the fusiform face area. Nat Neurosci. 2006;9:1177–1185. doi: 10.1038/nn1745. [DOI] [PubMed] [Google Scholar]
- 40.Petrella JR, Wang L, Krishnan S, et al. Cortical deactivation in mild cognitive impairment: High field strength functional MR imaging. Radiology. 2007;245:224–235. doi: 10.1148/radiol.2451061847. [DOI] [PubMed] [Google Scholar]
- 41.Swisher JD, Halko MA, Merabet LB, McMains SA, Somers DC. Visual topography of human intraparietal sulcus. J Neurosci. 2007;27:5326–5337. doi: 10.1523/JNEUROSCI.0991-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 43.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golland Y, Golland P, Bentin S, Malach R. Data-driven clustering reveals a fundamental subdivision of the human cortex into two global systems. Neuropsychologia. 2008;46:540–553. doi: 10.1016/j.neuropsychologia.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson SC, Schmitz TW, Moritz CH, et al. Activation of brain regions vulnerable to Alzheimer’s disease: The effect of mild cognitive impairment. Neurobiol Aging. 2006;27:1604–1612. doi: 10.1016/j.neurobiolaging.2005.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 47.Shannon BJ, Buckner RL. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J Neurosci. 2004;24:10084–10092. doi: 10.1523/JNEUROSCI.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson KA, Moran EK, Becker JA, Blacker D, Fischman AJ, Albert MS. Single photon emission computed tomography perfusion differences in mild cognitive impairment. J Neurol Neurosurg Psychiatry. 2007;78:240–247. doi: 10.1136/jnnp.2006.096800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ries ML, Schmitz TQ, Kawahara TN, Torgerson BM, Trivedi MA, Johnson SC. Task-dependent posterior cingulate activation in mild cognitive impairment. NeuroImage. 2006;29:485–492. doi: 10.1016/j.neuroimage.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Binder JR, Desai RH, Graves WW, Conant LL. Where Is the Semantic System? A Critical Review and Meta-Analysis of 120 Functional Neuroimaging Studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woodard JL, Seidenberg M, Nielson KH, et al. Semantic memory activation in amnestic mild cognitive impairment. Brain. 2009;132:2068–2078. doi: 10.1093/brain/awp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greenberg DL, Keane MM, Ryan L, Verfaellie M. Impaired Category Fluency in Medial Temporal Lobe Amnesia: The Role of Episodic Memory. J Neurosci. 2009;29:10900–10908. doi: 10.1523/JNEUROSCI.1202-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nelson JK, Reuter-Lorenz PA, Sylvester CYC, Jonides J, Smith EE. Dissociable neural mechanisms underlying response-based and familiarity-based conflict in working memory. Proc Natl Acad Sci USA. 2003;100:11171–11175. doi: 10.1073/pnas.1334125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hillary FG, Genova HM, Chiaravalloti ND, Rypma B, DeLuca J. Prefrontal modulation of working memory performance in brain injury and disease. Hum Brain Mapp. 2006;27:837–847. doi: 10.1002/hbm.20226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu YD, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440:91–95. doi: 10.1038/nature04262. [DOI] [PubMed] [Google Scholar]
- 57.Gobbini MI, Haxby JV. Neural response to the visual familiarity of faces. Brain Res Bull. 2006;71:76–82. doi: 10.1016/j.brainresbull.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 58.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 59.Chein JM, Schneider W. Neuroimaging studies of practice-related change: fMRI and meta-analytic evidence of a domain-general control network for learning. Cogn Brain Res. 2005;25:607–623. doi: 10.1016/j.cogbrainres.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 60.Rand-Giovannetti E, Chua EF, Driscoll AE, Schacter DL, Albert MS, Sperling RA. Hippocampal and neuocortical activation during repetitive encoding in older persons. Neurobiol Aging. 2006;27:173–182. doi: 10.1016/j.neurobiolaging.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 61.Seidenberg M, Guidotti L, Nielson KA, et al. Semantic memory activation in individuals at risk for developing Alzheimer disease. Neurology. 2009;73:612–620. doi: 10.1212/WNL.0b013e3181b389ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berthier ML, Green JP, Lara JP, et al. Memantine and constraint-induced aphasia therapy in chronic poststroke aphasia. Ann Neurol. 2009;65:577–585. doi: 10.1002/ana.21597. [DOI] [PubMed] [Google Scholar]


