Abstract
The concept of the neurovascular unit as the key brain component affected by stroke is controversial, because current definitions of this entity neglect mechanisms that control perfusion and reperfusion of arteries and arterioles upstream of the cerebral microcirculation. Indeed, although definitions vary, many researchers consider the neurovascular unit to be restricted to endothelial cells, neurons and glia within millimetres of the cerebral capillary microcirculation. This Perspectives article highlights the roles of vascular smooth muscle, endothelial cells and perivascular innervation of cerebral arteries in the initiation and progression of, and recovery from, ischaemic stroke. The concept of the vascular neural network—which includes cerebral arteries, arterioles, and downstream neuronal and glial cell types and structures—is introduced as the fundamental component affected by stroke pathophysiology. The authors also propose that the vascular neural network should be considered the main target for future therapeutic intervention after cerebrovascular insult.
Introduction
The currently accepted model of stroke pathophysiology is largely understood in terms of the neurovascular unit, which includes endothelial cells, astrocytes, pericytes and neurons associated with the capillary vasculature.1 In this model, vascular pathology is thought to develop in the neurovascular unit just before or during a stroke, and to progress throughout ischaemia and reperfusion. These processes culminate in tissue and vascular remodelling after stroke.
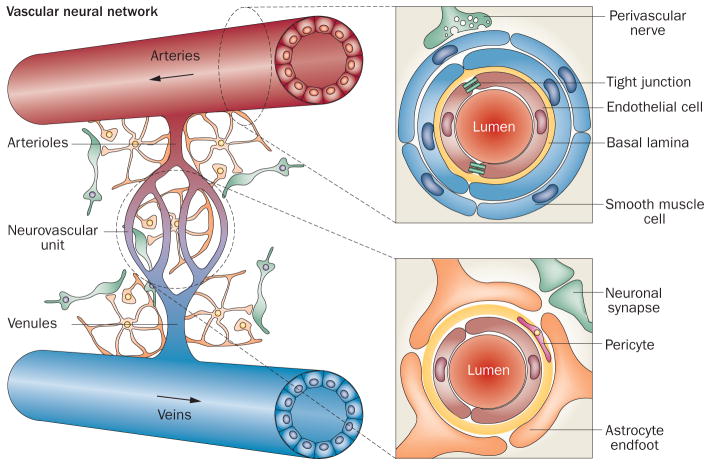
The concept of the neurovascular unit integrates neural and vascular cell types to help explain the failure of neuroprotective strategies, which target neuronal cells only and do not address vascular injury, which can be a major component of delayed stroke damage.2 The neurovascular unit model focuses largely on the areas immediately surrounding capillaries, where neural and vascular cells interact and influence each other, and excludes both downstream venous vasculature and upstream arterioles and smaller arteries.1,3 Consequently, the crucial roles of vascular smooth muscle cells, arterial endothelium and perivascular nerve fibres, and their involvement in vascular injury and compromised reperfusion, are not included in models of stroke pathophysiology that are based on the neurovascular unit. We propose, therefore, that endothelial cells, astrocytes, pericytes and neurons that are intimately associated with the cerebral capillaries—together with vascular smooth muscle cells, arterial endothelial cells and perivascular nerves connecting with arteries and arterioles upstream of the cerebral microcirculation—should be taken into account. In this article, we refer to this expanded group of structures as the vascular neural network (Figure 1), and suggest that it should be considered the fundamental structural and functional entity affected by cerebrovascular pathology. As such, we further propose that the vascular neural network should be a primary target for therapeutic intervention for stroke, as well as other traumatic brain injury and neurodegenerative disorders.
Figure 1.
The neurovascular unit as a component of the vascular neural network. The neurovascular unit consists of capillary endothelial cells, pericytes and basal lamina enveloped by astrocyte endfeet. Astrocytes communicate with adjacent neurons via metabolite exchange. The vascular neural network is larger than the neurovascular unit, as it also includes smooth muscle cells, noncapillary endothelial cells, perivascular nerves, fibroblasts, smooth muscle progenitor cells, and cells of the immune system—as well as collateral blood vessels, the rete vasorum, perivascular nerves and veins. The vascular neural network, therefore, comprises all cells and structures required to maintain cerebral blood flow under physiological and pathological conditions.
In this article, we discuss the limitations of the neurovascular unit model with regard to the functional role of cerebral arteries and their influence on downstream neural elements. We describe the vascular neural network model of stroke pathophysiology, and discuss its implications for the prevention of stroke, preservation of tissue perfusion during and after a stroke, and ultimately reduction of infarct volume.
The neurovascular unit
Ischaemic stroke was initially regarded as a vascular disease, and as such was categorized as a cerebrovascular disorder. During the 1990s, however, research into stroke began to focus on characterization of the underlying neuronal mechanisms, with the aim of identifying strategies to promote neuroprotection. Progress in the field was driven by a new understanding of the role of excitatory amino acids, N-methyl-D-aspartate receptor signalling and calcium channels in promoting neuronal cell death.2 Accumulating evidence that intracellular calcium overload has a critical role in post-stroke neuronal loss2 led to the development of multiple families of calcium-channel blockers for use in limiting tissue damage after stroke. Unfortunately, in clinical trials, virtually all these agents were ineffective.2 A weakness of this therapeutic approach was that neuronal preservation alone could not yield a benefit if poststroke reperfusion was compromised by vascular injury.2
Growing concerns about the inherent limitations of available neuroprotective strategies and the neurotoxic effects of recombinant tissue plasminogen activator (rtPA, a thrombolytic agent) supported the idea that neuroprotection should extend to pathophysiologically important neuron–astrocyte–capillary interactions,1,4 and led to the proposal that these cells and tissues should be considered a unique functional entity—the neurovascular unit. The concept of the neurovascular unit rapidly captured the attention of the stroke research community and became a key element in the understanding of stroke pathophysiology and drug development.1,5
An evolving concept
The neurovascular unit was initially proposed as a triad consisting of vascular smooth muscle, astrocytes and neurons in 1996.6 This concept was revised in 2002, during discussions at the first Stroke Progress Review Group meeting, by the substitution of endothelial cells for vascular smooth muscle cells.7 This updated definition of the neurovascular unit emphasized the complexity of interactions between all perivascular cell types,1 and integrated contemporaneous concepts of the blood–brain barrier (BBB) and the function of cerebrovascular endothelial cells with what was then understood about the roles of the basal membrane, astrocytic foot processes, pericytes, macrophages and other leukocytes.5
The neurovascular unit was a highly useful conceptual advance. However, different research groups characterized it dif ferently (Figure 2), which resulted in variable views of its role in cerebrovascular pathophysiology. The competing models of the neurovascular unit varied in two major respects. First, their physical size varied from a radius of a few hundred micrometres to more than a millimetre.3,8 The presumed functional advantage of the submillimetre scale was that all cells could communicate with each other easily and rapidly, and thus influence each other with great specificity.8 Second, the proposed models also varied with respect to the cell types included. Vascular smooth muscle cells were included in the original 1996 model of the neuron–astrocyte–vasculature triad,6 but were replaced by capillary endothelial cells in the revised definition published in 2002.7 Subsequent models added pericytes, oligodendrocytes and immune cells to the core triad of capillary-endothelial cells, astrocytes and neurons.4,5 Some authors continued to include vascular smooth muscle cells in the neurovascular unit,5 although the physiological importance of these cells was not emphasized, perhaps owing to contemporary ideas that arterial cell types were only minor players in stroke pathophysiology. This lack of emphasis on vascular smooth muscle cells was a logical consequence of the focus at that time on the neurovascular unit as a key determinant of BBB structure and function.4 Moreover, consideration of the neurovascular unit at the micrometre scale largely excluded the contributions of vascular cells to the pathophysiology of brain injury, instead emphasizing its contributions to reduction of infarction size, regeneration of new cells, and reorganization of the cellular environment to facilitate recovery. None of these physiological responses, however, could be achieved without the restoration of ad equate perfusion upstream of the injury.
Figure 2.
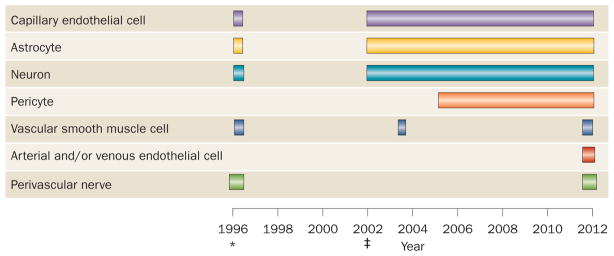
The evolving concept of the neurovascular unit. The concept of the neurovascular unit has changed considerably since its initial proposal as a triad of arteries, astrocytes and neurons15 in 1996. Vascular smooth muscle cells, endothelial cells and perivascular nerves were also included in the definition in some early publications. The definition jointly proposed by the NIH and National Institute of Neurological Disorders and Stroke9 in 2002 comprises endothelial cells, astrocytes and neurons, and subsequent publications highlighted physiological and pathophysiological roles for these cells within the neurovascular unit.*Indicates the initial 1996 publication. ‡Indicates the 2002 NIH–National Institute of Neurological Disorders and Stroke publication.
The role of vascular cell types
A prerequisite for successful poststroke reperfusion is a functional cerebrovascular network of small arteries and arterioles that supply the parenchymal capillaries. Capillaries in the brain arise from intraparenchymal arterioles that bifurcate repeatedly to form the cerebral capillary microcirculation.9 The intraparenchymal arterioles originate from pial arteries, penetrating arteries and nutrient arteries downstream of the middle cerebral, anterior cerebral, posterior cerebral and basilar arteries.9 All these arteries and their downstream arterioles include an essential functional cell type: the vascular smooth muscle cell. These multifunctional cells are organized in concentric layers around the basal lamina and make up the medial layer of the arterial wall.9
In all arteries, vascular smooth muscle cells exhibit a broad range of highly specialized functions, including migration, proliferation, secretion of extracellular matrix proteins and contraction.10 Although vascular smooth muscle cells were originally classified on the basis of morphology as either contractile or synthetic, it is now clear that these cells have multiple coexisting functions and phenotypic characteristics.10 Most importantly, vascular smooth muscle cells are highly dynamic, and their phenotype depends on the combined influences of multiple factors in the perivascular environment (Figure 3). In cerebrovascular injury, these properties can result in dedifferentiation of contractile smooth muscle cells with loss of myogenic tone and autoregulation, which can greatly compromise the vascular response to metabolic changes that require increased cerebral blood flow, which is essential for local neuronal homeostasis.11
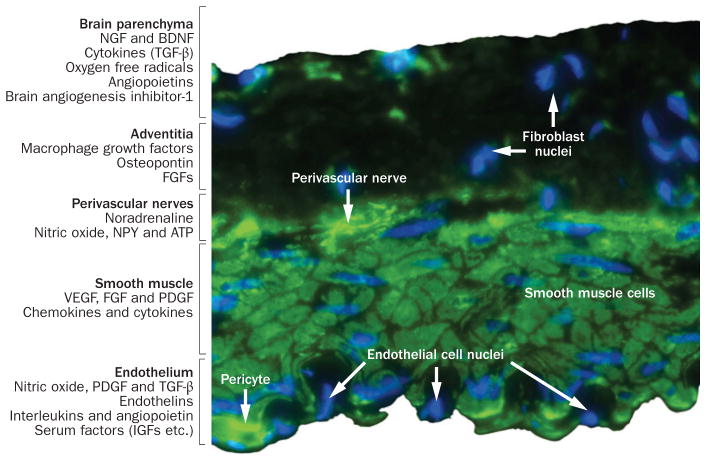
Figure 3.
Immunostained section of a cerebral artery showing the range of cell types present. The vessel lumen is lined with endothelial cells, and pericytes are visible in the subendothelial space. Vascular smooth muscle cells make up the medial layer, and perivascular nerves occur at the medial–adventitial border. The function and phenotype of these cell types are dynamically modulated by various factors released from brain parenchyma, fibroblasts and macrophages in the adventitia, perivascular nerves, vascular smooth muscle cells and endothelial cells (listed on the left). Abbreviations: IGF, insulin-like growth factor; BDNF, brain derived neurotrophic factor; FGF, fibroblast growth factor; NGF, nerve growth factor; NPY, neuropeptide Y; PDGF, platelet derived growth factor; TGF-β, transforming growth factor-β; VEGF, vascular endothelial growth factor.
Vascular function during cerebral injury is modulated through the release of active substances—such as platelet-derived growth factor, vascular endothelial growth factor, brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), angiopoietins, adenosine and glutamate—in the parenchyma.10 Changes in the levels of these factors can also initiate differentiation and migration of cerebrovascular pericytes, which can further modulate the structure and function of cerebral arteries.10 Dedifferentiation of contractile arterial smooth muscle cells to a non-contractile phenotype could also enable transient increases in blood pressure to reach the capillary microcirculation, elevate hydrostatic filtration pressure and, thereby, increase tissue oedema.10 This kind of injury can also lead to degradation of the adventitial layer and increased risk of vessel rupture.10
Perivascular nerve fibres are found at the junction of the medial and adventitial layers (Figures 1 and 3). These nerves use aminergic, cholinergic, peptidergic, purinergic, or nitridergic transmission and can promote vasoconstriction and vasodilatation, as well as smooth muscle growth and differentiation.12 The vasomotor functions of these nerves are critical to the regulation of cerebral perfusion, which suggests that damage to perivascular nerve fibres could impair blood flow to specific brain regions and compromise the crucial coupling between local metabolic rate and tissue perfusion, either during or after ischaemia. For example, subarachnoid haemorrhage significantly reduces both the density of nerve fibres and cerebrovascular tone,13 suggesting that such injury can lead to functional denervation of cerebral arteries. One rodent study using the middle cerebral artery occlusion model of ischaemic stroke has demonstrated that perivascular nerve fibres could have a critical and possibly protective role in reducing lesion volume.14 The reduction in lesion volume could be the result of not only short-term vasomotor effects, but also long-term effects of innervation on the growth, differentiation, and functional characteristics of cerebral arteries. Consequently, the functional denervation associated with ischaemic or haemorrhagic stroke could remove neural input to arterial smooth muscle, resulting in deleterious changes in the function and phenotype of cerebrovascular smooth muscle cells.13 Although perivascular innervation has long been recognized to have important influences on the structure and function of many arterial vessels, including those of the cerebral circulation,15 the consequences of functional denervation resulting from ischaemia are largely unexplored, despite their considerable potential as a therapeutic target. For these reasons, perivascular innervation should be included in models of cerebrovascular pathophysiology.
The endothelium releases numerous factors that can influence the proliferation and differentiation of adjacent vascular smooth muscle cells (Figure 3). In addition to the well-established vasomotor effects of endothelial factors such as nitric oxide and endothelin, these molecules exert powerful influences that regulate the phenotype of adjacent vascular smooth muscle cells.10 Large-vessel injury compromises the continuous release of these factors from the endothelium, thereby altering the phenotype—and, in turn, the contractility —of vascular smooth muscle cells. In addition, loss of endothelial factors after large vessel injury might increase permeability and cause loss of endothelial function in otherwise uninjured upstream arteries that supply the downstream capillaries, as well as loss of endothelial function in capillaries that comprise the BBB. Injury to the endothelium of small arteries promotes platelet aggregation and local clot formation, which exacerbates ischaemic vascular injury through serotonin release, which can in turn exert mitogenic and genomic effects on smooth muscle, resulting in changes to smooth muscle cell phenotype, arterial structure, and contractility.10
Another important component of almost all vasculature is the vasa vasorum, a network of small arterial vessels that supplies nutrients to the thick medial layers of large arteries.16 In the brain, cerebral arteries seem to lack a typical vasa vasorum and instead possess a rete vasorum connected to the subarachnoid space, through which cerebrospinal fluid can penetrate to the adventitial layer.17 Occlusion of the rete vasorum resulting from subarachnoid haemorrhage might, therefore, compromise both blood flow and nutrient supply to the brain arteries. Damage to the rete vasorum after cerebral ischaemia could also contribute to vasoparalysis and dysregulation of blood flow, but the importance of this effect remains largely unexplored. By contrast, strong evidence suggests an important role for the cerebral collateral circulation in recovery from stroke; in many patients, the extent of cerebral collateral circulation influences the volume of tissue damage resulting from ischaemia and the response to treatment.18 These features of the cerebral vasculature support the view that the pathophysiology of stroke involves many cell types and structures outside of those traditionally associated with the neurovascular unit.
Importantly, many disease processes that increase the risk of stroke also specifically affect arterial endothelial cells, vascular smooth muscle cells, and perivascular nerves innervating cerebral arteries. For example, the vascular inflammation associated with diabetes19 and arterial remodelling secondary to hypertension20 occurs in cerebral arteries and affects associated cell types. This evidence suggests that interventions that reverse these disease processes in the small cerebral arteries could reduce the risk of stroke and might also improve reperfusion after stroke. These findings further support the inclusion of vascular smooth muscle cells, endothelial cells and perivascular nerves among the cell types considered to be affected by stroke pathophysiology.
The vascular neural network
We propose the vascular neural network as a new paradigm that combines the original concept of the neurovascular unit with emerging understanding of the key roles of arterial smooth muscle cells, endothelial cells and perivascular nerves in cerebrovascular physiology and pathology. The physical components of the vascular neural network include small arteries downstream of the major cerebral arteries, arterioles, venules and small veins. The vascular neural network encompasses all cell types associated with these structures: not only the cells currently listed as components of the neurovascular unit (capillary endothelial cells, astrocytes, pericytes and neurons),4,5 but also cell types within the small arteries and veins that respectively supply and drain the neurovascular unit (Figure 1). The vascular neural network, therefore, expands the concept of the neurovascular unit to include multiple cell types with potentially important functional roles in stroke prevention, salvage of the lesion penumbra, restoration of tissue perfusion, promotion of cell regeneration and functional recovery after stroke (Box 1).
Box 1. The five ‘R’s of stroke therapy.
Five key aspects of the pathophysiology of stroke can be targeted therapeutically:
Retard the progression of pathological changes in the cerebral vasculature, by implementing stroke prevention measures and reducing risk factors
Reperfuse the ischaemic penumbra
Reduce tissue injury after a stroke
Regenerate new cells
Reorganise functional vascular and neural networks during recovery
The neurovascular unit incorporated aspects of stroke pathophysiology that can only be addressed by measures 3–5 listed above, whereas the vascular neural network incorporates all five aspects.
In our opinion, the concept of the vascular neural network improves upon the neurovascular unit model of stroke pathophysiology because most ischaemic stroke events affect major cerebral arteries and their secondary or tertiary branches—vessels that are not included in the neurovascular unit.21 A blood clot lodged in a cerebral capillary bed typically does not immediately cause a clinically evident stroke, but first increases vascular resistance to perfusion by the arteries or arterioles upstream of the neurovascular unit. Slightly larger clots that become trapped in arterioles also act first to increase local vascular resistance and reduce perfusion of the downstream neurovascular unit. Thus, most ischaemic stroke events do not initially occur within the neurovascular unit, but instead first affect arterioles, small arteries and extraparenchymal arteries, such as the sylvian, middle cerebral or pial arteries, which lie upstream of the neurovascular unit.21 Even lacunar infarcts in deep brain nuclei begin far upstream of the capillaries that are located in brain parenchyma, and might involve multiple intracerebral blood vessels.22 In addition, most cerebral haemorrhages result from rupture of major or penetrating arteries that bleed sufficiently to cause a noticeable stroke event.23 The micrometre scale of the neurovascular unit model of stroke pathophysiology focuses on the main effects of ischaemia at the capillary level, but does not take into account equally critical events that occur upstream of the capillaries, in cerebral arteries and arterioles.
The neurovascular unit includes three of the five key aspects of stroke pathophysiology that have potential for therapeutic targeting, as follows: reduction of tissue injury by normalization of the permeability of capillaries; regeneration of new cells; and reorganization of the vascular and neural microenvironment to promote functional recovery. The other two major categories of therapeutic targets for stroke (retardation of injury and promotion of reperfusion) are addressed only by the vascular neural network, which is the sole model to integrate all five aspects (Box 1).
The vascular neural network model might also be more appropriate than the neurovascular unit model for analysis of the pathophysiology of neurodegenerative disorders other than stroke. Just as large cerebral vessels are critically involved in ischaemic injury, they also have a key role in other brain diseases. Alzheimer disease, Parkinson disease, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy syndrome,24 gliomas,25 multiple sclerosis and other autoimmune diseases of the CNS8 all have major components of cerebrovascular dysfunction. As suggested above, cerebrovascular dysfunction in these settings could result from phenotypic transformation of cerebrovascular smooth muscle cells and impaired blood flow to metabolically active brain regions, which is a characteristic of many of these disorders. The vascular neural network is, therefore, a useful integrated model relevant to all neurodegenerative brain disorders that involve both the cerebral vasculature and the downstream neurovascular unit.
Implications for treatment
The purpose of establishing a new pathophysiological paradigm of stroke is to guide interventions to prevent stroke, reduce brain injury during acute stroke and to promote functional recovery after stroke. In common with the neurovascular unit, the vascular neural network includes targets that fall into key categories of stroke treatment to reduce infarction, regenerate new cells and reorganize new neuronal microenvironments. The two treatment categories unique to the vascular neural network are those that aim to retard acute ischaemia and help reperfuse the ischaemic brain. These last two categories are discussed below.
Stroke prevention
A key aim of stroke prevention is to retard the progression of cerebrovascular pathology by reducing risk factors, such as hypertension, diabetes, dyslipidaemia and smoking, all of which affect mainly the smaller arteries and arterioles that are included in the vascular neural network.26 At present, the primary FDA-approved agent used for stroke treatment is rtPA, which digests intravascular thrombi,1 such as those that occur predominantly in small arteries. Carotid endarterectomy and stenting,27 and treatment with direct thrombin inhibitors and factor Xa inhibitors to prevent atrial fibrillation,28 are additional interventions designed to reduce the risk of stroke by decreasing the likelihood of intravascular embolism and subsequent occlusion of small cerebral arteries. Together, these strategies serve to both reduce the frequency of stroke and retard the progression of cerebrovascular pathology, with the aim of limiting the severity of injury after a stroke.
Reperfusion
Reperfusion after an ischaemic stroke event involves restoration of blood flow in major cerebral arteries and, more importantly, smaller cerebral arteries and arterioles. Surgical interventions such as aneurysm clipping or coiling can be used to prevent rebleeding after subarachnoid haemorrhage,29 and mechanical clot-retrieving devices30 or chemical agents such as rtPA1 are used to reopen occluded arteries. Small cerebral arteries and arterioles, however, can lose their autoregulation capability within hours after a stroke event2 as a result of phenotypic transformation of vascular smooth muscle or endothelial cells following ischaemia–reperfusion injury. Moreover, sudden restoration of perfusion through a major artery might lead to hyperperfusion, vascular injury, disruption of gap junctions in arterial endothelium, brain oedema or even haemorrhagic transformation of the ischaemic region.4 Careful monitoring and control of reperfusion once patency has been restored is critical, therefore, to minimize ischaemia–reperfusion injury and restore optimal cerebrovascular function. Once again, these approaches involve cerebral arteries and arterioles that are upstream of the traditional neurovascular unit, but are included in the vascular neural network.
Conclusions and future directions
Application of the vascular neural network paradigm of stroke pathophysiology presents an opportunity to identify how vasoactive, chemical and mechanical factors simultaneously influence the function of cell types in both the neurovascular unit and the upstream vasculature. This expanded model promotes improved understanding of the vulnerability of the cerebral vasculature to injury and loss of function, and of how recovery of vascular smooth muscle function after cerebral injury can facilitate penumbral rescue and preservation of the downstream neurovascular unit.4 The vascular neural network is, therefore, a more comprehensive and robust model of cerebrovascular pathophysiology than is the neurovascular unit. As such, we suggest that the vascular neural network should be considered the principal territory affected by stroke pathophysiology.
More research is, however, needed to define the interactions between cell types in the vascular neural network during early and late stages of the response to an ischaemic insult. Interactions between smooth muscle cells, arterial endothelial cells and perivascular nerves, and their influences on downstream capillary endothelial cells, astrocytes, pericytes and neurons, should be studied systematically to elucidate how and when these cells communicate. Interestingly, factors such as NGF and BDNF promote survival of cell types in both the neurovascular unit and the upstream vasculature, which suggests that the therapeutic potential of these factors should be explored. Therapeutic strategies that enable clot dissolution while minimizing the risk of haemorrhagic transformation are also needed.
Many factors that might influence stroke pathophysiology—including whether small cerebral veins are involved in the control of reperfusion, and the role of the rete vasorum in prevention and treatment of, and recovery from, ischaemic stroke—remain undefined. Studies are also needed to investigate how the function of the rete vasorum is influenced by diabetes or hypertensive injury. Improved understanding of the time course of phenotypic changes in arterial smooth muscle cells after stroke might enable such changes to be controlled, ultimately leading to targeted reperfusion of the affected area. The extent to which smooth muscle injury contributes to stroke pathophysiology is not yet known, although stroke-induced injury to arterial and arteriolar smooth muscle and arterial endothelium could potentially influence both poststroke cerebral perfusion and subsequent stroke recovery. On a smaller scale, we do not know how gap junctions and tight-junction proteins are affected by stroke. In addition to addressing these gaps in our knowledge, evolution in our thinking about stroke must continue with the goal of improving our understanding of stroke-related cerebral injury. Ultimately, these efforts should facilitate the development of pharmacological strategies that target all elements of the vascular neural network, including the vascular smooth muscle cells, arterial endothelium and perivascular nerves that serve to build, maintain and regulate cerebral arteries.
Acknowledgments
The authors’ research is supported by grants to J. H. Zhang (NIH NS43338), J. Badaut (NIH HD061946), J. Tang (NIH NS060936) and W. J. Pearce (NIH NS076945 and HD031226).
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
J. H. Zhang, J. Badaut and W. J. Pearce wrote the article. J. H. Zhang, J. Badaut, J. Tang and W. J. Pearce researched the data for the article. J. H. Zhang, J. Badaut, J. Tang, A. Obenaus, R. Hartman and W. J. Pearce provided substantial contributions to discussion of the content and reviewing and/or editing of the manuscript before submission.
References
- 1.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–414. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg MD. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology. 2008;55:363–389. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.del Zoppo GJ. The neurovascular unit in the setting of stroke. J Intern Med. 2010;267:156–171. doi: 10.1111/j.1365-2796.2009.02199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lo EH, Broderick JP, Moskowitz M. A tPA and proteolysis in the neurovascular unit. Stroke. 2004;35:354–356. doi: 10.1161/01.STR.0000115164.80010.8A. [DOI] [PubMed] [Google Scholar]
- 5.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 6.Cohen Z, Bonvento G, Lacombe P, Hamel E. Serotonin in the regulation of brain microcirculation. Prog Neurobiol. 1996;50:335–362. doi: 10.1016/s0301-0082(96)00033-0. [DOI] [PubMed] [Google Scholar]
- 7.Grotta JC, et al. Report of the Stroke Progress Review Group. National Institute of Neurological Disorders and Stroke; 2002. [online], http://www.ninds.nih.gov/find_people/groups/stroke_prg/StrokePRGreport-4-23-02.pdf. [Google Scholar]
- 8.Zlokovic BV. The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Lee RM. Morphology of cerebral arteries. Pharmacol Ther. 1995;66:149–173. doi: 10.1016/0163-7258(94)00071-a. [DOI] [PubMed] [Google Scholar]
- 10.Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol. 2012;74:13–40. doi: 10.1146/annurev-physiol-012110-142315. [DOI] [PubMed] [Google Scholar]
- 11.Croll SD, Wiegand SJ. Vascular growth factors in cerebral ischemia. Mol Neurobiol. 2001;23:121–135. doi: 10.1385/MN:23:2-3:121. [DOI] [PubMed] [Google Scholar]
- 12.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2006;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- 13.Alabadi JA, et al. Changes in the adrenergic mechanisms of cerebral arteries after subarachnoid hemorrhage in goats. Neurosurgery. 1994;34:1027–1033. doi: 10.1227/00006123-199406000-00011. discussion 1033–1034. [DOI] [PubMed] [Google Scholar]
- 14.Diansan S, Shifen Z, Zhen G, Heming W, Xiangrui W. Resection of the nerves bundle from the sphenopalatine ganglia tend to increase the infarction volume following middle cerebral artery occlusion. Neurol Sci. 2010;31:431–435. doi: 10.1007/s10072-010-0238-0. [DOI] [PubMed] [Google Scholar]
- 15.Bevan RD, Tsuru H, Bevan JA. Cerebral artery mass in the rabbit is reduced by chronic sympathetic denervation. Stroke. 1983;14:393–396. doi: 10.1161/01.str.14.3.393. [DOI] [PubMed] [Google Scholar]
- 16.Mulligan-Kehoe MJ. The vasa vasorum in diseased and nondiseased arteries. Am J Physiol Heart Circ Physiol. 2010;298:H295–H305. doi: 10.1152/ajpheart.00884.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zervas NT, Liszczak TM, Mayberg MR, Black PM. Cerebrospinal fluid may nourish cerebral vessels through pathways in the adventitia that may be analogous to systemic vasa vasorum. J Neurosurg. 1982;56:475–481. doi: 10.3171/jns.1982.56.4.0475. [DOI] [PubMed] [Google Scholar]
- 18.Shuaib A, Butcher K, Mohammad AA, Saqqur M, Liebeskind DS. Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. Lancet Neurol. 2011;10:909–921. doi: 10.1016/S1474-4422(11)70195-8. [DOI] [PubMed] [Google Scholar]
- 19.Kampoli AM, et al. Potential pathogenic inflammatory mechanisms of endothelial dysfunction induced by type 2 diabetes mellitus. Curr Pharm Des. 2011;17:4147–4158. doi: 10.2174/138161211798764825. [DOI] [PubMed] [Google Scholar]
- 20.Manolio TA, Olson J, Longstreth WT. Hypertension and cognitive function: pathophysiologic effects of hypertension on the brain. Curr Hypertens Rep. 2003;5:255–261. doi: 10.1007/s11906-003-0029-6. [DOI] [PubMed] [Google Scholar]
- 21.Manjila S, et al. Evidence-based review of primary and secondary ischemic stroke prevention in adults: a neurosurgical perspective. Neurosurg Focus. 2011;30:E1. doi: 10.3171/2011.2.FOCUS1164. [DOI] [PubMed] [Google Scholar]
- 22.Behrouz R, Malek AR, Torbey MT. Small vessel cerebrovascular disease: the past, present, and future. Stroke Res Treat. 2012;2012:839151. doi: 10.1155/2012/839151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balami JS, Buchan AM. Complications of intracerebral haemorrhage. Lancet Neurol. 2012;11:101–118. doi: 10.1016/S1474-4422(11)70264-2. [DOI] [PubMed] [Google Scholar]
- 24.Ayata C. CADASIL: experimental insights from animal models. Stroke. 2010;41:S129–S134. doi: 10.1161/STROKEAHA.110.595207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J, et al. Glioma-induced remodeling of the neurovascular unit. Brain Res. 2009;1288:125–134. doi: 10.1016/j.brainres.2009.06.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuklina EV, Tong X, George MG, Bansil P. Epidemiology and prevention of stroke: a worldwide perspective. Expert Rev Neurother. 2012;12:199–208. doi: 10.1586/ern.11.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajamani K, Chaturvedi S. Stroke prevention—surgical and interventional approaches to carotid stenosis. Neurotherapeutics. 2011;8:503–514. doi: 10.1007/s13311-011-0052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Dell KM, Igawa D, Hsin J. New oral anticoagulants for atrial fibrillation: a review of clinical trials. Clin Ther. 2012;34:894–901. doi: 10.1016/j.clinthera.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 29.Rose MJ. Aneurysmal subarachnoid hemorrhage: an update on the medical complications and treatments strategies seen in these patients. Curr Opin Anaesthesiol. 2011;24:500–507. doi: 10.1097/ACO.0b013e32834ad45b. [DOI] [PubMed] [Google Scholar]
- 30.Hussain MS, et al. Mechanical thrombectomy for acute stroke with the alligator retrieval device. Stroke. 2009;40:3784–3788. doi: 10.1161/STROKEAHA.108.525618. [DOI] [PubMed] [Google Scholar]