Abstract
The mammalian fetus is highly adapted for growth in a low-O2 environment in which arterial O2 tensions average near 30 mm Hg. Acute decreases in O2 tension below this value elicit vasodilatation, but the responses are blunted compared to those observed in adults. Chronic hypoxia in the fetus stimulates a pattern of cerebrovascular remodeling that results in an increased wall thickness and decreased overall contractility and also depresses the capacity for cerebral vasodilatation through decreases in NO release, soluble guanylate cyclase activity, and expression of PKG substrates. Many of these hypoxic effects appear to be homeostatic and may be mediated by VEGFs, which increase in direct response to hypoxia and, in turn, can dramatically alter the expression and function of multiple contractile proteins in cerebrovascular smooth muscle through both endothelium-dependent and endothelium-independent effects on large artery smooth muscle.
1 Introduction
The near universal importance of O2 as an electron acceptor has given rise to a remarkable diversity of mechanisms that mediate homeostatic adaptation to limited O2 availability. Adaptation to hypoxia is essential for many pathophysiological processes, such as ischemia and wound repair, but one of the best examples is the developing mammalian fetus. Normally, arterial O2 tensions in a healthy fetus average near 30 mm Hg, a situation which Eastman described more than 50 years ago as “Mount Everest in utero” [1]. To thrive in this environment, the fetus is equipped with a unique isoform of hemoglobin that has a higher O2 affinity than adult hemoglobin, and thus can “extract” O2 from maternal hemoglobin in the placenta [2]. Owing to this and other adaptations, the fetal cardiovascular system exhibits a clear ability to match tissue blood flow, and cerebral perfusion in particular, to metabolic activity under a broad variety of physiological conditions [3][4].
2 Responses to Acute Hypoxia
Acute hypoxic stress can elicit a compensatory cerebral vasodilatation in the healthy fetus, but the magnitude of this response is significantly smaller than observed in adults [3][4]. This attenuation is due not only to a reduced release of tissue vasodilator metabolites in response to hypoxia, but also to a reduced direct effect of hypoxia on fetal cerebrovascular smooth muscle [5]. Whereas the release of vasoactive molecules from the vascular endothelium typically contributes to hypoxic vasodilatation in adult cerebral arteries [6], this contribution is also diminished in immature cerebral arteries [7]. Supplementing these reduced fetal hypoxic cerebrovascular responses, however, are powerful systemic fetal reflexes known as “brain sparing” responses, which redistribute cardiac output in favor of the brain in response to hypoxia [8]. Fetal tissues also typically exhibit a lower metabolic rate for oxygen and contain mitochondria more resistant to hypoxic damage than those of adult tissues [9]. Thus, the fetal cardiovascular system is well adapted to maintain cerebral oxygen homeostatis, even during episodes of acute systemic hypoxia.
3 Responses to Chronic Hypoxia
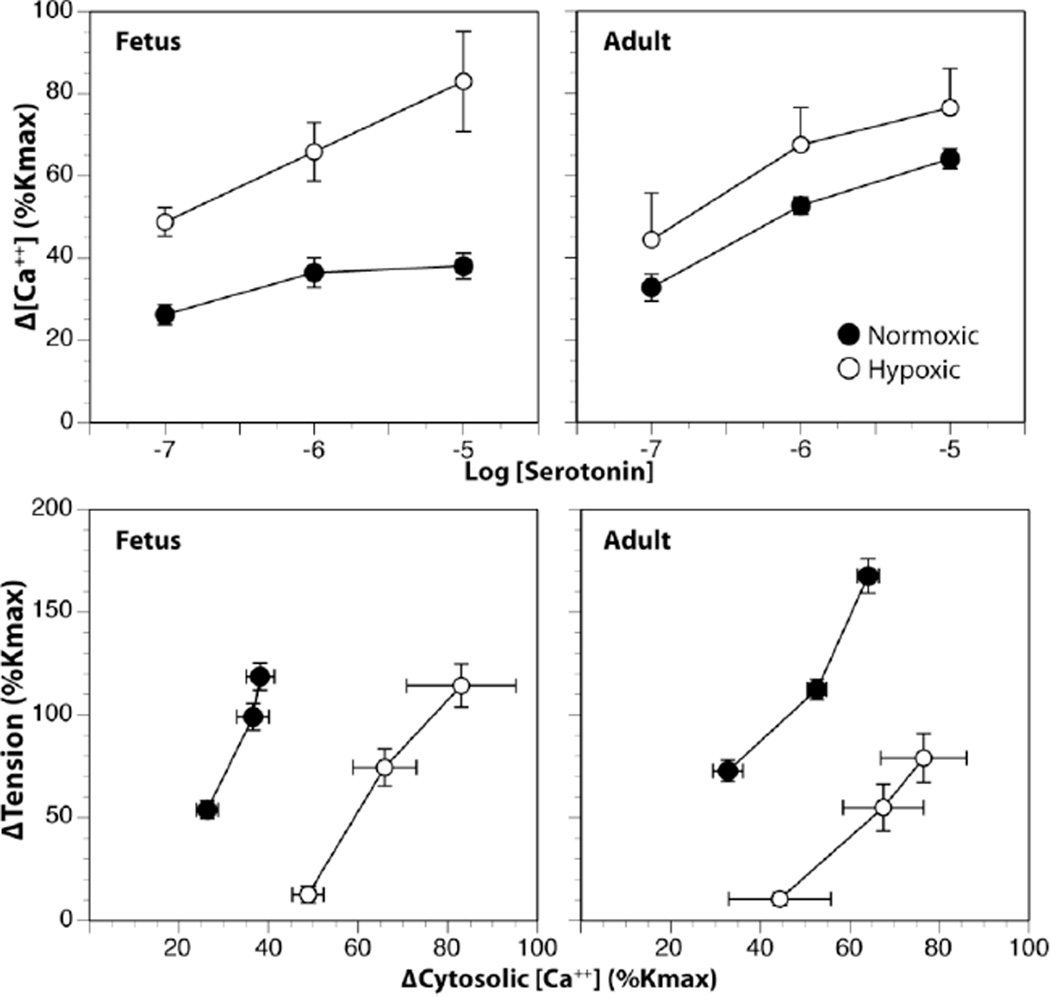
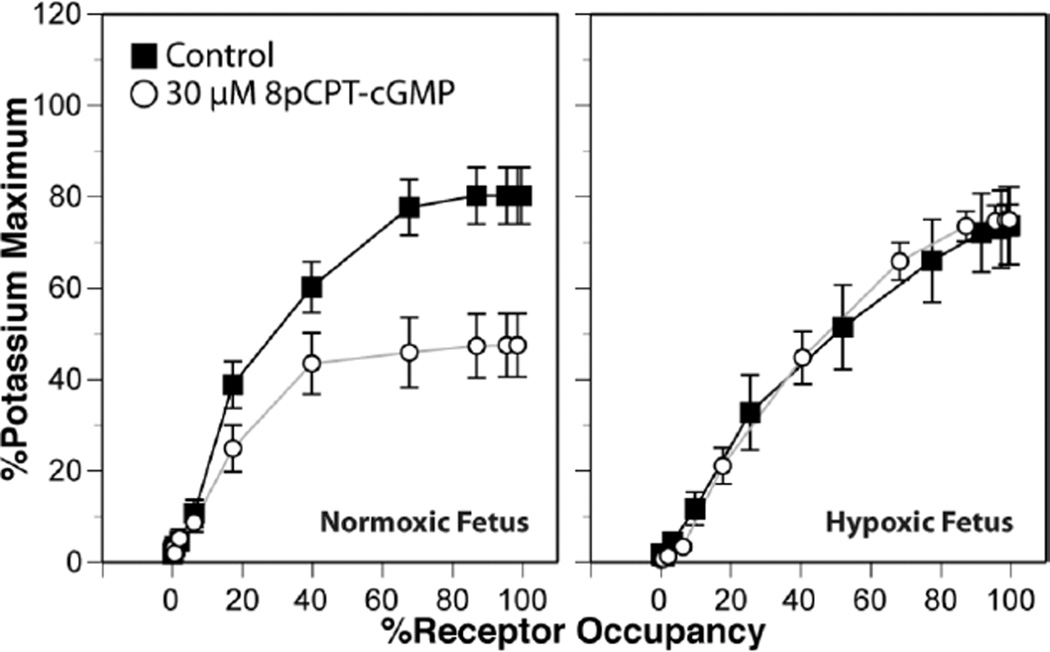
In contrast to acute fetal hypoxia, which is typically caused by either maternal hypoxia or abrupt reductions in placental perfusion, chronic fetal hypoxia is clinically more common and has a wider variety of causes. Placental insufficiency, placentia abruptia, maternal infection or inflammation, maternal hypertension, undernutrition, alcohol abuse, and smoking all can contribute to chronic fetal hypoxia [10]. In response to chronic hypoxia, fetal cerebral arteries undergo dramatic remodeling responses that enlarge both the endothelial and smooth muscle cells of fetal cerebral arteries [11]. Functionally, chronic hypoxia enhances agonist-induced calcium mobilization, but reduces overall cerebrovascular contractility though significant changes in myofilament calcium sensitivity [12][13](Figure 1). The capacity for vasodilatation is also reduced by chronic hypoxia, and this effect involves decreases in endothelial NO production and vascular soluble guanylate cyclase activity [11][14]. Interestingly, chronic hypoxia has little effect on the abundance or activity of protein kinase G (PKG), the downstream effector for NO-induced changes in vascular cGMP. Even so, chronic hypoxia ablates the ability of PKG to inhibit contractility (Figure 2), suggesting that chronic hypoxia decreases the availability of substrates that mediate the effects of PKG on cerebrovascular contractility. The identities of these substrates are currently unknown, but remain the topic of active investigation. Confocal immunohistochemistry studies further suggest that decreased PKG substrate availability may also involve a more heterogeneous distribution of PKG expression throughout the artery wall.
Fig. 1.
Effects of chronic hypoxia on Ca++ mobilization and myofilament Ca++ sensitivity in fetal and adult cerebral arteries. Acclimatization to chronic hypoxia for 110 days at an altitude of 3820 m enhanced serotonin-induced increases in cytosolic Ca++ (upper panels) but depressed myofilament sensitivity to Ca++ (lower panels) in main branch middle cerebral arteries taken from term (140 d) fetal and non-pregnant adult sheep (18–24 mo old). Cytosolic Ca++ and contractile force were measured simultaneously in fura-2 loaded artery segments mounted in a Jacso CAF-110 photometer. Ca++ sensitivity was indicated by the slopes of the relations between the changes in tension (expressed relative to the maximum contractile response to 120 mM K+) and cytosolic Ca++ produced in response to serotonin. Error bars indicate SEM for N≥6.
Fig. 2.
Chronic hypoxia ablates the ability of Protein Kinase G to inhibit serotonergic contractile tone (expressed relative to the maximum contractile tone produced in response to 120 mM K+) in ovine fetal middle cerebral arteries. Concentration-response determinations for serotonin were determined in vitro in the presence and absence of a cell permeant and non-metabolizable activator of Protein Kinase G, and then converted to occupancy-response curves using parallel measurements of agonist binding affinity. PKG activation potently inhibited serotonergic tone independent of changes in serotonergic receptor density or affinity in arteries from normoxic, but not chronically hypoxic, term fetuses. Chronic hypoxia was imposed on the fetuses by maintaining pregnant ewes at 3820m for the final 110 days of gestation. Error bars indicate SEM for N≥6.
4 VEGF and Hypoxic Vascular Adaptation
Despite the many well-documented effects of chronic hypoxia on fetal cerebral artery structure and function, the main molecular mechanisms driving these changes remain largely unidentified. Among the many growth factors that could potentially mediate the cerebrovascular effects of chronic hypoxia, the most promising candidates are the Vascular Endothelial Growth Factors (VEGFs). This family of peptide growth factors has close similarity to the Platelet Derived Growth Factor family and arises from multiple genes with numerous splicing variants [15]. Most importantly, the main member of the VEGF family, VEGF-A165, is upregulated in response to increases in Hypoxia Inducible Factor, a transcription factor that increases in direct response to hypoxia [16]. Uncertainty about the ability of VEGFs to mediate hypoxic remodeling arises mainly from the view that VEGF acts primarily on endothelial cells to stimulate angiogenesis [15]. However, recent evidence suggests that VEGF may also exert trophic effects on non-endothelial cell types including neurons of both the central [17] and peripheral [18] nervous systems. Most importantly, VEGF has recently been shown to influence vascular differentiation through a direct effect on VEGF receptors in smooth muscle cells [19]. These findings support the novel hypothesis that hypoxic changes in the structure and function of large artery smooth muscle may involve direct effects of VEGF.
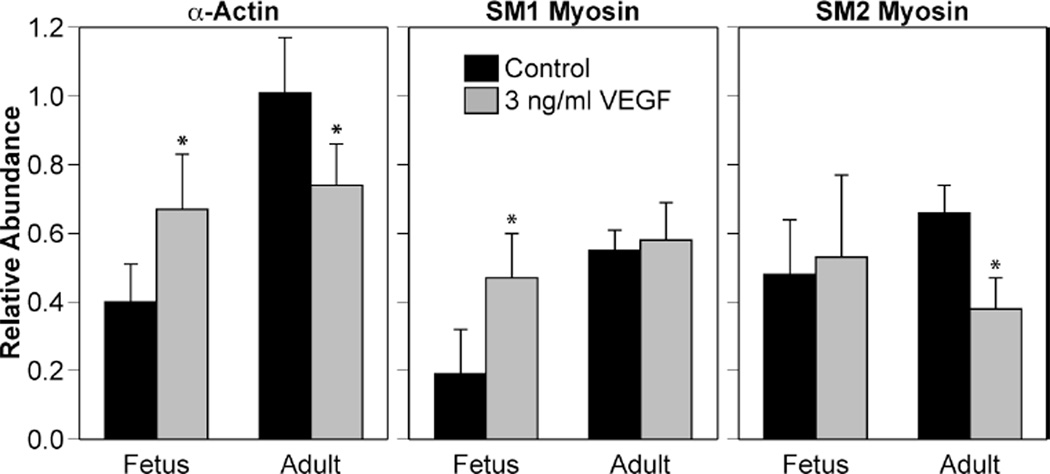
To assess the potential of VEGF as a trophic factor in large artery smooth muscle, ovine carotid arteries harvested under sterile conditions from term fetal (140 d) and non-pregnant adult sheep were denuded of endothelium, then organ cultured (37 °C, 95% O2, 5% CO2) under serum starvation conditions in DMEM to align the cell cycle status of all cells in the artery wall. The 24-hour period of starvation also facilitated diffusional extraction of endogenous trophic factors present in the tissue at the time of harvest. Following starvation, the arteries then were maintained in organ culture for an additional 24 hours in the presence or absence of a low physiological concentration (3 ng/ml) of VEGF-A165. This 3 ng/ml concentration was 10–30 fold less than used in many cell culture studies and was selected because it approximated the endogenous concentrations present in adult ovine arteries, as revealed by Western blotting. Organ culture for 24 hours with 3 ng/ml VEGF-A165 clearly upregulated expression of certain contractile proteins in the fetal arteries, but decreased other proteins in adult arteries (Figure 3), thus demonstrating the vasotrophic potential of VEGF-A165 in large artery smooth muscle. Most interestingly, organ culture with VEGF-A165 influenced protein distribution most prominently in the smooth muscle immediately adjacent to the basal lamina, as indicated by fluorescent immunohistochemistry. The reasons why reactivity to VEGF-A165 were dependent on age and location are unclear but most probably arise from differences in either the types or densities of VEGF receptors expressed, and/or the coupling of VEGF receptors to gene expression; many of the kinases that mediate the nuclear effects of VEGF exhibit major age-related changes that occur gradually throughout postnatal maturation [15].
Fig. 3.
VEGF alters contractile protein expression in ovine arteries in an age-dependent manner. In endothelium-denuded ovine carotids, 24 hours of organ culture with VEGF enhanced expression of α-actin and the SM1 myosin isoform in fetal arteries, but decreased α-actin and SM2 myosin in adult arteries. Error bars indicate SEM for n≥4. Asterisks indicate VEGF values significantly different than control at P<0.05.
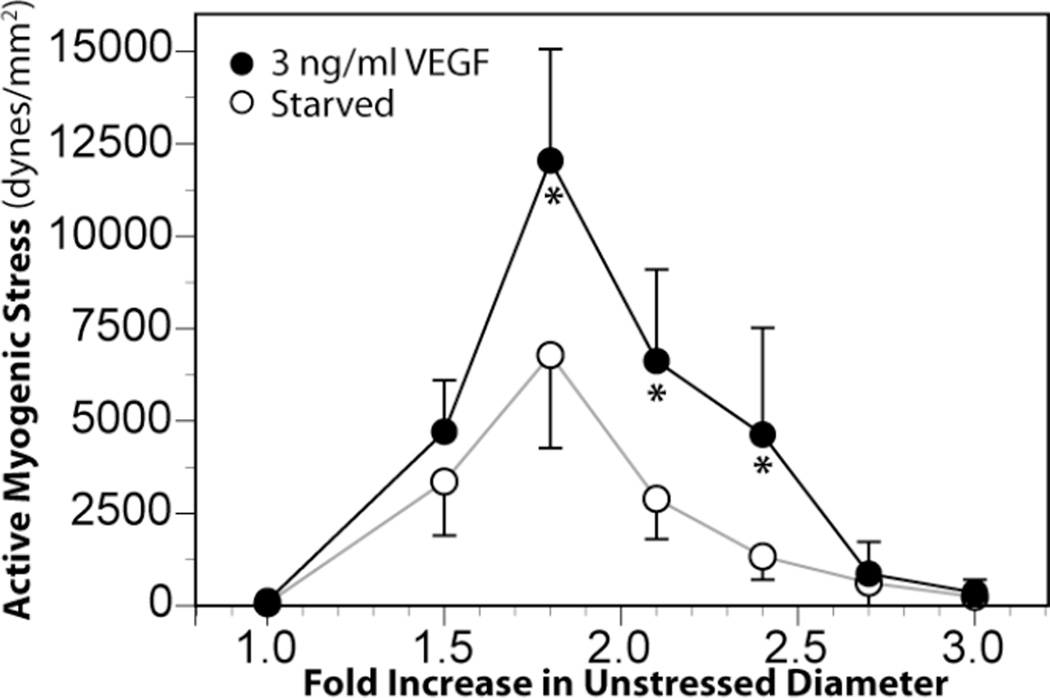
To assess the functional impact of organ culture with VEGF-A165, active stress-strain relations were determined in wire-mounted fetal middle cerebral arteries that had been cultured with VEGF-A165. Artery strain was calculated as the ratio of working diameter divided by unstressed diameter where unstressed diameter was measured at a near-zero initial tension (30 mg). Artery stresses were calculated as the contractile tensions in dynes divided by cross-sectional areas in mm2, which were measured as the products of artery wall thicknesses and segment lengths measured via microscope. Working wall thicknesses were corrected for stretch at each strain level examined. Myogenic stresses were calculated as the differences in total artery stresses measured at rest in normal sodium Krebs solution (37 °C, pH 7.4) before and after freezing the arteries in liquid N2 and equilibration in 3 mM EGTA. These in vitro measurements of stretch-induced contractile stresses revealed that serum starvation for 48 hours significantly reduced myogenic reactivity to progressive stretch and peak myogenic stress compared to fresh arteries. More importantly, these measurements also demonstrated that 24 hours of serum starvation followed by 24 hours of organ culture in a low physiological concentration (3 ng/ml) of VEGF can enhance fetal cerebrovascular contractility through direct effects on smooth muscle (Figure 4). Because the extent of strain required to produce maximum myogenic stress was identical in serum starved and VEGF treated arteries, the results also suggest that the effects of VEGF on the structure and orientation of the extracellular matrix were not as prominent as effects on the contractile apparatus. Consistent with this interpretation, organ culture with VEGF-A165 significantly increased the relative abundance of both smooth muscle α-actin and SM1 myosin in fetal but not adult cerebral arteries (Figure 3). These findings strongly support the hypothesis that VEGF helps mediate the remodeling effect of chronic hypoxia on fetal cerebral arteries.
Fig. 4.
Organ culture with VEGF enhanced myogenic tone in fetal middle cerebral arteries. Term fetal ovine middle cerebral arteries denuded of endothelium were organ cultured for 24 hours with a low concentration of VEGF-A165. Treatment with VEGF significantly enhanced peak myogenic tone compared to serum starvation alone. This effect was not observed in adult middle cerebral arteries. Error bars indicate SEM for n≥7. Asterisks indicate VEGF values significantly greater than Starved at P<0.05.
5 Summary & Conclusions
The fetus is highly adapted for growth in a low-O2 environment. As a consequence of this adaptation, cerebral vasodilator responses to acute hypoxia are dampened in both fetal vascular smooth muscle and endothelium. Chronic hypoxia stimulates extensive cerebrovascular remodeling that increases wall thickness but decreases overall contractility, largely through depressed myofilament Ca++ sensitivity. Chronic hypoxia also depresses endothelium-dependent vasodilatation through decreases in NO release, soluble guanylate cyclase activity, and expression of PKG substrates. Many of the cerebrovascular effects of chronic hypoxia appear to be homeostatic and may be mediated by VEGFs, which increase in direct response to hypoxia and, in turn, can directly influence cerebrovascular smooth muscle through endothelium-independent effects to alter the expression and function of multiple contractile proteins. Taken together, these findings demonstrate that the fetal cerebral vasculature is highly adapted to thrive in the hypoxic environment characteristic of in utero life, and suggest that further study of the molecular mechanisms mediating fetal adaptation to both physiological and pathophysiological perturbations hold great promise for improvements in strategies for management of the at-risk fetus and neonate.
References
- 1.Eastman NJ. Mount Everest in utero. Am J Obstet Gynecol. 1954;67:701–711. doi: 10.1016/0002-9378(54)90098-8. [DOI] [PubMed] [Google Scholar]
- 2.Bank A. Regulation of human fetal hemoglobin: new players, new complexities. Blood. 2006;107:435–443. doi: 10.1182/blood-2005-05-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blood AB, Hunter CJ, Power GG. Adenosine mediates decreased cerebral metabolic rate and increased cerebral blood flow during acute moderate hypoxia in the near-term fetal sheep. J Physiol. 2003;553:935–945. doi: 10.1113/jphysiol.2003.047928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishida N, Blood AB, Hunter CJ, Bragg S, Williams J, Pearce WJ, Power GG. Role of prostanoids in the regulation of cerebral blood flow during normoxia and hypoxia in the fetal sheep. Pediatr Res. 2006;60:524–529. doi: 10.1203/01.pdr.0000242268.99726.53. [DOI] [PubMed] [Google Scholar]
- 5.Pearce WJ, Ashwal S. Developmental changes in thickness, contractility, and hypoxic sensitivity of newborn lamb cerebral arteries. Pediatr Res. 1987;22:192–196. doi: 10.1203/00006450-198708000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Pearce WJ, Ashwal S, Cuevas J. Direct effects of graded hypoxia on intact and denuded rabbit cranial arteries. Am J Physiol. 1989;257:H824–H833. doi: 10.1152/ajpheart.1989.257.3.H824. [DOI] [PubMed] [Google Scholar]
- 7.Zurcher SD, Ong-Veloso GL, Akopov SE, Pearce WJ. Maturational modification of hypoxic relaxation in ovine carotid and cerebral arteries: role of endothelium. Biol Neonate. 1998;74:222–232. doi: 10.1159/000014028. [DOI] [PubMed] [Google Scholar]
- 8.Salihagic-Kadic A, Medic M, Jugovic D, Kos M, Latin V, Kusan Jukic M, Arbeille P. Fetal cerebrovascular response to chronic hypoxia--implications for the prevention of brain damage. J Matern Fetal Neonatal Med. 2006;19:387–396. doi: 10.1080/14767050600637861. [DOI] [PubMed] [Google Scholar]
- 9.Larsen GA, Skjellegrind HK, Vinje ML, Berg-Johnsen J. Mitochondria are more resistant to hypoxic depolarization in the newborn than in the adult brain. Neurochem Res. 2008;33:1894–1900. doi: 10.1007/s11064-008-9664-2. [DOI] [PubMed] [Google Scholar]
- 10.Rees S, Harding R, Walker D. An adverse intrauterine environment: implications for injury and altered development of the brain. Int J Dev Neurosci. 2008;26:3–11. doi: 10.1016/j.ijdevneu.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 11.Williams JM, Pearce WJ. Age-dependent modulation of endothelium-dependent vasodilatation by chronic hypoxia in ovine cranial arteries. J Appl Physiol. 2006;100:225–232. doi: 10.1152/japplphysiol.00221.2005. [DOI] [PubMed] [Google Scholar]
- 12.Teng GQ, Williams J, Zhang L, Purdy R, Pearce WJ. Effects of maturation, artery size, and chronic hypoxia on 5-HT receptor type in ovine cranial arteries. Am J Physiol. 1998 Sep;275:R742–R753. doi: 10.1152/ajpregu.1998.275.3.R742. [DOI] [PubMed] [Google Scholar]
- 13.Nauli SM, Williams JM, Gerthoffer WT, Pearce WJ. Chronic hypoxia modulates relations among calcium, myosin light chain phosphorylation, and force differently in fetal and adult ovine basilar arteries. J Appl Physiol. 2005;99:120–127. doi: 10.1152/japplphysiol.01131.2004. [DOI] [PubMed] [Google Scholar]
- 14.Pearce WJ, Williams JM, White CR, Lincoln TM. Effects of chronic hypoxia on soluble guanylate cyclase activity in fetal and adult ovine cerebral arteries. J Appl Physiol. 2009;107:192–199. doi: 10.1152/japplphysiol.00233.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 16.Nilsson I, Shibuya M, Wennstrom S. Differential activation of vascular genes by hypoxia in primary endothelial cells. Exp Cell Res. 2004;299:476–485. doi: 10.1016/j.yexcr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Storkebaum E, Lambrechts D, Carmeliet P. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays. 2004;26:943–954. doi: 10.1002/bies.20092. [DOI] [PubMed] [Google Scholar]
- 18.Marko SB, Damon DH. VEGF promotes vascular sympathetic innervation. Am J Physiol Heart Circ Physiol. 2008;294:H2646–H2652. doi: 10.1152/ajpheart.00291.2008. [DOI] [PubMed] [Google Scholar]
- 19.Osada-Oka M, Ikeda T, Imaoka S, Akiba S, Sato T. VEGF-enhanced proliferation under hypoxia by an autocrine mechanism in human vascular smooth muscle cells. J Atheroscler Thromb. 2008;15:26–33. doi: 10.5551/jat.e533. [DOI] [PubMed] [Google Scholar]