Abstract
As atherosclerosis is still one of the major causes of death in Western populations, it is important to identify those individuals who are at increased risk for the disease so that aggressive treatment may be administered as early as possible. Following the understanding that oxidative stress has a pivotal role in the development and progression of atherosclerosis, many polymorphisms in genes that are related to redox systems were examined for their association with increased risk for cardiovascular disease (CVD). Although many polymorphisms were studied, only a handful showed consistent relevance to CVD in different trials. This article focuses on six of these polymorphisms, examining their effect on the risk for CVD as well as their effect on protein expression and function. Reports regarding pharmacogenetic implications of these polymorphisms, where such exist, are discussed as well.
Keywords: Cardiovascular disease, CVD, Redox, Genetics, Oxidative stress, Vitamins
Introduction
Atherosclerosis is a major cause of death in western societies. For the past 30 years interventional therapies have focused on lowering low-density lipoprotein cholesterol (LDL-C) levels, which were shown to be closely correlated with the risk of CVD [1]. Further research has allowed the identification of other factors important for the development of atherosclerosis, one of which is oxidative stress. Today, the role of oxidative stress in the atherogenic process is well established. Common risk factors, such as endothelial dysfunction, dyslipidemia, smoking, obesity and diabetes, are all associated with increased production of reactive oxygen species (ROS), which are considered to promote atherogenesis, as they lead to the oxidation of LDL in the vessel wall and, consequently, to the conversion of macrophages to foam cells [2]. Another important development in the field of atherosclerosis prevention is the identification of polymorphisms that are associated with an increased risk for the development of CVD. These polymorphisms are found in genes that are relevant to the disease and result in changes in protein expression, function, or stability, compromising normal vascular physiology. Specifically, such polymorphisms have been found in enzymes of redox systems, a handful of which were shown to be clinically relevant and are discussed below.
Endothelial Nitric Oxide Synthase and Vascular Physiology
Nitric oxide (NO) is known to have a pivotal role in preserving the integrity of the vascular bed and in maintaining normal cardiovascular function. The function of NO is to inhibit platelet activation and aggregation, maintain the anti-thrombotic and anti-coagulation properties of the endothelium, regulate vascular smooth muscle cell (VSMC) proliferation vessel vasodilation by relaxing VSMCs, and protect the vessel wall from inflammatory processes. NO also acts as an antioxidant, inhibiting pro-oxidative reactions catalyzed by H2O2 [3].
NO is synthesized in vivo by the nitric oxide synthase (NOS) enzymes, which include three isoforms. The enzyme endothelial nitric oxide synthase (eNOS) is synthesized from the gene NOS3, which is found at chromosomal locus 7q35-36 [4]. eNOS expression is not unique to the endothelium, and it can also be found in other cells, such as red blood cells and leukocytes [5]. Acting as a membrane-bound homodimer [6], eNOS catalyzes production of NO from L-arginine, a reaction that utilizes a variety of co-factors [7]. eNOS is constitutively expressed in the endothelium, but its activity is regulated by various factors including fatty-acid modification, protein-protein interaction, calcium and calmodulin, and phosphorylation. Altogether, these factors determine NO production by the endothelium and its release to the bloodstream and the vessel wall [8].
Due to the importance of eNOS and NO in normal vascular physiology, the coding region of NOS3 was searched for polymorphisms that might be correlated with changes in enzyme expression and activity. The single nucleotide polymorphism (SNP) G894T in the coding region, which results in a missense mutation, Glu298Asp, has been extensively studied. The precise effect of this polymorphism on eNOS function is not well understood. The enzymatic characteristics (Vmax, Km, and Ki for various inhibitors) appear to be similar for both the 298Glu and the 298Asp proteins [9]. Although initial trials have described decreased endothelial function in carriers of the 298Asp allele [10], these results were later contradicted [11]. More recently, this polymorphism was identified as an important determinant of coronary collateral vessel development [12].
Expression of eNOS was found to be altered by the promoter SNP T(−786)C. The −786 C allele was associated with decreased mRNA levels of NOS3, most likely due to the preferential binding of the inhibitory transcription factor protein A1 to the −786 C allele [13]. This decreased mRNA expression results in decreased NO production and endothelial function [11].
An intronic polymorphism that has gained much attention is the 27 base pair tandem repeat in intron 4. This tandem repeat appears four times in one allele, denoted a, and five times in the second allele, denoted b. The evidence regarding the effect of this polymorphism on eNOS activity is lacking. Although the b allele was shown to be associated with increased protein expression [14] and plasma NO concentration [15], the a allele was shown to correlate with increased specific activity [14]. It has also been suggested that this polymorphism is found in linkage disequilibrium with other functional polymorphisms, a theory that was supported by studies showing linkage disequilibrium between this polymorphism and the T(−786)C polymorphism [14].
A recent meta-analysis has found a significant effect for the Glu298Asp and the T(−786)C polymorphisms on coronary artery disease (CAD), with a significant combined effect as well. These associations were more prominent in the non-Asian population. The 4b/a polymorphism was not associated with CAD in this meta-analysis [16]. These results are similar to those reported in a previous meta-analysis that found a mild effect for the three polymorphisms on CVD. However, this previous meta-analysis reported that when larger trials were examined, these associations were either lost or were no longer significant. It also reported that no association was found between eNOS polymorphisms and other CVD outcomes such as stroke and carotid stenosis [17].
Glutathione Peroxidase
Glutathione (GSH) is a tripeptide, γ-L-glutamyl-L-cysteinyl-glycine that is enzymatically synthesized by the addition of glutamate to cysteine, followed by the addition of glycine. It is a common cellular and plasma antioxidant, involved in the scavenging of reactive oxygen species (ROS), lipid hydrogen peroxides, and other radicals. This antioxidant activity is facilitated by enzymes of the glutathione peroxidase (GPx) family, all of which couple the oxidation and formation of glutathione disulfide (GSSG) to the reduction of oxidative agents. GPx can also release NO from S-nitroso-L-glutathione (GSNO), thereby increasing NO availability [18]. The GPx family includes at least six isoforms, which differ in their activity, tissue-specific expression, and cellular localization [19•].
GPx-3
The GPx-3 isoform was first identified in plasma and is the only GPx that is present in extracellular fluids. Its activity in the vasculature is important for maintaining the availability of NO and preventing its scavenging by ROS [20]. Additionally, GPx protects fibrinogen from modification by ROS and reactive nitrogen species, modifications that lead to increased thrombogenicity [21]. A study of families with familial childhood stroke has linked decreased plasma GPx activity to increased thrombogenicity, due to increased scavenging of NO by ROS, leading to decreased NO bioavailability and resulting in increased platelet aggregation and activation [22]. Subsequent studies demonstrated that the decrease in GPx activity was linked to a polymorphism in the gene GPX3, which is situated on chromosomal locus 5q32. The polymorphic allele presented with seven SNPs in the promoter region, all found in tight linkage disequilibrium, and was shown to result in decreased transcriptional activity [21]. Carriers of this allele were shown to have increased risk for central venous thrombosis [23•] and arterial ischemic stroke [21].
GPx-1
GPx-1, also known as cellular GPx, was the first enzyme of the family to be isolated and is the most common of all cellular GPx. It was initially discovered in erythrocytes, where it was believed to protect the cells from hemoglobin-associated oxidative stress. In addition to its role in mitigating ROS-associated oxidative stress, GPx-1 appears to take part in various cellular processes, including modulation of apoptosis, protein phosphorylation, and NF-κB activation [24]. Four polymorphisms were found in the GPX1 gene, located on chromosomal locus 3p21, all in strong linkage disequilibrium. Two of the polymorphisms, A-602 G and C2T, are not exonic and were found to significantly decrease mRNA expression. The Pro198-Leu missense mutation in exon 2 and the polyalanine polymorphism Ala5/Ala6 in exon 1 were found to decrease protein activity. Type 2 diabetes mellitus (DM) patients that carried the 198Leu allele were found to have increased intimal thickness and a higher prevalence of CVD [25]. Subsequent studies demonstrated that the 198Leu polymorphism was associated with increased coronary artery calcium score [26] in DM patients as well. Additionally, an earlier study found that the Ala5/Ala6 polymorphism resulted in a significantly increased risk for CAD [27]. Although no prospective studies were performed regarding the relationship of GPx-1 polymorphisms and CVD, a large study prospective study found an inverse correlation between CVD and erythrocyte GPx-1 activity [28].
Methyltetrahydrofolate Reductase and Homocysteine
Homocysteine (Hcy) is situated at the crossroads of several metabolic pathways. Synthesis of Hcy starts with the activation of methionine by ATP, a step that is followed by the loss of a methyl group and enzymatic hydrolysis. Betaine-homocysteine methyltransferase catalyzes the remethylation of Hcy back to homocysteine in the liver. In other tissues, this process is catalyzed by methionine synthase, which relies upon methyltetrahydrofolate as a methyl donor, and vitamin B12 as a co-factor. Methylenetetrahydrofolate reductase (MTHFR) is responsible for the production of methyltetrahydrofolate, a process that requires B2 as a co-factor. When in excess, cystathionine β-synthase, in the presence of vitamin B6, catalyzes the trans-sulfuration of Hcy to form cystathionine, that can later on be converted to cysteine [29]. A pathologic elimination of Hcy and its conversion to Hcy-thiolactone occurs when Hcy is mistakenly recognized as methionine by methionyl-tRNA synthetase (MetRS) [30].
Both Hcy and its metabolites were discovered to possess pro-atherogenic effects, including modulation of thrombosis, lipid metabolism and peroxidation, cell survival, proliferation, and apoptosis. As a consequence of its ability to form amide bonds with lysine residues, Hcy-thiolactone can interact with various proteins causing their n-homocysteinylation and altering their solubility and activity. This process is known to occur with paraoxonase 1 (PON1), which hydrolyzes Hcy-thiolactone and oxidized phospholipids. N-homocysteinylation of PON1 causes its inactivation, thus decreasing HDL associated antioxidant activity and HDL function [30]. Additionally, hyperhomocysteinemia has been associated with diminished expression of HDL-related proteins such as apolipoprotein A1 (ApoA1) and lecithin-cholesterol acyltransferase (LCAT), and increased HDL catabolism, both leading to decreased high-density lipoprotein (HDL) levels and activity [31]. Another lipoprotein that was shown to undergo n-homocysteinylation by Hcy-thiolactone is apolipoprotein B100 (ApoB100), which is the major component of low density lipoprotein (LDL). Modification of ApoB100 by Hcy-thiolactone causes protein aggregation, making it toxic to endothelial cells [30].
Reacting with copper to produce H2O2 and other ROS, Hcy was identified as a pro-oxidative agent. Although Hcy is also capable of promoting the production of NO, which scavenges Hcy and glutathione, which in turn scavenges H2O2, the saturability of these reactions renders them ineffective when facing prolonged exposure to high Hcy levels [32]. Promotion of a pro-coagulatory state and decreased fibrinolysis both contribute to the pro-thrombotic effects of Hcy and its metabolites. When exposed to Hcy, endothelial cells shift to a pro-coagulatory state, increasing the production of tissue factor and factor V, and attenuating the synthesis and activation of anti-coagulatory factors, including heparin sulfate, protein C, NO, and prostacyclins [32]. Activation of tissue plasminogen activator is inhibited as well, leading to decreased fibrinolysis. During the latter, n-homocysteinylation of fibrinogen occurs, rendering it more resistant to fibrinolysis [30].
Hcy and Hcy-thiolactone negatively interact with endothelial cells by affecting pathways other than coagulation and thrombolysis. Endothelial cells treated with Hcy thiolactone or Hcy exhibited increased apoptosis [33] and decreased proliferation [34]. Hyperhomocysteinemia was observed to cause impairment of endothelial-dependent vasodilation, probably as a result of injury to the endothelium, increased oxidative status, and decreased NO availability. Hcy can also cause thickening of the vessel wall due to its ability to accelerate vascular smooth muscle cell (VSMCs) [35] proliferation and increase collagen production [32].
The connection between hyperhomocysteinemia and CVD was first implicated in 1969, with the observation that children suffering from inherited cystathionine β-synthase deficiency presenting with homocystinuria tend to suffer from cardiovascular diseases as well [36]. Since then, the relationship between elevated Hcy and CVD has been extensively studied, with the evidence pointing toward a strong relationship between the two, a relationship that is independent of other risk factors for CVD [37]. Therefore, Hcy-reducing treatments, including mainly vitamins from the B group, were examined for their ability to reduce the risk for CVD among individuals suffering from hyperhomocysteinemia. Although supplementation did lead to a decrease in homocysteine levels, in most studies this decrease was not accompanied by a lesser risk for CVD [38]. Furthermore, a potential harmful effect of vitamin supplementation was suggested by some studies [39]. An exception to these results is the effect of B vitamin supplementation on stroke, where reduced risk was observed [40]. Searching for an answer to this conundrum, it has been proposed that Hcy is a marker of other pathologic phenomena that occur in CVD. Alternatively, it has been suggested that the short durations of Hcy-lowering treatments and the confounding effect of grain fortification with folate shifted the results of the clinical trials. Another explanation for the discrepancy is that Hcy reduction may only be helpful in earlier stages of CVD [3].
The discovery of the relationship between CBS deficiency, hyperhomocysteinemia, and CVD has led to the search for other polymorphisms and mutations that may interrupt normal Hcy metabolism. The most widely studied polymorphism is that of MTHFR, where a C to T substitution occurs at position 677 (C677T). This is a missense polymorphism, the enzyme produced by it being thermolabile and with decreased reactivity. The frequency of the polymorphic 677 T allele ranges from 30% to 40%, with 10% homozygosity. In folate deficiency, TT homozygotes were noted to suffer from hyperhomocysteinemia [32]. Although a strong relationship between the C677T polymorphism and CVD was suggested in initial studies, the failure to replicate these results lead to the notion that the C677T polymorphism is only a minor risk factor for CVD [41].
Paraoxonase
The paraoxonase gene family (PON1, PON2 and PON3) is clustered on a single chromosomal locus 7q21.3. Although they are highly homologous both in nucleotide sequence (70% homology) and amino acid sequence (60% homology), they are extensively varied with regard to their expression profile. PON1 and PON3 are expressed mostly in the liver and are associated with HDL in the plasma. PON2, on the other hand, is expressed in a variety of tissues and is localized to the nuclear membrane and endoplasmic reticulum (ER) [42•]. Although the ability to hydrolyze organophosphates is unique to PON1, all PON enzymes share the ability to metabolize different lactones [43], some of which are formed by peroxidation of phospholipids [42]. This enzymatic activity is supposedly essential for maintaining cell membrane integrity, protecting it from different endogenous and exogenous toxins. The localization of PON1 to the same HDL subfraction as clusterin supports this hypothesis, as the latter is also assumed to be involved in cell membrane protection [44]. A recent trial has suggested a role for the PON enzymes in the innate immune system, as they are able to hydrolyze the quorum-sensing signal molecule N-acyl-homoserine-lactone [42•].
It is well appreciated that HDL is able to attenuate the development of atherosclerosis by various mechanisms, which include the removal of cholesterol from vessel wall cells and its transport back to the liver, a process known as reverse cholesterol transport (RCT), and attenuating LDL oxidation. The various HDL anti-atherogenic activities are catalyzed by enzymes that are associated with the HDL particle. PON1 has been shown to diminish LDL oxidation [45] and prevent the oxidized LDL (oxLDL)-associated pro-inflammatory response, the latter most likely related to its ability to metabolize lipid peroxides [46]. PON1 is also vital for the continuous normal function of HDL, as it prevents its oxidation. This was also shown in PON1 knockout mice, whose HDL was less able to limit lipid peroxidation and LDL-induced inflammation [47]. These mice were also more susceptible to the development of atherosclerosis in both dietary and genetic models [47, 48]. Mice overexpressing PON1 were found to be more resistant to the development of atherosclerosis compared to their wild-type littermates, suggesting a therapeutic potential for PON1 elevation [49].
Although genetic polymorphisms were found in all members of the PON gene family, research in the area of CVD has focused on the enzyme PON1, where several polymorphisms, both in the coding region and the promoter region, were identified. The promoter polymorphism C (−107)T was shown to have a striking effect on gene transcription, as the affinity between the transcription factor Sp1 and the −107 C allele is significantly higher compared to the affinity between Sp1 and the −107 T allele. This disparity translates into increased plasma PON1 activity and concentration in carriers of the −107 C allele [50]. However, in spite of the difference in protein concentration and activity, this polymorphism did not prove to be clinically significant with regard to CVD [51]. It was recently shown that although the C(−107)T polymorphism does not affect the risk for CVD, it may modulate the extent of the disease as measured by the number of coronary arteries undergoing stenosis [52].
The Glu192Arg missense mutation has also been extensively studied. The exchange of glutamine to arginine results in a decrease in serum PON activity and concentration. This may be caused by a diminished affinity of the Arg192 PON for the HDL, resulting in decreased protein stability and activity [53]. Of all PON1 polymorphisms, only the Glu192Arg polymorphism was associated with CVD in a large meta-analysis. However, the external validity of this result was questioned due to lack of a significant association between this polymorphism and CVD in large trials, and also because of possible publication bias [51]. This polymorphism has also been associated with stroke in a recently published meta-analysis [54•]. Due to the many polymorphisms in PON1 and the failure to show any association with CVD, the relationship between PON1 phenotype (concentration and activity) and CVD was studied. Indeed, PON1 activity was demonstrated to be a predictor of CVD, regardless of its genotype [55]. A large trial linking PON1 genotype to PON phenotype has shown that not only do both the Glu192Arg genotype and PON phenotype determine the risk for CVD, but also that the PON1 genotype is a predictor of PON serum activity and concentration [56].
Superoxide Dismutase
The superoxide dismutase family of proteins consists of three members, all of which rely on various metals as cofactors and act as antioxidants in both intracellular and extracellular compartments, scavenging mostly superoxide anions, but other oxidants as well that are formed during physiologic and pathologic biological processes. SOD1, the first enzyme to be discovered, is dependent on copper (Cu) and Zinc (Zn) for its activity and is localized to the cytosol and nucleus. SOD2, also known as manganese-SOD (MnSOD), is the mitochondrial form of SOD and is responsible for scavenging O2- ion formed as a byproduct of aerobic metabolism. SOD3 is the extracellular member of the SOD family (EC-SOD), and like SOD1, its metal cofactors are Cu and Zn [57].
MnSOD
As mentioned above, SOD2 is most abundant in the mitochondria. Of all three SODs enzymes, only MnSOD was found to be critical for survival of both eukaryotes and other aerobic organisms. Mice with MnSOD deficiency suffer from growth retardation and die shortly after birth with neurodegeneration, dilated cardiomyopathy, steatosis, and skeletal muscle defects. Mitochondrial injury in these mice, assessed by changes in both structure and function of the citric acid cycle, has been observed as well [57, 58]. Several polymorphisms have been discovered in the SOD2 gene, situated on chromosomal locus 6q25 [57]. Of all these polymorphisms, the Ala16Val missense mutation was found to be relevant for cardiovascular disease. This polymorphism disrupts the secondary structure of MnSOD, decreasing its activity by approximately 40% and decreasing its transport to the mitochondria. Most of the studies conducted on this polymorphism were with diabetes patients, showing that the 16Val allele is associated with increased diabetic coronary heart disease in women [59] and diabetic nephropathy [60]. In the non-diabetes population, this polymorphism was associated with vasospastic angina pectoris [61, 62].
Haptoglobin-Mediated and Hemoglobin-Mediated Oxidative Stress
Haptoglobin (Hp) is an acute phase, plasma-born glycoprotein that is produced mainly by hepatocytes, but also by other cell types including adipocytes and lung cells, and is well-known for its ability to tightly bind free hemoglobin (Hb) following its release from erythrocytes [63]. Hp plasma concentrations are high, ranging from 0.3 mg/mL to 3.0 mg/mL, yielding an Hp/Hb molar ratio of 400:1 and allowing effective Hb scavenging even when hemolysis occurs and Hb levels steeply increase. This activity of Hp is important for the prevention of iron loss via Hb filtration by the glomeruli and renal damage. Following its formation, Hp-Hb complex is taken up by various scavenger receptors in the liver and other tissues. One such receptor is the CD163 receptor, present on macrophages and Kupffer cells. Scavenging of Hb by Hp is also critical for preventing Hb-mediated oxidative damage. Extracorpuscular Hb can release its heme iron, which acts as a potent oxidizing agent and can generate free radicals, leading to the production of ROS that exert oxidative damage to their surroundings. By shielding Hb from its surroundings, Hp prevents this deleterious cascade. Additionally, Hp protects the Hb globin from oxidation by the heme iron, enabling effective clearance of Hb by the CD163 receptor [64•].
The Hp gene is located at chromosomal locus 16q22, and has two known alleles in humans: Hp1, whose allele frequency is 0.4, and Hp2, whose allele frequency is 0.6 in most Western populations. As the alleles are in a Hardy-Weinberg equilibrium, the frequency of Hp genotypes is 16% Hp 1–1, 48% Hp 2–1, and 36% Hp 2–2 [63]. It is hypothesized that early in human evolution a duplication of exons 3 and 4 of the Hp 1 allele occurred, resulting in the formation of the Hp 2 allele. This duplication includes a cysteine residue located in exon 3 that participates in the formation of a disulfide bridge between Hp monomers, thus making the Hp 2 monomer divalent whereas the Hp 1 monomer remains monovalent. As a result, there is a dramatic difference in the size and structure of the Hp protein in the serum. The monovalent Hp 1 monomer can bind a single Hp molecule, leading to the formation of dimers in the Hp 1–1 genotype. The Hp 2 monomer is able to bind two Hp molecules, resulting in the formation of cyclic Hp 2–2 polymers in the Hp 2–2 genotype. In the Hp 2–1 genotype, heteromeric polymers are formed, with Hp1 proteins at each end of an Hp2 linear polymer. Although Hp 1–1 dimers are known to exist in the plasma of Hp 2–1 individuals, Hp 2–2 cyclic polymers have not been detected [65].
With regard to CVD, the Hp polymorphism has a disease-specific interaction with DM. In the general population, it has been suggested that individuals with the Hp 1–1 genotype are more prone to developing of CVD [66]. However, in the presence of DM, the Hp 2–2 genotype is a significant risk factor for CVD, conferring a twofold to fivefold increase in the risk of CVD compared to Hp 2–1 or Hp 1–1 genotypes [67•]. This observation was supported by in vivo studies in transgenic mice, in which there was an increased tendency of diabetic Hp 2–2 mice to develop retinopathy, nephropathy, and atherosclerosis compared to diabetic Hp 1–1 mice [3].
The unique interaction between the Hp 2–2 genotype and DM appears to stem from the interaction of the Hp 2–2 protein with Hb. Increased extravascular and intravascular hemolysis is known to occur in DM, leading to an increase in Hp-Hb complexes in plasma and tissues. As was discussed above, Hp is regarded as an antioxidant due to its ability to bind Hb and prevent the release of heme iron and the initiation of a free radical chain reaction. However, the ability to act as an antioxidant differs greatly between the Hp genotypes [68]. Whereas Hp 1–1 and Hp 2–2 have similar affinities for Hb [69], the ability of the Hp 2–2 protein to shield the heme iron from its aqueous surroundings is poor compared to that of the Hp 1–1 protein. Oxidation and glycation of Hb, which commonly occur in DM, further amplify these differences. Oxidative stress evoked by Hp 2-Hb complexes in Chinese hamster ovary (CHO) cells expressing the CD163 receptor cultured in hyperglycemic conditions was greater compared to that evoked by Hp1-Hb under similar conditions. Tissue and plasma redox–active iron was decreased in Hp 1–1 DM mice and humans compared to Hp 2–2 DM individuals, demonstrating the diminished ability of the Hp 2–2 protein to neutralize Hb [70]. The increase in plasma oxidative stress is also demonstrated by the reduction in antioxidants such as vitamin C in the serum of Hp2-2 DM individuals [65]. Additionally, hyperglycemia and increased oxidative stress both result in diminished expression of CD163 on the surface of macrophages, which is the result of plasma membrane shedding and decreased transcription [71]. Finally, the rate of clearance of the Hp-Hb-2-2 complex by the CD163 receptor is reduced compared to that of the Hp-Hb-1-1, resulting in the extended presence of Hp-Hb in tissues in Hp 2–2 individuals [69]. This situation is confounded by the decreased expression of CD163 in Hp 2–2 individuals [72].
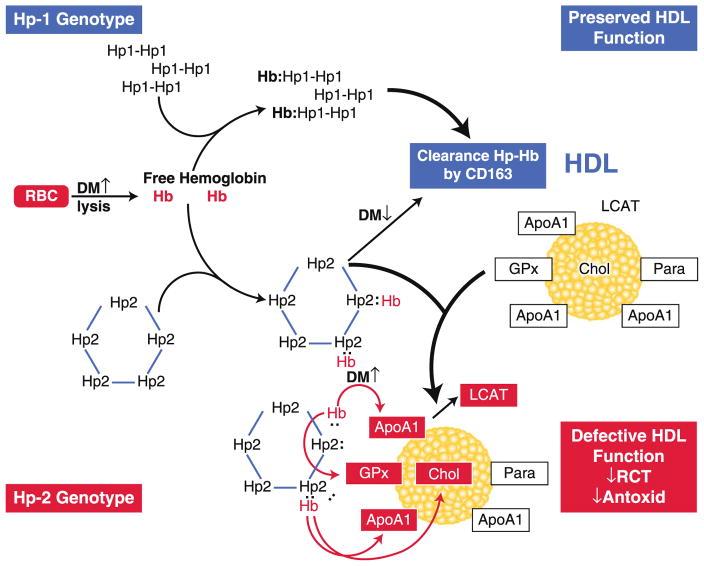
Another novel aspect of the Hp2-2–associated oxidative stress in diabetes is related to the association of Hp with HDL. Either free or Hb bound, Hp appears to be a part of the HDL proteome. There is more Hp 2–2 associated with HDL compared to Hp 1–1 due to its polymeric nature and also due to the impaired clearance of Hp-Hb in Hp 2–2 individuals with DM. This, along with the lesser antioxidant ability of Hp2-2, makes the HDL of Hp 2–2 individuals with DM exposed to higher levels of oxidative stress and results in an increase in HDL-associated lipid peroxides. These oxidative alterations result in a decrease in HDL function, such as cholesterol esterification and cholesterol efflux, and may result in the formation of pro-atherogenic HDL particles in DM individuals with the Hp 2–2 genotype (Fig. 1) [73]. This pathway provides a mechanistic rationale for the pharmacogenetic interaction between vitamin E and the Hp 2–2 genotype, whereby vitamin E significantly reduces the risk for CVD in diabetic individuals with Hp 2–2 [74].
Fig. 1.
The interaction between haptoglobin (Hp) genotype and diabetes mellitus (DM) leading to high-density lipoprotein (HDL) dysfunction. Hemoglobin (Hb) released intravascularly from red blood cells (RBC) is rapidly bound by Hp protein to form an Hp-Hb complex. In Hp 2–2 diabetic individuals, the complex is cleared more slowly than in Hp 1–1 diabetic individuals by the scavenger receptor CD163. The Hp-Hb complex can bind to apolipoprotein A1 (ApoA1) in HDL, with increased binding of Hp 2-2–Hb occurring due its increased affinity for HDL and its increased plasma concentration. The Hp 2-2–Hb complex, but not the Hp 1-1–Hb complex, when bound to HDL can produce reactive oxygen species that can oxidize protein (eg, ApoA1, glutathione peroxidase [GPx], and lecithin: cholesterol acyl transferase [LCAT]) and lipid components (cholesterol [Chol]) of HDL and render the HDL dysfunctional (because of decreased reversed cholesterol transport [RCT] and antioxidant activity), pro-atherogenic, and pro-thrombotic. Antioxid—antioxidant; Para—paraoxynase. (From Asleh et al. [73]. Correction of HDL dysfunction in individuals with diabetes and the haptoglobin 2–2 genotype. Diabetes 2008;57:2794–800)
Identification of Polymorphisms in Redox Genes in Genome-Wide Association Studies
The effect of the polymorphisms mentioned above was tested in trials where one or a small number of candidate gene polymorphisms were tested for their correlation with CVD. A non-biased approach to identify possible genetic contributions to CVD risk has been attempted in genome-wide association studies (GWAS) using high-density screening with a panel of single nucleotide polymorphisms that can scan the entire human genome. It is interesting to note that most of the genes discussed above were not identified by GWAS as significant for the prediction of CVD risk. Similar to other studies, the MTHFR C677T polymorphism was identified as a determinant of plasma homocysteine levels in GWAS [75]. Polymorphisms in the NOS3 gene were observed to have an effect on CVD risk in a single GWAS [76].
Conclusions
With the increasing capacity to perform genomic association studies, many genetic polymorphisms are being investigated with regard to their association with various diseases. However, most of them do not show significance when tested in different populations.
This may be explained by the inability to specify the effect of a certain polymorphism on protein activity, expression, or stability. Thus, these polymorphisms may be in linkage disequilibrium with other genetic markers that have an impact on disease progression, a linkage that may perhaps not exist in all populations. Most of the genes examined in this article fall in this category. Having a direct effect on redox systems, these polymorphisms maintain their significant relationship with CVD even in the face of population stratification (Table 1).
Table 1.
Summary of redox genes that modulate risk for cardiovascular disease
While identification of at-risk individuals is an important step in personalized medicine, it becomes valuable only when it is accompanied by a personalized treatment scheme, which may include a more aggressive pharmacologic intervention, or closer monitoring of the patient, as is customarily done for carriers of genetic polymorphisms associated with colorectal cancer. As is demonstrated by the pharmacogenetic relationship between vitamin E and the Hp genotype in the setting of DM, the identification of at-risk individuals allows for early intervention and clear benefit in terms of reduced complications from CVD.
Acknowledgments
This work was supported by grants from the Israel-US Binational Science Foundation, the Juvenile Diabetes Foundation, and the National Institutes of Health (NIH RO1DK085226).
Footnotes
Disclosure All of the authors have received grants to their institution from the National Institutes of Health and the Juvenile Diabetes Research Foundation.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104(4):503–16. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 2.Kondo T, Hirose M, Kageyama K. Roles of oxidative stress and redox regulation in atherosclerosis. J Atheroscler Thromb. 2009;16(5):532–8. doi: 10.5551/jat.1255. [DOI] [PubMed] [Google Scholar]
- 3.Farbstein D, Levy AP. The genetics of vascular complications in diabetes mellitus. Cardiol Clin. 2010;28(3):477–96. doi: 10.1016/j.ccl.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marsden PA, Heng HH, Scherer SW, Stewart RJ, Hall AV, Shi XM, et al. Structure and chromosomal localization of the human constitutive endothelial nitric oxide synthase gene. J Biol Chem. 1993;268(23):17478–88. [PubMed] [Google Scholar]
- 5.Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res. 2007;75(2):247–60. doi: 10.1016/j.cardiores.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrew PJ, Mayer B. Enzymatic function of nitric oxide synthases. Cardiovasc Res. 1999;43(3):521–31. doi: 10.1016/s0008-6363(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 7.Napoli C, Ignarro LJ. Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Arch Pharm Res. 2009;32(8):1103–8. doi: 10.1007/s12272-009-1801-1. [DOI] [PubMed] [Google Scholar]
- 8.Napoli C, Ignarro LJ. Polymorphisms in endothelial nitric oxide synthase and carotid artery atherosclerosis. J Clin Pathol. 2007;60 (4):341–4. doi: 10.1136/jcp.2006.040550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald DM, Alp NJ, Channon KM. Functional comparison of the endothelial nitric oxide synthase Glu298Asp polymorphic variants in human endothelial cells. Pharmacogenetics. 2004;14 (12):831–9. doi: 10.1097/00008571-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Naber CK, Baumgart D, Altmann C, Siffert W, Erbel R, Heusch G. eNOS 894 T allele and coronary blood flow at rest and during adenosine-induced hyperemia. Am J Physiol. 2001;281(5):H1908–1912. doi: 10.1152/ajpheart.2001.281.5.H1908. [DOI] [PubMed] [Google Scholar]
- 11.Rossi GP, Taddei S, Virdis A, Cavallin M, Ghiadoni L, Favilla S, et al. The T-786 C and Glu298Asp polymorphisms of the endothelial nitric oxide gene affect the forearm blood flow responses of Caucasian hypertensive patients. J Am Coll Cardiol. 2003;41(6):938–45. doi: 10.1016/s0735-1097(02)03011-5. [DOI] [PubMed] [Google Scholar]
- 12.Gulec S, Karabulut H, Ozdemir AO, Ozdol C, Turhan S, Altin T, et al. Glu298Asp polymorphism of the eNOS gene is associated with coronary collateral development. Atherosclerosis. 2008;198(2):354–9. doi: 10.1016/j.atherosclerosis.2007.09.037. [DOI] [PubMed] [Google Scholar]
- 13.Miyamoto Y, Saito Y, Nakayama M, Shimasaki Y, Yoshimura T, Yoshimura M, et al. Replication protein A1 reduces transcription of the endothelial nitric oxide synthase gene containing a −786 T–>C mutation associated with coronary spastic angina. Hum Mol Genet. 2000;9(18):2629–37. doi: 10.1093/hmg/9.18.2629. [DOI] [PubMed] [Google Scholar]
- 14.Wang XL, Sim AS, Wang MX, Murrell GA, Trudinger B, Wang J. Genotype dependent and cigarette specific effects on endothelial nitric oxide synthase gene expression and enzyme activity. FEBS Lett. 2000;471(1):45–50. doi: 10.1016/s0014-5793(00)01356-9. [DOI] [PubMed] [Google Scholar]
- 15.Jeerooburkhan N, Jones LC, Bujac S, Cooper JA, Miller GJ, Vallance P, et al. Genetic and environmental determinants of plasma nitrogen oxides and risk of ischemic heart disease. Hypertension. 2001;38(5):1054–61. doi: 10.1161/hy1101.092967. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Wu X, Li X, Feng G, He L, Shi Y. The endothelial nitric oxide synthase gene is associated with coronary artery disease: a meta-analysis. Cardiology. 2010;116(4):271–8. doi: 10.1159/000316063. [DOI] [PubMed] [Google Scholar]
- 17.Casas JP, Cavalleri GL, Bautista LE, Smeeth L, Humphries SE, Hingorani AD. Endothelial nitric oxide synthase gene polymorphisms and cardiovascular disease: a HuGE review. Am J Epidemiol. 2006;164(10):921–35. doi: 10.1093/aje/kwj302. [DOI] [PubMed] [Google Scholar]
- 18.Comhair SA, Erzurum SC. The regulation and role of extracellular glutathione peroxidase. Antioxid Redox Signal. 2005;7(1–2):72–9. doi: 10.1089/ars.2005.7.72. [DOI] [PubMed] [Google Scholar]
- 19•.Margis R, Dunand C, Teixeira FK, Margis-Pinheiro M. Glutathione peroxidase family - an evolutionary overview. FEBS J. 2008;275(15):3959–3970. doi: 10.1111/j.1742-4658.2008.06542.x. This is an excellent review of the glutathione peroxidase family of proteins and their functions. [DOI] [PubMed] [Google Scholar]
- 20.Voetsch B, Loscalzo J. Genetic determinants of arterial thrombosis. Arterioscler Thromb Vasc Biol. 2004;24(2):216–29. doi: 10.1161/01.ATV.0000107402.79771.fc. [DOI] [PubMed] [Google Scholar]
- 21.Voetsch B, Jin RC, Bierl C, Benke KS, Kenet G, Simioni P, et al. Promoter polymorphisms in the plasma glutathione peroxidase (GPx-3) gene: a novel risk factor for arterial ischemic stroke among young adults and children. Stroke. 2007;38(1):41–9. doi: 10.1161/01.STR.0000252027.53766.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenet G, Freedman J, Shenkman B, Regina E, Brok-Simoni F, Holzman F, et al. Plasma glutathione peroxidase deficiency and platelet insensitivity to nitric oxide in children with familial stroke. Arterioscler Thromb Vasc Biol. 1999;19(8):2017–23. doi: 10.1161/01.atv.19.8.2017. [DOI] [PubMed] [Google Scholar]
- 23•.Voetsch B, Jin RC, Bierl C, Deus-Silva L, Camargo EC, Annichino-Bizacchi JM, Handy DE, Loscalzo J. Role of promoter polymorphisms in the plasma glutathione peroxidase (GPx-3) gene as a risk factor for cerebral venous thrombosis. Stroke. 2008;39(2):303–307. doi: 10.1161/STROKEAHA.107.490094. This article demonstrates the importance of glutathione peroxidase polymorphism in stroke risk. [DOI] [PubMed] [Google Scholar]
- 24.Lei XG, Cheng WH, McClung JP. Metabolic regulation and function of glutathione peroxidase-1. Annu Rev Nutr. 2007;27:41–61. doi: 10.1146/annurev.nutr.27.061406.093716. [DOI] [PubMed] [Google Scholar]
- 25.Hamanishi T, Furuta H, Kato H, Doi A, Tamai M, Shimomura H, et al. Functional variants in the glutathione peroxidase-1 (GPx-1) gene are associated with increased intima-media thickness of carotid arteries and risk of macrovascular diseases in japanese type 2 diabetic patients. Diabetes. 2004;53(9):2455–60. doi: 10.2337/diabetes.53.9.2455. [DOI] [PubMed] [Google Scholar]
- 26.Nemoto M, Nishimura R, Sasaki T, Hiki Y, Miyashita Y, Nishioka M, et al. Genetic association of glutathione peroxidase-1 with coronary artery calcification in type 2 diabetes: a case control study with multi-slice computed tomography. Cardiovasc Diabetol. 2007;6:23. doi: 10.1186/1475-2840-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winter JP, Gong Y, Grant PJ, Wild CP. Glutathione peroxidase 1 genotype is associated with an increased risk of coronary artery disease. Coron Artery Dis. 2003;14(2):149–53. doi: 10.1097/00019501-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L, et al. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med. 2003;349(17):1605–13. doi: 10.1056/NEJMoa030535. [DOI] [PubMed] [Google Scholar]
- 29.Finkelstein JD. Methionine metabolism in mammals. J Nutr Biochem. 1990;1(5):228–37. doi: 10.1016/0955-2863(90)90070-2. [DOI] [PubMed] [Google Scholar]
- 30.Jakubowski H. The pathophysiological hypothesis of homocysteine thiolactone-mediated vascular disease. J Physiol Pharmacol. 2008;59 (Suppl 9):155–67. [PubMed] [Google Scholar]
- 31.Obeid R, Herrmann W. Homocysteine and lipids: S-adenosyl methionine as a key intermediate. FEBS Lett. 2009;583(8):1215–25. doi: 10.1016/j.febslet.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 32.Nygard O, Vollset SE, Refsum H, Brattstrom L, Ueland PM. Total homocysteine and cardiovascular disease. J Intern Med. 1999;246 (5):425–54. doi: 10.1046/j.1365-2796.1999.00512.x. [DOI] [PubMed] [Google Scholar]
- 33.Kerkeni M, Tnani M, Chuniaud L, Miled A, Maaroufi K, Trivin F. Comparative study on in vitro effects of homocysteine thiolactone and homocysteine on HUVEC cells: evidence for a stronger proapoptotic and proinflammative homocysteine thiolactone. Mol Cell Biochem. 2006;291(1–2):119–26. doi: 10.1007/s11010-006-9204-9. [DOI] [PubMed] [Google Scholar]
- 34.Chang PY, Lu SC, Lee CM, Chen YJ, Dugan TA, Huang WH, et al. Homocysteine inhibits arterial endothelial cell growth through transcriptional downregulation of fibroblast growth factor-2 involving G protein and DNA methylation. Circ Res. 2008;102 (8):933–41. doi: 10.1161/CIRCRESAHA.108.171082. [DOI] [PubMed] [Google Scholar]
- 35.Tsai JC, Wang H, Perrella MA, Yoshizumi M, Sibinga NE, Tan LC, et al. Induction of cyclin A gene expression by homocysteine in vascular smooth muscle cells. J Clin Investig. 1996;97(1):146–53. doi: 10.1172/JCI118383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56(1):111–28. [PMC free article] [PubMed] [Google Scholar]
- 37.Van Guelpen B, Hultdin J, Johansson I, Witthoft C, Weinehall L, Eliasson M, et al. Plasma folate and total homocysteine levels are associated with the risk of myocardial infarction, independently of each other and of renal function. J Intern Med. 2009;266(2):182–95. doi: 10.1111/j.1365-2796.2009.02077.x. [DOI] [PubMed] [Google Scholar]
- 38.Albert CM, Cook NR, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. Jama. 2008;299(17):2027–36. doi: 10.1001/jama.299.17.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354(15):1567–77. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Qin X, Demirtas H, Li J, Mao G, Huo Y, et al. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet. 2007;369(9576):1876–82. doi: 10.1016/S0140-6736(07)60854-X. [DOI] [PubMed] [Google Scholar]
- 41.Lewis SJ, Ebrahim S, Davey Smith G. Meta-analysis of MTHFR 677 C->T polymorphism and coronary heart disease: does totality of evidence support causal role for homocysteine and preventive potential of folate? BMJ (Clinical research ed. 2005;331 (7524):1053. doi: 10.1136/bmj.38611.658947.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42•.Shih DM, Lusis AJ. The roles of PON1 and PON2 in cardiovascular disease and innate immunity. Current opinion in lipidology. 2009;20(4):288–292. doi: 10.1097/MOL.0b013e32832ca1ee. This is an excellent review of paraoxonase biology and its relationship to cardiovascular disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Draganov DI, Teiber JF, Speelman A, Osawa Y, Sunahara R, La Du BN. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res. 2005;46(6):1239–47. doi: 10.1194/jlr.M400511-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Durrington PN, Mackness B, Mackness MI. Paraoxonase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21(4):473–80. doi: 10.1161/01.atv.21.4.473. [DOI] [PubMed] [Google Scholar]
- 45.Mackness MI, Arrol S, Durrington PN. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991;286(1–2):152–4. doi: 10.1016/0014-5793(91)80962-3. [DOI] [PubMed] [Google Scholar]
- 46.Watson AD, Berliner JA, Hama SY, La Du BN, Faull KF, Fogelman AM, et al. Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J Clin Investig. 1995;96(6):2882–91. doi: 10.1172/JCI118359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shih DM, Gu L, Xia YR, Navab M, Li WF, Hama S, et al. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998;394(6690):284–7. doi: 10.1038/28406. [DOI] [PubMed] [Google Scholar]
- 48.Shih DM, Xia YR, Wang XP, Miller E, Castellani LW, Subbanagounder G, et al. Combined serum paraoxonase knockout/apolipoprotein E knockout mice exhibit increased lipoprotein oxidation and atherosclerosis. J Biol Chem. 2000;275(23):17527–35. doi: 10.1074/jbc.M910376199. [DOI] [PubMed] [Google Scholar]
- 49.Tward A, Xia YR, Wang XP, Shi YS, Park C, Castellani LW, et al. Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation. 2002;106(4):484–90. doi: 10.1161/01.cir.0000023623.87083.4f. [DOI] [PubMed] [Google Scholar]
- 50.Deakin S, Leviev I, Brulhart-Meynet MC, James RW. Paraoxonase-1 promoter haplotypes and serum paraoxonase: a predominant role for polymorphic position - 107, implicating the Sp1 transcription factor. Biochem J. 2003;372(Pt 2):643–9. doi: 10.1042/BJ20021670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wheeler JG, Keavney BD, Watkins H, Collins R, Danesh J. Four paraoxonase gene polymorphisms in 11212 cases of coronary heart disease and 12786 controls: meta-analysis of 43 studies. Lancet. 2004;363(9410):689–95. doi: 10.1016/S0140-6736(04)15642-0. [DOI] [PubMed] [Google Scholar]
- 52.Najafi M, Gohari LH, Firoozrai M. Paraoxonase 1 gene promoter polymorphisms are associated with the extent of stenosis in coronary arteries. Thromb Res. 2009;123(3):503–10. doi: 10.1016/j.thromres.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Gaidukov L, Rosenblat M, Aviram M, Tawfik DS. The 192R/Q polymorphs of serum paraoxonase PON1 differ in HDL binding, lipolactonase stimulation, and cholesterol efflux. J Lipid Res. 2006;47(11):2492–502. doi: 10.1194/jlr.M600297-JLR200. [DOI] [PubMed] [Google Scholar]
- 54•.Dahabreh IJ, Kitsios GD, Kent DM, Trikalinos TA. Paraoxonase 1 polymorphisms and ischemic stroke risk: A systematic review and meta-analysis. Genet Med. 2010;12(10):606–615. doi: 10.1097/GIM.0b013e3181ee81c6. This article is a recent comprehensive meta-analysis of paraoxonase polymorphisms and their relationship. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jarvik GP, Hatsukami TS, Carlson C, Richter RJ, Jampsa R, Brophy VH, et al. Paraoxonase activity, but not haplotype utilizing the linkage disequilibrium structure, predicts vascular disease. Arterioscler Thromb Vasc Biol. 2003;23(8):1465–71. doi: 10.1161/01.ATV.0000081635.96290.D3. [DOI] [PubMed] [Google Scholar]
- 56.Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. Jama. 2008;299(11):1265–76. doi: 10.1001/jama.299.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miao L, St Clair DK. Regulation of superoxide dismutase genes: implications in disease. Free Radic Biol Med. 2009;47(4):344–56. doi: 10.1016/j.freeradbiomed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lebovitz RM, Zhang H, Vogel H, Cartwright J, Jr, Dionne L, Lu N, et al. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci USA. 1996;93(18):9782–7. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones DA, Prior SL, Tang TS, Bain SC, Hurel SJ, Humphries SE, Stephens JW. Association between the rs4880 superoxide dismutase 2 (C>T) gene variant and coronary heart disease in diabetes mellitus. Diabetes Res Clin Pract. 2010 doi: 10.1016/j.diabres.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 60.Mollsten A, Marklund SL, Wessman M, Svensson M, Forsblom C, Parkkonen M, et al. A functional polymorphism in the manganese superoxide dismutase gene and diabetic nephropathy. Diabetes. 2007;56(1):265–9. doi: 10.2337/db06-0698. [DOI] [PubMed] [Google Scholar]
- 61.Fujimoto H, Kobayashi H, Ogasawara K, Yamakado M, Ohno M. Association of the manganese superoxide dismutase polymorphism with vasospastic angina pectoris. J Cardiol. 2010;55 (2):205–10. doi: 10.1016/j.jjcc.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 62.Fujimoto H, Taguchi J, Imai Y, Ayabe S, Hashimoto H, Kobayashi H, et al. Manganese superoxide dismutase polymorphism affects the oxidized low-density lipoprotein-induced apoptosis of macrophages and coronary artery disease. Eur Heart J. 2008;29 (10):1267–74. doi: 10.1093/eurheartj/ehm500. [DOI] [PubMed] [Google Scholar]
- 63.Bowman BH, Kurosky A. Haptoglobin: the evolutionary product of duplication, unequal crossing over, and point mutation. Advances in human genetics. 1982;12:189–261. 453–184. doi: 10.1007/978-1-4615-8315-8_3. [DOI] [PubMed] [Google Scholar]
- 64•.Levy AP, Asleh R, Blum S, Levy NS, Miller-Lotan R, Kalet-Litman S, Anbinder Y, Lache O, Nakhoul FM, Asaf R, et al. Haptoglobin: Basic and Clinical Aspects. Antioxidants & redox signaling. 2009 doi: 10.1089/ars.2009.2793. This is an excellent summary of work showing basic mechanisms and clinical studies showing the importance of the haptoglobin polymorphism in predicting risk of CVD in diabetes. [DOI] [PubMed] [Google Scholar]
- 65.Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem. 1996;42 (10):1589–600. [PubMed] [Google Scholar]
- 66.Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G. Haptoglobin and risk of myocardial infarction, stroke, and congestive heart failure in 342,125 men and women in the Apolipoprotein MOrtality RISk study (AMORIS) Annals of medicine. 2009:1–11. doi: 10.1080/07853890903089453. [DOI] [PubMed] [Google Scholar]
- 67•.Milman U, Blum S, Shapira C, Aronson D, Miller-Lotan R, Anbinder Y, Alshiek J, Bennett L, Kostenko M, Landau M, et al. Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2–2 genotype: a prospective double-blinded clinical trial. Arteriosclerosis, thrombosis, and vascular biology. 2008;28(2):341–347. doi: 10.1161/ATVBAHA.107.153965. This prospective, placebo-controlled pharmacogenomic study showed that vitamin E provides significant cardiovascular protection to individuals with diabetes and the haptoglobin 2–2 genotype. [DOI] [PubMed] [Google Scholar]
- 68.Melamed-Frank M, Lache O, Enav BI, Szafranek T, Levy NS, Ricklis RM, et al. Structure-function analysis of the antioxidant properties of haptoglobin. Blood. 2001;98(13):3693–8. doi: 10.1182/blood.v98.13.3693. [DOI] [PubMed] [Google Scholar]
- 69.Asleh R, Marsh S, Shilkrut M, Binah O, Guetta J, Lejbkowicz F, et al. Genetically determined heterogeneity in hemoglobin scavenging and susceptibility to diabetic cardiovascular disease. Circ Res. 2003;92(11):1193–200. doi: 10.1161/01.RES.0000076889.23082.F1. [DOI] [PubMed] [Google Scholar]
- 70.Asleh R, Guetta J, Kalet-Litman S, Miller-Lotan R, Levy AP. Haptoglobin genotype- and diabetes-dependent differences in iron-mediated oxidative stress in vitro and in vivo. Circ Res. 2005;96(4):435–41. doi: 10.1161/01.RES.0000156653.05853.b9. [DOI] [PubMed] [Google Scholar]
- 71.Timmermann M, Hogger P. Oxidative stress and 8-iso-prostaglandin F(2alpha) induce ectodomain shedding of CD163 and release of tumor necrosis factor-alpha from human monocytes. Free Radic Biol Med. 2005;39(1):98–107. doi: 10.1016/j.freeradbiomed.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 72.Boyle JJ, Harrington HA, Piper E, Elderfield K, Stark J, Landis RC, et al. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. Am J Pathol. 2009;174 (3):1097–108. doi: 10.2353/ajpath.2009.080431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Asleh R, Blum S, Kalet-Litman S, Alshiek J, Miller-Lotan R, Asaf R, et al. Correction of HDL dysfunction in individuals with diabetes and the haptoglobin 2–2 genotype. Diabetes. 2008;57(10):2794–800. doi: 10.2337/db08-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Farbstein D, Levy AP. Pharmacogenomics and the prevention of vascular complications in diabetes mellitus. Therapy. 2009;6 (4):531–8. [Google Scholar]
- 75.Pare G, Chasman DI, Parker AN, Zee RR, Malarstig A, Seedorf U, et al. Novel associations of CPS1, MUT, NOX4, and DPEP1 with plasma homocysteine in a healthy population: a genome-wide evaluation of 13 974 participants in the Women’s Genome Health Study. Circ Cardiovasc Genet. 2009;2(2):142–50. doi: 10.1161/CIRCGENETICS.108.829804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O’Donnell CJ, Cupples LA, D’Agostino RB, Fox CS, Hoffmann U, Hwang SJ, Ingellson E, Liu C, Murabito JM, Polak JF, et al. Genome-wide association study for subclinical atherosclerosis in major arterial territories in the NHLBI’s Framingham Heart Study. BMC Med Genet. 2007;8 (Suppl 1):S4. doi: 10.1186/1471-2350-8-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]