Abstract
Background and Aims
Despite their relatively high content of saturated fat, studies of dairy product intake and the risk of cardiovascular disease have often yielded null or inverse results. The use of fatty acid biomarkers to reflect dairy intake could elucidate this association. This study aims to evaluate the association between dairy intake, assessed by adipose pentadecanoic (15:0) and heptadecanoic (17:0) fatty acids and food frequency questionnaire (FFQ), and the risk of nonfatal myocardial infarction (MI), in a matched case-control study of Costa Rican adults (n=3630).
Methods and Results
The association was examined using conditional logistic regression, adjusted for potential confounders. The associations of adipose tissue 15:0 and 17:0 with the risk of MI were not statistically significant (for 15:0: multivariate-adjusted OR for 5th quintile vs. 1st =1.14 (95% CI= 0.85, 1.53), p-value for linear trend=0.77; for 17:0: multivariate-adjusted OR for 5th quintile vs. 1st =1.15 (95% CI= 0.88, 1.51), p-value for linear trend=0.18). The association between the FFQ measure of dairy intake and MI showed evidence of a possible threshold effect, with a protective association observed for all but the top quintile of the exposure distribution.
Conclusion
Dairy product intake as assessed by adipose tissue 15:0, 17:0, and by FFQ is not associated with a linear increase in the risk of MI in the study population. It is possible that the adverse effect of saturated fat in dairy products on cardiovascular health is offset by presence of beneficial nutrients.
Keywords: biomarkers, dairy, myocardial infarction
Introduction
Dairy products have long been recognized as a crucial source of energy, protein, calcium, and other essential nutrients in the human diet (1). They are also rich in cholesterol and saturated fatty acids, which are widely believed to increase the risk of cardiovascular disease by promoting atherogenesis (2). In support of that hypothesis, evidence from human intervention trials has linked intake of butter to an increase in both total and low-density lipoprotein (LDL) cholesterol (3). The hypercholesterolemic effect of dairy, however, was not consistently replicated in studies using milk, yogurt, or other fermented milk products (4, 5).
Evidence from observational studies is also conflicting. Recent systematic reviews of prospective cohort studies of dairy intake, assessed by food frequency questionnaire (FFQ) or dietary record, concluded that dairy food intake was not consistently associated with adverse cardiovascular outcomes in a variety of adult populations (6–8). Case-control studies using FFQ measures as exposure have reported either a decrease in cardiovascular risk (9, 10) or a non-significant association (11). Studies using biomarkers of dairy intake have also yielded discrepant results, ranging from robust protective associations in case-control studies of Scandinavian populations (12, 13) to a significant positive association between the proportion of 15:0 fatty acid in plasma and the risk of ischemic heart disease in the Nurses’ Health Study cohort (14). Although differences in study design and exposure ascertainment may explain some of the heterogeneity, it is likely that the atherogenic effect of dairy may be counterbalanced by other bioactive components that are actually protective of cardiovascular disease, namely conjugated linoleic acid (CLA), calcium, vitamin D, and others (15).
In recent years, the use of biomarkers in nutritional epidemiology has become more widespread. Advantages of using adipose tissue biomarkers to characterize long-term nutritional intake include slow turnover, absence of recall bias, lack of response to conditions of acute disease, and others (16). Several studies identified the proportions of 15:0 and 17:0 fatty acids in human adipose tissue as valid biomarkers of long-term dairy intake, as these fatty acids cannot be synthesized endogenously and are specific for dairy fat in most populations (17–19). However, because adipose tissue 15:0 and 17:0 focus on a sole dietary constituent, they do not account for the role of other dairy components in disease pathogenesis. As errors associated with biomarker ascertainment are independent of errors associated with FFQ or dietary record data, combining dairy intake estimates from both methods provides the most comprehensive and powerful method of exposure ascertainment (16).
In this study we assessed the hypothesis that dairy intake, measured by adipose tissue pentadecanoic (15:0) and heptadecanoic (17:0) fatty acids as well as FFQ data, is associated with the risk of first nonfatal myocardial infarction (MI) in a population-based case-control study of Costa Rican adults.
Methods
Study population
The study population, described in detail in several publications, consisted of Hispanics who resided in the Central Valley of Costa Rica between 1994 and 2004 (20–23). Cases of first nonfatal acute MI were ascertained by two independent cardiologists in the participating hospitals and deemed eligible if they met the World Health Organization criteria (24), survived hospitalization, were under 75 years of age on the day of their first MI, and able to answer the questionnaire. Eligible cases were matched by 5-year age group, sex, and area of residence to population controls identified randomly using data from the National Census and Statistics Bureau of Costa Rica. Participation was 98% for cases and 88% for controls. All participants provided written informed consent. The study has been approved by the Human Subjects Committee of the Harvard School of Public Health and the University of Costa Rica.
Data collection
After the cases were discharged from the hospital, all cases and controls received home visits, during which trained study workers collected lifestyle and medical history data, anthropometric measurements, and biological specimens. Self-reported dairy intake during the past year was assessed using an FFQ specifically designed and validated in the Costa Rican population (25). Specifically, dairy intake was defined to include intake of butter, buttermilk, cheeses (including mozzarella-style cheese, cottage cheese, and cream cheese), cream, ice cream, lactocrema (a mixture of butter and margarine), milk (whole, 1%, and 2%), and yogurt.
Physical activity information was collected using a questionnaire described in more detail in previous publications (23). Briefly, participants reported the average frequency and time spent on several occupational and leisure time activities during the last year. Energy expenditure for each activity was calculated as the product of frequency, time, and intensity measured in METS (metabolic equivalents, defined as the energy expenditure for sitting quietly or approximately kJ · kg body−1 · h−1) (23). Smoking and alcohol intake were measured using questionnaires. Anthropometric measurements, including abdominal obesity as operationalized by the waist-to-hip ratio, were collected the morning after an overnight fast by trained fieldworkers while subjects wore light clothing and no shoes. Measurements were performed in duplicate, with the average used in the analysis. Finally, income was measured by showing participants index cards with ranges of income (in US dollars per month) and asking them to select the appropriate index card.
Biological samples were collected following an overnight fast. Subcutaneous adipose biopsies, collected following an overnight fast, were performed with a 16-gauge needle using a modification of the method proposed by Beynen and Katan (26). Fatty acids from adipose tissue were quantified by gas-liquid chromatography and used as a biomarker of dairy intake over the past two or more years (16). Total separation of trans isomers was achieved to distinguish three 18:1 isomers, three 18:2 isomers and one CLA isomer.
Statistical analysis
A total of 3630 participants had complete data on adipose tissue fatty acids and potential confounders. The indicator method was used to address missing values of the income variable. After deleting participants with missing information, remaining cases were re-matched to controls to preserve the design of the study.
Data from the Costa Rica Study were analyzed using the SAS software package (Version 9.2; SAS Institute Inc, Cary, NC). To assess the statistical significance of differences in general characteristics and potential confounders, we used paired t-tests for continuous variables and McNemar’s tests for categorical variables. Partial Spearman’s correlation coefficients were used to determine associations between adipose tissue biomarkers and self-reported dairy intake. Least squares means adjusted for age, sex, waist-to-hip ratio, and smoking were calculated to assess the association between self-reported dairy intake and adipose tissue biomarkers among controls. Exposures were defined as adipose tissue 15:0 and 17:0, as well as self-reported dairy intake, divided into quintiles. The associations between exposures and nonfatal MI was evaluated using conditional logistic regression, adjusted for age, sex, area of residence (by matching), income, smoking status, total energy intake, physical activity, waist-to-hip ratio, alcohol intake, as well as self-reported history of hypertension, diabetes, and hypercholesterolemia. For both adipose tissue and self-reported exposures, additional models were fit adjusting for all of the above covariates plus adipose tissue CLA and trans fatty acids, and intake of calcium intake and saturated fats. Tests for linear trends across quintiles were conducted using logistic regression models, with the median value of the corresponding quintile as the predictor.
Results
The general characteristics of the study population are summarized by case/control status in Table 1. Neither adipose tissue 15:0 nor 17:0 nor self-reported dairy intake (adjusted for total energy intake) differed significantly by disease status. Cases had higher frequencies of MI risk factors, including smoking, low physical activity, lower monthly income, abdominal obesity, and a history of hypertension, diabetes, and hypercholesterolemia. Additionally, cases were more likely to report a diet high in calories, saturated fat, cholesterol, and trans fat, but low in polyunsaturated fat.
Table 1.
Characteristics of the Costa Rica Study population by case-control status (n=3630).
| Variable | Cases (n=1815) | Controls (n=1815) |
|---|---|---|
| Age, years | 58.5±10.9 | 58.2±11.2 |
| % Female | 27 | 27 |
| Monthly household income, USD a | 505 ±394 | 581±424 |
| % Current smokers a | 40 | 21 |
| Alcohol intake, g/d a | 7.1±18.5 | 6.1±14.5 |
| % History of hypertension a | 39 | 30 |
| % History of diabetes a | 25 | 15 |
| % History of hypercholesterolemia a | 30 | 27 |
| Waist-to-hip ratio a | 0.97±0.07 | 0.95±0.07 |
| Physical activity, MET a | 34.2±15.7 | 35.4±15.3 |
| Adipose tissue fatty acids, % of total | ||
| 15:0 | 0.21±0.07 | 0.21±0.07 |
| 17:0 | 0.20±0.05 | 0.20±0.05 |
| Daily dietary intake | ||
| Total energy, kcal a | 2692±939 | 2435±750 |
| Saturated fat, % energy a | 12.5±3.1 | 11.6±2.9 |
| Polyunsaturated fat, % energy a | 6.9±2.4 | 7.2±2.4 |
| trans fat, % energy a | 1.29±0.60 | 1.25±0.59 |
| Cholesterol, mg/1000kcal a | 349±231 | 292±171 |
| Dairy products, g/d a,b | 244±270 | 237±227 |
P-value < 0.05, from paired t-test for continuous variables or McNemar’s test for categorical variables.
Adjusted for total energy intake using the residuals method
The age-standardized distribution of potential confounders by quintile of dairy product intake, as assessed by both FFQ and adipose tissue 15:0 and 17:0, is presented in Table 2. The range of median dairy intake extended from 24 g/day in the lowest quintile to 593 g/day in the highest quintile. Participants with a history of diabetes or hypercholesterolemia were more likely to be in higher quintiles of dairy intake as ascertained by all measures of exposure. Finally, participants in higher quintiles of dairy intake were less likely to be current smokers and consumed less alcohol.
Table 2.
Characteristics of the Costa Rica Study by quintile of dairy intake among controls, standardized by age (n=1815).
| Variable | FFQ dairy intakea, g/day | Adipose tissue 15:0, % of total fatty acids | Adipose tissue 17:0, % of total fatty acids | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | |
| Median quintile value | 24 | 176 | 593 | 0.13 | 0.21 | 0.30 | 0.14 | 0.20 | 0.27 |
| Monthly household income, USD | 582 | 571 | 590 | 526 | 581 | 609 | 548 | 571 | 628 |
| % Current smokers | 23 | 21 | 19 | 32 | 19 | 13 | 25 | 19 | 20 |
| Alcohol intake, g/d | 11 | 4 | 5 | 11 | 5 | 3 | 9 | 5 | 4 |
| % History of hypertension | 23 | 31 | 30 | 28 | 29 | 32 | 32 | 31 | 25 |
| % History of diabetes | 9 | 16 | 16 | 13 | 15 | 17 | 14 | 12 | 16 |
| % History of hypercholesterolemia | 22 | 23 | 29 | 22 | 27 | 32 | 25 | 28 | 25 |
| Waist-to-hip ratio | 0.96 | 0.95 | 0.94 | 0.96 | 0.95 | 0.94 | 0.95 | 0.95 | 0.95 |
| Physical activity, MET | 37 | 34 | 36 | 35 | 36 | 35 | 34 | 35 | 37 |
| Adipose tissue fatty acids | |||||||||
| trans fatty acids | 3.4 | 3.5 | 3.6 | 3.5 | 3.5 | 3.7 | 3.3 | 3.6 | 3.8 |
| ALA | 0.67 | 0.67 | 0.61 | 0.63 | 0.68 | 0.64 | 0.60 | 0.67 | 0.68 |
| CLA | 0.50 | 0.54 | 0.63 | 0.54 | 0.54 | 0.64 | 0.59 | 0.56 | 0.53 |
| Daily dietary intake | |||||||||
| Total energy, kcal | 2921 | 2131 | 2620 | 2444 | 2409 | 2485 | 2393 | 2422 | 2516 |
| Saturated fat, % energy | 11 | 11 | 13 | 11 | 12 | 12 | 11 | 12 | 12 |
| Polyunsaturated fat, % energy | 7.2 | 7.6 | 6.6 | 6.8 | 7.2 | 7.1 | 6.8 | 7.3 | 7.3 |
| Cholesterol, mg/1000kcal | 341 | 259 | 343 | 288 | 276 | 303 | 267 | 283 | 324 |
| Calcium, mga | 649 | 773 | 1277 | 732 | 871 | 1024 | 816 | 858 | 920 |
Adjusted for total energy using the residuals method
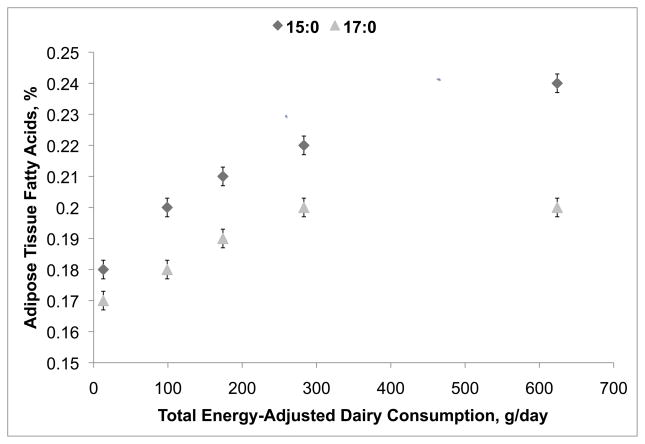
The correlation of dairy intake with adipose tissue biomarkers was strong for 15:0 (Spearman’s ρ= 0.34, one-sided p-value <0.0001), but lower for 17:0 (Spearman’s ρ= 0.16, one-sided p-value <0.0001). Age-, sex- and waist-to-hip ratio-adjusted adipose tissue 15:0 and 17:0 increased across dairy intake quintiles, although at higher intake levels the correlations reached a plateau (Figure 1). Energy-adjusted dairy intake was strongly correlated with intake of calcium (Spearman’s ρ= 0.75; one-sided p-value <0.0001). There was a weak correlation between adipose tissue CLA (Spearman’s ρ= 0.24, one-sided p-value <0.0001) and energy-adjusted dairy intake. Similarly, there were weak correlations between calcium and adipose tissue CLA with adipose tissue biomarkers (for 15:0: Spearman’s ρ= 0.33 and 0.16 respectively, one-sided p-values <0.0001; for 17:0: Spearman’s ρ= 0.21 and −0.12 respectively, one-sided p-values <0.0001).
Figure 1.
Relationship between adipose tissue dairy biomarkers and dairy intake among controls (n=1815). Least-square means and standard errors of adipose tissue fatty acids, plotted against quintiles of calorie-adjusted weighted dairy product intake, adjusted for age, sex, and waist-to-hip ratio. Diamonds and triangles indicate least-squares means of adipose 15:0 and 17:0 respectively; bars indicate 95% CIs for these means.
Table 3 presents the analyses examining associations between long-term dairy intake assessed by FFQ as well as adipose tissue 15:0 and 17:0 and the risk of nonfatal MI in the study population. In the models adjusted only for age, sex, and area of residence by matching, dairy intake as assessed by FFQ was not linearly associated with the risk of MI. However, a threshold effect was observed, with a statistically significant protective association for quintiles 2, 3, and 4, and a statistically insignificant increase in the odds of MI in the 5th quintile. Upon further adjustment for income, smoking, physical activity, waist-to-hip ratio, alcohol intake, as well as for adipose tissue alpha-linolenic acid (ALA), and history of hypertension, hypercholesterolemia, and diabetes, the linear association between dairy intake as assessed by FFQ and the risk of MI stayed non-significant (multivariate-adjusted OR for 5th vs. 1st quintile= 0.82, 95% CI= (0.64, 1.04), p-value for linear trend= 0.61) but the threshold effect remained. To evaluate the effect of specific dairy components, we also adjusted for adipose tissue trans fatty acids as well as for intake of saturated fats (as a % of total energy), which yielded a protective relation for 5th vs. 1st quintile of dairy intake (multivariate-adjusted OR= 0.68, 95% CI= (0.53, 0.87), p-value for linear trend= 0.19). However, further adjustment for protective dairy components, namely dietary intake of calcium and adipose tissue CLA, did not result in a significant estimate of association (multivariate-adjusted OR for 5th vs. 1st quintile= 0.78, 95% CI= (0.56, 1.08), p-value for linear trend= 0.34).
Table 3.
Dairy product intake and the risk of nonfatal MI in the Costa Rica Study (n= 3630).
| Model | OR (95% CI) | P-value For Linear Trend | ||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Dairy intake (FFQ)a | ||||||
| Age/sex/residence adjusted | 1.00 (referent) | 0.72 (0.59, 0.88) | 0.74 (0.60, 0.92) | 0.67 (0.54, 0.83) | 0.83 (0.67, 1.03) | 0.60 |
| Multivariate-adjusted model 1b | 1.00 (referent) | 0.73 (0.57, 0.92) | 0.75 (0.59, 0.96) | 0.72 (0.57, 0.92) | 0.82 (0.64, 1.04) | 0.61 |
| Multivariate-adjusted model 2c | 1.00 (referent) | 0.71 (0.56, 0.90) | 0.75 (0.59, 0.95) | 0.68 (0.53, 0.87) | 0.68 (0.53, 0.87) | 0.19 |
| Multivariate-adjusted model 3d | 1.00 (referent) | 0.74 (0.58, 0.94) | 0.79 (0.61, 1.01) | 0.75 (0.57, 0.98) | 0.78 (0.56, 1.08) | 0.34 |
| 15:0 | ||||||
| Age/sex/residence adjusted | 1.00 (referent) | 1.20 (0.97, 1.49) | 1.01 (0.82, 1.26) | 0.95 (0.75, 1.19) | 0.89 (0.70, 1.11) | 0.06 |
| Multivariate-adjusted model 4e | 1.00 (referent) | 1.31 (1.02, 1.68) | 1.23 (0.95, 1.58) | 1.10 (0.84, 1.43) | 1.03 (0.79, 1.36) | 0.54 |
| Multivariate-adjusted model 5f | 1.00 (referent) | 1.30 (1.02, 1.67) | 1.19 (0.92, 1.54) | 1.06 (0.81, 1.39) | 0.95 (0.72, 1.25) | 0.23 |
| Multivariate-adjusted model 6g | 1.00 (referent) | 1.33 (1.03, 1.71) | 1.26 (0.97, 1.64) | 1.19 (0.90, 1.57) | 1.14 (0.85, 1.53) | 0.77 |
| 17:0 | ||||||
| Age/sex/residence adjusted | 1.00 (referent) | 1.01 (0.82, 1.24) | 1.05 (0.85, 1.30) | 1.01 (0.82, 1.26) | 1.20 (0.97, 1.49) | 0.10 |
| Multivariate-adjusted model 4e | 1.00 (referent) | 1.03 (0.81, 1.31) | 1.15 (0.90, 1.46) | 1.26 (0.98, 1.62) | 1.33 (1.03, 1.72) | 0.01 |
| Multivariate-adjusted model 5f | 1.00 (referent) | 1.01 (0.79, 1.29) | 1.11 (0.87, 1.41) | 1.21 (0.94, 1.56) | 1.24 (0.96, 1.61) | 0.05 |
| Multivariate-adjusted model 6g | 1.00 (referent) | 1.00 (0.78, 1.27) | 1.07 (0.84, 1.37) | 1.17 (0.91, 1.52) | 1.15 (0.88, 1.51) | 0.18 |
Adjusted for total energy intake using the residuals method
Model 1: Adjusted for age/sex/residence by matching as well as for income, smoking, physical activity, waist-to-hip ratio, alcohol intake, adipose tissue ALA, and history of hypertension, hypercholesterolemia, or diabetes.
Model 2: Adjusted for all the above plus adipose tissue trans fatty acids and dietary intake of saturated fats (as a % of total energy).
Model 3: Adjusted for all the above plus adipose tissue CLA and dietary intake of calcium (adjusted for total energy using the residuals method).
Model 4: Adjusted for age/sex/residence by matching as well as for income, smoking, physical activity, waist-to-hip ratio, adipose tissue ALA, total energy intake, alcohol intake, and history of hypertension, hypercholesterolemia, or diabetes.
Model 5: Adjusted for all the above plus adipose tissue trans fatty acids and dietary intake of saturated fats (as a proportion of total energy intake).
Model 6: Adjusted for all the above plus adipose tissue CLA and dietary intake of calcium (adjusted for total energy using the residuals method).
Adipose tissue 15:0 and 17:0 were not significantly associated with the risk of MI in models adjusted only for matching factors. Further adjustment for income, smoking, physical activity, waist-to-hip ratio, adipose tissue ALA, total energy intake, alcohol intake, and history of hypertension, hypercholesterolemia, and diabetes yielded a significant estimate of association for 17:0 fatty acid (OR for 5th vs. 1st quintile= 1.33, 95% CI= (1.03, 1.72), p-value for linear trend= 0.01) but not for 15:0. For both adipose tissue biomarkers, additional adjustment for adipose tissue trans fatty acids and intake of saturated fats resulted in weaker estimates of association for 5th vs. 1st quintile of dairy intake (for 15:0: OR = 0.95, 95% CI= (0.72, 1.25), p-value for linear trend= 0.23; for 17:0: OR= 1.24, 95% CI= (0.96, 1.61), p-value for linear trend= 0.05). Finally, in the fully adjusted models including adipose tissue CLA and intake of calcium, adipose tissue 15:0 and 17:0 were not significantly associated with the risk of the outcome: (for 15:0: OR=1.14, 95% CI= (0.85, 1.53), p-value for linear trend= 0.77, for 17:0: OR=1.15, 95% CI= (0.88, 1.51), p-value for linear trend= 0.18).
Discussion
We have shown that dairy intake, as ascertained by FFQ and adipose tissue 15:0 and 17:0 fatty acids, was not linearly associated with the risk of MI in the Costa Rica Study. However, the association between the FFQ measure of dairy intake and MI showed evidence of a possible threshold effect, with a protective association observed for all but the top quintile of the exposure distribution. Furthermore, our estimates of association were attenuated after adjustment for known atherogenic factors (trans fats and saturated fats) but increased after adjustment for known protective components such as calcium and CLA, suggesting that the cumulative effect of dairy products is likely to involve a balance of factors.
There are several reasons for the discrepancies between the adipose tissue 15:0 and 17:0 fatty acids results, as well as between biomarker and FFQ results. Specifically, our data showed that adipose tissue 17:0 is less correlated with diet than 15:0, suggesting that it may reflect yet unknown metabolic processes in addition to long-term dairy intake. Additionally, both 15:0 and 17:0 are specific for dairy fats, while the FFQ measure includes effects of other potentially cardioprotective components of dairy. Furthermore, biomarker measures are more indicative of long-term intake and less prone to recall bias than FFQ measures. Finally, chance remains a possible explanation for the observed associations.
The association between dairy intake and cardiovascular health is likely to emerge from a balance of protective and risk factors. Although the evidence from animal and human studies is inconclusive and often conflicting, several distinct mechanisms have been proposed involving individual nutrients and their putative interactions. On the protective side, dairy is a rich source of calcium, magnesium, potassium and vitamin D, which have been linked to a reduction in risk of cardiovascular disease in a variety of populations (15, 27). A recent review of experimental and observational studies has shown that increased intake of dairy minerals, combined with a low-sodium diet, has anti-hypertensive effects in a variety of populations (27). The effects of vitamin D on cardiovascular health are also likely to involve blood pressure regulation, particularly by suppressing the renin gene on the renin-angiotensin pathway. However, data from the Women’s Health Initiative show no reduction in either blood pressure or the risk of developing hypertension over 7 years of follow-up associated with vitamin D supplementation (28).
On the other hand, dairy is a rich source of dietary cholesterol, trans fatty acids, and saturated fatty acids, which are believed to increase the risk of heart disease by promoting hyperlipidemia and atherogenesis. Results from the Nurses’ Health Study indicate that higher ratios of full-fat to low-fat dairy intake as well as higher plasma 15:0 are associated with an increase in risk of coronary heart disease (14, 29). On the contrary, a previous analysis of the Costa Rica Study found that adipose tissue 9c,11t isomer of CLA was associated with a decreased risk of MI and may offset the adverse effects of dairy saturated fat intake on cardiovascular risk (30).
The strengths of our study include smaller errors in dairy intake measurements associated with the use of combined biomarker and FFQ data, large sample size, and high participation rates. Although recall bias is a possibility in our study, we believe it is unlikely as the participants did not demonstrate a high level of awareness of dietary determinants of heart disease, instead reporting stress as the most likely cause of their MI. We have adjusted for potential confounders such as age, sex, area of residence, dietary factors, smoking, alcohol, physical activity, and socioeconomic status, but the possibility of residual confounding cannot be completely excluded. Also, our outcome variable definition was restricted to first nonfatal MI, and thus the results cannot be generalized to the broader concept of cardiovascular disease. Finally, the observational nature of the Costa Rica Study precludes establishment of causal relationships between study variables.
In summary, we have shown that long-term dairy intake is not associated with adverse cardiovascular outcomes in the Costa Rica Study. The mechanisms underlying the potential association between dairy intake and cardiovascular health are complex and clearly warrant more research.
Acknowledgments
This study was supported by grant HL60692 from the National Institutes of Health.
Acronyms used
- LDL
low-density lipoprotein
- FFQ
food frequency questionnaire
- CLA
conjugated linoleic acid
- MI
myocardial infarction
- OR
odds ratio
- ALA
alpha-linolenic acid
- CI
confidence interval
Footnotes
All authors read and approved the final manuscript. All authors report no conflicts of interest. No supplementary online material has been submitted.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
S Aslibekyan, Email: stella@brown.edu.
H Campos, Email: hcampos@hsph.harvard.edu.
A Baylin, Email: abaylin@umich.edu.
References
- 1.Donovan S. Milk composition and its implications in the adult diet. P Nutr Soc. 1983;42:375–384. doi: 10.1079/pns19830045. [DOI] [PubMed] [Google Scholar]
- 2.Mann JI. Diet and risk of coronary heart disease and type 2 diabetes. Lancet. 2002;360:783–789. doi: 10.1016/s0140-6736(02)09901-4. [DOI] [PubMed] [Google Scholar]
- 3.Steinmetz KA, Childs MT, Stimson C, Kushi LH, McGovern PG, Potter JD, et al. Effect of consumption of whole milk and skim milk on blood lipid profiles in healthy men. Am J Clin Nutr. 1994;59:612–618. doi: 10.1093/ajcn/59.3.612. [DOI] [PubMed] [Google Scholar]
- 4.Rossouw JE, Burger EM, Vandervyver P, Ferreira JJ. The effect of skim milk, yogurt, and full cream milk on human serum lipids. Am J Clin Nutr. 1981;34:351–356. doi: 10.1093/ajcn/34.3.351. [DOI] [PubMed] [Google Scholar]
- 5.Thompson LU, Jenkins DJ, Amer MA, Reichert R, Jenkins A, Kamulsky J. The effect of fermented and unfermented milks on serum cholesterol. Am J Clin Nutr. 1982;36:1106–1111. doi: 10.1093/ajcn/36.6.1106. [DOI] [PubMed] [Google Scholar]
- 6.Gibson RA, Makrides M, Smithers LG, Voevodin M, Sinclair AJ. The effect of dairy foods on CHD: a systematic review of prospective cohort studies. Br J Nutr. 2009;102:1267–1275. doi: 10.1017/S0007114509371664. [DOI] [PubMed] [Google Scholar]
- 7.German JB, Gibson RA, Krauss RM, Nestel P, Lamarche B, van Staveren WA, et al. A reappraisal of the impact of dairy foods and milk fat on cardiovascular disease risk. Eur J Nutr. 2009;48:191–203. doi: 10.1007/s00394-009-0002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elwood PC, Pickering JE, Givens DI, Gallacher JE. The consumption of milk and dairy foods and the incidence of vascular disease and diabetes: an overview of the evidence. Lipids. doi: 10.1007/s11745-010-3412-5. In print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biong AS, Rebnord HM, Fimreite RL, Trygg KU, Ringstad J, Thelle DS, et al. Intake of dairy fat and dairy products, and risk of myocardial infarction: A case-control study. Int J Food Sci Nutr. 2008;59:155–165. doi: 10.1080/09637480701532521. [DOI] [PubMed] [Google Scholar]
- 10.Kontogianni MD, Panagiotakos DB, Chrysohoou C, Pitsavos C, Stefanadis C. Modelling dairy intake on the development of acute coronary syndromes: the CARDIO2000 study. Eur J Cardiovasc Prev Rehabil. 2006;13:791–797. doi: 10.1097/01.hjr.0000219115.48285.33. [DOI] [PubMed] [Google Scholar]
- 11.Tavani A, Gallus S, Negri E, La Vecchia C. Milk, dairy products, and coronary heart disease. J Epidemiol Community Health. 2002;56:471–472. doi: 10.1136/jech.56.6.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biong AS, Veierod MB, Ringstad J, Thelle DS, Pedersen JI. Intake of milk fat, reflected in adipose tissue fatty acids and risk of myocardial infarction: a case-control study. Eur J Clin Nutr. 2006;60:236–244. doi: 10.1038/sj.ejcn.1602307. [DOI] [PubMed] [Google Scholar]
- 13.Warensjo E, Jansson JH, Berglund L, Boman K, Ahren B, Weinehall L, et al. Estimated intake of milk fat is negatively associated with cardiovascular risk factors and does not increase the risk of a first acute myocardial infarction: a prospective case-control study. Br J Nutr. 2004;91:635–642. doi: 10.1079/BJN20041080. [DOI] [PubMed] [Google Scholar]
- 14.Sun Q, Ma J, Campos H, Hu FB. Plasma and erythrocyte biomarkers of dairy fat intake and risk of ischemic heart disease. Am J Clin Nutr. 2007;86:929–937. doi: 10.1093/ajcn/86.4.929. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Manson JE, Buring JE, Lee IM, Sesso HD. Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension. 2008;51:1073–1079. doi: 10.1161/HYPERTENSIONAHA.107.107821. [DOI] [PubMed] [Google Scholar]
- 16.Baylin A, Kabagambe EK, Siles X, Campos H. Adipose tissue biomarkers of fatty acid intake. Am J Clin Nutr. 2002;76:750–757. doi: 10.1093/ajcn/76.4.750. [DOI] [PubMed] [Google Scholar]
- 17.Brevik A, Veierod MB, Drevon CA, Andersen LF. Evaluation of the odd fatty acids 15:0 and 17:0 in serum and adipose tissue as markers of intake of milk and dairy fat. Eur J Clin Nutr. 2005;59:1417–1422. doi: 10.1038/sj.ejcn.1602256. [DOI] [PubMed] [Google Scholar]
- 18.Wolk A, Furuheim M, Vessby B. Fatty acid composition of adipose tissue and serum lipids are valid biological markers of dairy fat intake in men. J Nutr. 2001;131:828–833. doi: 10.1093/jn/131.3.828. [DOI] [PubMed] [Google Scholar]
- 19.Wolk A, Vessby B, Ljung H, Barrefors P. Evaluation of a biological marker of dairy fat intake. Am J Clin Nutr. 1998;68:291–295. doi: 10.1093/ajcn/68.2.291. [DOI] [PubMed] [Google Scholar]
- 20.Campos H, Baylin A, Willett WC. alpha-linolenic acid and risk of nonfatal acute myocardial infarction. Circulation. 2008;118:339–345. doi: 10.1161/CIRCULATIONAHA.107.762419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornelis MC, El-Sohemy A, Kabagambe EK, Campos H. Coffee, CYP1A2 genotype, and risk of myocardial infarction. JAMA. 2006;295:1135–1141. doi: 10.1001/jama.295.10.1135. [DOI] [PubMed] [Google Scholar]
- 22.Kabagambe EK, Baylin A, Ruiz-Narvaez E, Rimm EB, Campos H. Alcohol intake, drinking patterns, and risk of nonfatal acute myocardial infarction in Costa Rica. Am J Clin Nutr. 2005;82:1336–1345. doi: 10.1093/ajcn/82.6.1336. [DOI] [PubMed] [Google Scholar]
- 23.Campos H, Siles X. Siesta and the risk of coronary heart disease: results from a population-based, case-control study in Costa Rica. Int J Epidemiol. 2000;29:429–437. [PubMed] [Google Scholar]
- 24.Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project: registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation. 1994;90:583–612. doi: 10.1161/01.cir.90.1.583. [DOI] [PubMed] [Google Scholar]
- 25.Kabagambe EK, Baylin A, Allan DA, Siles X, Spiegelman D, Campos H. Application of the method of triads to evaluate the performance of food frequency questionnaires and biomarkers as indicators of long-term dietary intake. Am J Epidemiol. 2001;154:1126–1135. doi: 10.1093/aje/154.12.1126. [DOI] [PubMed] [Google Scholar]
- 26.Beynen A, Katan M. Rapid sampling and long-term storage of subcutaneous adipose-tissue biopsies for determination of fatty acid composition. Am J Clin Nutr. 1985;42:317–322. doi: 10.1093/ajcn/42.2.317. [DOI] [PubMed] [Google Scholar]
- 27.Kris-Etherton PM, Grieger JA, Hilpert KF, West SG. Milk products, dietary patterns and blood pressure management. J Am Coll Nutr. 2009;28:103S–119S. doi: 10.1080/07315724.2009.10719804. [DOI] [PubMed] [Google Scholar]
- 28.Margolis KL, Ray RM, Van Horn L, Manson JE, Allison MA, Black HR, et al. Effect of calcium and vitamin D supplementation on blood pressure: the Women’s Health Initiative randomized trial. Hypertension. 2008;52:847–855. doi: 10.1161/HYPERTENSIONAHA.108.114991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu FB, Stampfer MJ, Manson JE, Ascherio A, Colditz GA, Speizer FE, et al. Dietary saturated fats and their food sources in relation to the risk of coronary heart disease in women. Am J Clin Nutr. 1999;70:1001–1008. doi: 10.1093/ajcn/70.6.1001. [DOI] [PubMed] [Google Scholar]
- 30.Smit LA, Baylin A, Campos H. Conjugated linoleic acid in adipose tissue and risk of myocardial infarction. Am J Clin Nutr. 2010;92:34–40. doi: 10.3945/ajcn.2010.29524. [DOI] [PMC free article] [PubMed] [Google Scholar]