Abstract
Probiotics have been shown to exert beneficial effects in the context of different diseases including inflammatory bowel diseases (IBD). However, clinical use of probiotics is hampered by lack of understanding of the protective mechanisms and by safety concerns regarding the application of high numbers of live bacteria in patients. The identification of protective microbial structure-function relationships might enable to overcome these restraints and might lead to innovative therapies using the isolated active microbial structures. In our study, we aimed to characterize the protective mechanisms of VSL#3, a clinically relevant probiotic mixture in IBD. We found Lactobacillus casei/paracasei-produced lactocepin to selectively degrade pro-inflammatory chemokines, resulting in reduced immune cell infiltration and reduced inflammation in experimental IBD models. As immune cell recruitment is a major proinflammatory mechanism our findings suggest that lactocepin might be of broad therapeutic relevance in an array of inflammatory diseases like IBD, allergic skin inflammation and psoriasis.
Keywords: lactocepin, prtP, CEP, IBD, probiotic, inflammation, chemokines, protease
Introduction
An extensive bulk of experimental data and an array of clinical studies indicate that specific probiotics might be a good alternative or adjunct therapy in the context of inflammatory bowel disease (IBD). However, the therapeutical application of probiotics is still hampered by uncertainties concerning appropriate strain selection, timing and dosage in the context of different IBD indications (Ulcerative Colitis, Crohn Disease or pouchitis, acute inflammation or remission, location and severity of the inflammation). In addition, there are still safety concerns regarding the uptake of huge amounts of live bacteria in IBD patients that are characterized by immune dysregulation and compromised intestinal barrier functions.1 Besides the lack of extensive strain specific clinical data, all these problems boil down to the lack of mechanistic understanding of probiotic efficacy. In contrast to the use of defined pharmaceuticals like corticosteroids, NSAIDs, immunosuppressive drugs or biologics that are known or even designed to target specific immune functions of the host, the protective structure-function relationships underlying the observed anti-inflammatory effects of specific probiotics are largely unknown. The identification of probiotic structure-function relationships is therefore a clear prerequisite for the targeted clinical use of probiotics or isolated probiotic structures in the future.
Recent studies already identified various microbial structures (e.g., proteins, cell surface components, metabolites, DNA) that beneficially affect the health of the host via highly diverse mechanisms.2-4 These microbial structures were found to either affect other microbes or to modulate host functions. The exact nature of these probiotic structure-function relationships has important implications for the potential therapeutic use of the respective microbe or microbial structure. Microbial structure-microbe relationships are exemplified by the finding that a Lactobacillus salivarius UCC118-produced bacteriocin (Abp118) mediates the observed prevention of systemic Listeria monocytogenes infection by Lactobacillus salivarius.4 This finding clearly indicates that the protective effect of potential Lactobacillus salivarius applications is restricted to the prevention of infections with Abp118-sensitive pathogens, demonstrating the major relevance of probiotic structure-function relationships for the development of effective probiotic therapies. Concerning direct microbe-host relationships, microbial structures were found to affect various intestinal barrier and immune functions. Bifidobacteria-produced acetate was found to reduce the mortality of EHEC-infected gnotobiotic mice by strengthening the intestinal epithelial barrier, resulting in reduced translocation of Shiga toxin into the systemic circulation. Importantly, this protective effect could be reproduced by feeding acetylated starch to germfree mice, proving that isolated microbial structures can be sufficient to mediate protection.5 In the context of IBD, polysaccharide A (PSA) of the intestinal commensal Bacteroides fragilis was also found to be sufficient to prevent and cure experimental colitis, probably via the induction of IL10-producing regulatory CD4+T cells.6-8 Furthermore, a secreted protein of Lactobacillus (L) rhamnosus GG, p40, was identified to reduce IEC apoptosis by epidermal growth factor receptor activation.9,10 Feeding isolated and encapsulated p40 to mice suffering from chemically-induced colitis resulted in significant reduction of IEC apoptosis and colonic inflammation.11 This result shows that isolated microbial proteins like p40 have the potential to exert protective functions in the intestine, provided that they are protected from the harsh conditions in the upper gastrointestinal tract. In summary, these findings demonstrate that tracking down probiotic effects to the mechanistic level is not only necessary to enable rational use of probiotics but can also result in the detection of promising new agents for the treatment of IBD.
PrtP-Encoded Lactocepin as Anti-Inflammatory Microbial Structure in the Context of IBD
In our study, we aimed to elucidate the protective mechanisms underlying the anti-inflammatory effects of the probiotic mixture VSL#3 that has been proven to be clinically relevant in the prevention and treatment of pouchitis12 as well as in the treatment of ulcerative colitis.13,14 Initial screening experiments using VSL#3 or the eight single bacterial strains (Bifidobacterium (B) breve, B. infantis, B. longum, Streptococcus thermophilus, L. acidophilus, L. bulgaricus, L. paracasei (L.p), L. plantarum) revealed that the complete mixture and the single strain L.p reduce TNF-induced secretion of the proinflammatory chemokine interferon-induced protein 10 (IP-10) in intestinal epithelial cells (IEC).15 The T-cell recruiting chemokine IP-10 is not only known to be strongly upregulated in inflamed intestinal tissue of IBD patients16,17 but has been shown to play a major proinflammatory role in experimental IBD. The neutralization of IP-10 via anti-IP-10 antibodies resulted in significantly reduced inflammation in DSS-treated mice and IL10−/− mice.18-20 The selective inhibitory effect of L.p on IP-10 was therefore thought to be of significant anti-inflammatory relevance. Subsequent stimulation experiments in order to analyze the underlying protective structure-function relationship revealed that the reduction of TNF-induced IP-10 is mediated by a cell surface attached and secreted protein of the probiotic strain. Mechanistically, we were not able to detect any impact of L.p on TNF-induced IP-10 expression in IEC, whereas the active protein in L.p supernatants (CM) was found to mediate loss of existing IEC-surface attached and secreted IP-10 in TNF-pre-activated IEC. These findings clearly indicated that the observed loss of IP-10 is due to an IEC-independent, direct effect of the active probiotic protein on the chemokine. Indeed, cell free experiments with CM and recombinant IP-10 revealed that the observed loss of IP-10 is due to IP-10 degradation by an L.p-derived serine protease. Further experiments revealed that the probiotic protease targets an array of additional proinflammatory chemokines like I-TAC and Eotaxin. In contrast, cytokines like IL-6 and IL-10 as well as IEC viability and barrier function were unaffected. This selective anti-inflammatory substrate profile suggested that the probiotic protease might potentially be used as a safe and efficient anti-inflammatory agent in vivo. In order to identify the active protease, chromatographic fractionation of L.p supernatants and subsequent LC-MS-MS analysis of the fractions were performed, suggesting prtP-encoded lactocepin to be the active anti-inflammatory structure. PrtP-encoded lactocepins are cell envelope proteases (CEPs) of lactobacilli and lactococci that are mainly known for the degradation of caseins and that play an important role in cheese production. The hypothesis that this long known protease is the anti-inflammatory structure of L.p was finally proven by IP-10 cleavage assays using immunoprecipitated L.p lactocepin. The selective degradation of proinflammatory chemokines by prtP-encoded lactocepin was therefore identified as anti-inflammatory structure-function relationship of L.p (Fig. 1). In order to test the physiological relevance of this newly identified probiotic mechanism, we generated a lactocepin-deficient mutant (L.c prtPdis) of a transformable human fecal L. casei (L.c) isolate that had been found to secrete prtP-encoded lactocepin with analogous anti-IP-10 activity as L.p. Feeding studies in inflamed T cell transferred RAG2−/− mice revealed significantly reduced cecal IP-10 levels, significantly reduced T cell and neutrophil infiltration (CD3+ and MPO-positive cells) and significantly reduced histopathological cecal inflammation in L.c fed mice compared with L.c prtPdis-fed mice.21 These results demonstrate that prtP-encoded lactocepin is indeed a physiologically relevant anti-inflammatory bacterial protease in the context of IBD that may, at least in part, be responsible for the observed anti-inflammatory effects of VSL#3 in experimental and clinical studies.
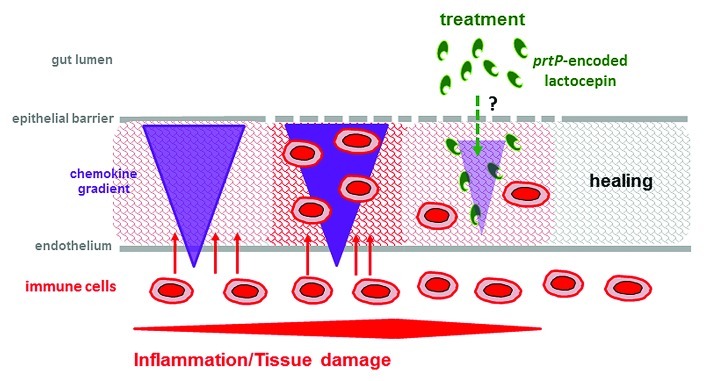
Figure 1. Anti-inflammatory mechanism of prtP-encoded lactocepin. Chronic inflammatory diseases like IBD, allergy and autoimmune diseases are characterized by a vicious circle of chemokine secretion, immune cell recruitment and activity and tissue damage including barrier disruption. PrtP-encoded lactocepin produced by L. casei/L. paracasei interferes with this vicious circle by specifically degrading proinflammatory chemokines, resulting in reduced infiltration of immune cells and potentially healing. However, the location of luminal lactocepin production and the mechanisms underlying its penetration into the mucosal tissue remain to be elucidated.
However, the characterization of this anti-inflammatory structure-function relationship raises several questions concerning the mode of action of prtP-encoded lactocepin in the intestine. Considering the fact that the intestinal lumen harbours large amounts of various bacterial and host proteases that degrade IP-10 (unpublished data) the underlying reasons for the specific anti-inflammatory effect of prtP-encoded lactocepin remain to be elucidated. One hypothesis is based on the assumption that prtP-encoded lactocepin degrades tissue distributed chemokines in the mucosa and therefore, the protective effect is dependent on the ability of prtP-encoded lactocepin to penetrate into the inflamed target tissue. The penetration might be facilitated by bacterial carriers that adhere to the intestinal mucosa and secrete prtP-encoded lactocepin in close proximity to the target tissue. Interestingly, the expression of prtP-encoded lactocepin seems to positively affect mucosal adherence in itself, as L.c showed better mucosal adherence than L.c prtPdis in monocolonized mice.21 The possible penetration of bacteria-produced proteins into inflamed intestinal mucosal tissue has already been proven in a study using TNF-nanobody-secreting Lactococcus lactis22 and is presumably due to disturbed intestinal barrier functions in inflammation.
Furthermore, it is unclear why L.c-produced lactocepin was found to be protective in the cecum whereas the inflammation of the distal colon did not differ between L.c and L.c prtPdis-fed T cell transferred RAG2−/− mice.21 This discrepancy in responsiveness had already been observed in studies using VSL#3 in IL10−/− mice15 and might be due to the different morphology, different fecal passage kinetics and/or the reduced water content in the colon.23 It remains to be determined whether these factors impair the proteolytic activity of prtP-encoded lactocepin, reduce the accessibility of mucosal attachment sites for lactocepin-expressing bacteria or hamper the expression of prtP-encoded lactocepin, resulting in insufficient protective activity of prtP-encoding bacteria in the colon.
In light of these questions, it will be highly interesting to investigate the anti-inflammatory impact of isolated encapsulated lactocepin on experimental IBD. On the one hand, the application of the isolated protective protease allows the uptake of defined high amounts of prtP-encoded lactocepin, circumventing the potential problem of insufficient prtP-encoded lactocepin expression of the probiotic bacteria in the intestinal tract. On the other hand, appropriate bacterial carriers might be necessary for the protective effect. However, the outcome of these experimental studies will answer the question whether isolated prtP-encoded lactocepin can be considered as a promising new anti-inflammatory agent in the context of IBD (Table 1). If bacterial carriers are found to be necessary for the protective effect, prtP-encoded lactocepin might also be applied via defined bacterial carriers, e.g., Lactococcus lactis, that are approved for the application in humans.24
Table 1. Questions in the context of the newly identified anti-inflammatory activity of lactocepin.
| Summary of open questions | ||||||
|---|---|---|---|---|---|---|
| |
|
|
|
|
|
|
| |
Intestinal prtP-expression? |
|
|
|
||
| |
‒ localization (luminal, mucosal, intestinal compartments) |
|
||||
| |
‒ expression level (health, inflammation, probiotic supplementation) |
|||||
| |
|
|
|
|
|
|
| |
Substrate specificity of prtP? |
|
|
|
||
| |
‒ substrate binding residues, folding |
|
|
|
||
| |
‒ lactobacillus vs. lactococcus prtP |
|
|
|
||
| |
‒ related CEPs (prtB, prtH, prtR, prtS) |
|
|
|
||
| |
|
|
|
|
|
|
| |
Therapeutical relevance of isolated prtP-encoded lactocepin? |
|
||||
| |
‒ oral application in IBD |
|
|
|
|
|
| |
‒ topical application in inflammatory skin diseases |
|
|
|||
| |
‒ injections (i.v., joints, subcutaneous) |
|
|
|||
Substrate Profiles and Expression Levels of prtP-Encoded Lactocepin are Strain-Specific
The newly observed anti-IP-10 activity of L.p and L.c prtP-encoded lactocepin raises questions concerning the substrate specificity of highly similar prtP-encoded lactocepins from other lactobacilli as well as lactococci. In addition, the anti-chemokine activity of less similar CEPs like prtB, prtH, prtR and prtS, which are expressed by other Lactobacillus species like L. delbrueckii, L. rhamnosus and L. helveticus, remain to be elucidated (Table 1).25,26 First analysis in this context revealed that most tested L. casei/L. paracasei strains encode functional IP-10-degrading prtP-encoded lactocepin (Fig. 2) whereas the anti-IP-10 activity of lactococcal prtP-encoded lactocepin seems to be strain specific (unpublished data). The amino acid residues that determine whether a specific prtP-encoded lactocepin degrades IP-10 remain to be determined. Earlier work in the context of casein cleavage already indicated that the catalytic (PR), insertion (I) and A-domain contribute to the substrate specificity of prtP-encoded lactocepin (Fig. 3).26
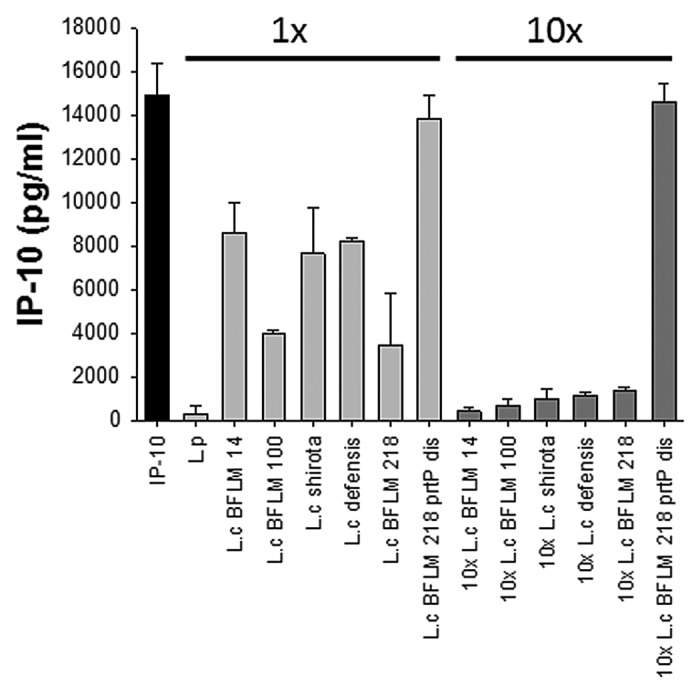
Figure 2. Screening of lactobacilli for anti-IP-10 activity. Human fecal L.casei isolates (L.c BFLM14, L.c BFLM 100, L.c BFLM 218) and commercial strains (L.c shirota, L.c defensis) were screened for their ability to degrade murine IP-10. All tested strains secrete low amounts of IP-10-degrading prtP-encoded lactocepin compared with L.p. The anti-IP-10 activity was strongly increased after cultivation in higher cell densities (1x = 5x107 cfu/ml, 10x = 5x108 cfu/ml). L.c BFLM 218 prtPdis served as negative control.
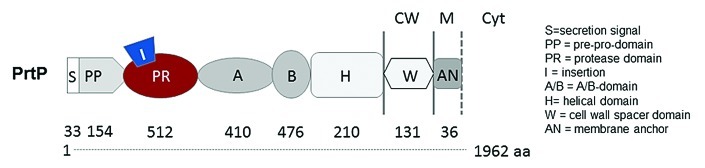
Figure 3. Domain structure of prtP-encoded lactocepin. PrtP-encoded lactocepins from lactobacilli and lactococci are highly similar but show different substrate specificities even within the same species. It has been found that the catalytic domain (PR), the insertion domain (I) and the A domain play a role in the casein specificity of certain prtP-encoded lactocepins. However, it is not known which residues of the active secreted protease (PR to H domain) determine the anti-inflammatory substrate profile of L.p-derived prtP-encoded lactocepin.
Regarding the expression level of prtP-encoded lactocepin, most strains were found to be less active than L.p and higher bacterial numbers are required to produce similar anti-IP-10 activity (Fig. 2). Besides this high intraspecies variability, environmental factors were found to play an important role in the regulation of prtP expression, as e.g., L.p BL23 does not secrete detectable amounts of lactocepin in CM whereas it produces proteolytically active lactocepin in milk.21 The mere presence of prtP does therefore clearly not serve as an indicator for the anti-inflammatory potential of a specific bacterial strain. The enormous complexity of prtP-regulation and substrate specificity is underlined by the bulk of work that has already been published in the field of food technology.27-29 The newly identified anti-inflammatory effect of prtP-encoded lactocepin in IBD raises additional questions concerning the regulation of prtP expression in the intestinal tract (Table 1). Strains of L. casei/L. paracasei are present in the human intestinal microbiota30-32 and it will therefore be interesting to determine intestinal prtP expression levels in health and disease as well as in different intestinal compartments before vs. after the additional administration of bacteria like L.p, that are known to express high levels of prtP-encoded lactocepin in vitro.
Therapeutic Application of prtP-Encoded Lactocepin in Extraintestinal Inflammation
The identification of selective chemokine degradation by prtP-encoded lactocepin allows the assumption that prtP-encoded lactocepin might be protective in an array of chronic inflammatory diseases. The reason for this hypothesis is that the vicious circle of dysregulated high secretion of proinflammatory chemokines that induces increased recruitment and therefore activity of immune effector cells in the tissue, which in turn results in tissue destruction and even more chemokine secretion (Fig. 1), is thought to drive the inflammation in chronic inflammatory diseases like allergic diseases, psoriasis, lupus erythematosus and arthritis. As prtP-encoded lactocepin specifically degrades an array of proinflammatory chemokines that play an important role in these diseases,33-37 the purified bacterial protease might potentially be used as a new anti-inflammatory pharmaceutical agent. PrtP-encoded lactocepin might be applied topically to reduce inflammatory skin diseases or it might even be injected e.g., to reduce arthritis. To date, these possible applications of prtP-encoded lactocepin are merely hypothetical and require extensive studies regarding safety and efficacy in the respective physiological context before they can be considered as a real therapeutic option. It will also be of major importance to determine the accessibility of the respective target tissue as well as the stability of the selective proteolytic activity of prtP-encoded lactocepin under physiological conditions using appropriate model systems. Our proof of concept study in the context of IBD revealed that intraperitoneally injected prtP-encoded lactocepin reduces the infiltration of immune cells into the inflamed ileum,21 underlining the significant anti-inflammatory potential of isolated prtP-encoded lactocepin.
Summary
The characterization of the specific degradation of pro-inflammatory chemokines by prtP-encoded lactocepin as anti-inflammatory probiotic structure-function relationship opens the door toward the development of anti-inflammatory therapies based on prtP-expressing bacteria or isolated prtP-encoding lactocepin in a broad range of chronic inflammatory diseases. The study therefore serves as an example for how the large array of already observed probiotic effects could be used in order to identify protective microbial structures which, in the best of all cases, might be used as new pharmaceutical agents in the future.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/23444
References
- 1.Sanders ME, Akkermans LM, Haller D, Hammerman C, Heimbach J, Hörmannsperger G, et al. Safety assessment of probiotics for human use. Gut Microbes. 2010;1:164–85. doi: 10.4161/gmic.1.3.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lebeer S, Vanderleyden J, De Keersmaecker SC. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol. 2010;8:171–84. doi: 10.1038/nrmicro2297. [DOI] [PubMed] [Google Scholar]
- 3.Jijon H, Backer J, Diaz H, Yeung H, Thiel D, McKaigney C, et al. DNA from probiotic bacteria modulates murine and human epithelial and immune function. Gastroenterology. 2004;126:1358–73. doi: 10.1053/j.gastro.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Corr SC, Li Y, Riedel CU, O’Toole PW, Hill C, Gahan CG. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc Natl Acad Sci U S A. 2007;104:7617–21. doi: 10.1073/pnas.0700440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–7. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 6.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–5. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 7.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107:12204–9. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen Y, Torchia ML, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe. 2012;12:509–20. doi: 10.1016/j.chom.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem. 2002;277:50959–65. doi: 10.1074/jbc.M207050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562–75. doi: 10.1053/j.gastro.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan F, Cao H, Cover TL, Washington MK, Shi Y, Liu L, et al. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J Clin Invest. 2011;121:2242–53. doi: 10.1172/JCI44031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holubar SD, Cima RR, Sandborn WJ, Pardi DS. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis. Cochrane Database Syst Rev. 2010:CD001176. doi: 10.1002/14651858.CD001176.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Sood A, Midha V, Makharia GK, Ahuja V, Singal D, Goswami P, et al. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7:1202–9, 1209, e1. doi: 10.1016/j.cgh.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, Giorgetti GM, et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2010;105:2218–27. doi: 10.1038/ajg.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoermannsperger G, Clavel T, Hoffmann M, Reiff C, Kelly D, Loh G, et al. Post-translational inhibition of IP-10 secretion in IEC by probiotic bacteria: impact on chronic inflammation. PLoS One. 2009;4:e4365. doi: 10.1371/journal.pone.0004365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostvik AE, Vb Granlund A, Bugge M, Nilsen NJ, Torp SH, Waldum HL, et al. Enhanced expression of CXCL10 in inflammatory bowel disease: Potential role of mucosal toll-like receptor 3 stimulation. Inflamm Bowel Dis. 2012 doi: 10.1002/ibd.23034. [DOI] [PubMed] [Google Scholar]
- 17.Schroepf S, Kappler R, Brand S, Prell C, Lohse P, Glas J, et al. Strong overexpression of CXCR3 axis components in childhood inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1882–90. doi: 10.1002/ibd.21312. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki S, Yoneyama H, Suzuki K, Suriki H, Aiba T, Watanabe S, et al. Blockade of CXCL10 protects mice from acute colitis and enhances crypt cell survival. Eur J Immunol. 2002;32:3197–205. doi: 10.1002/1521-4141(200211)32:11<3197::AID-IMMU3197>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 19.Hyun JG, Lee G, Brown JB, Grimm GR, Tang Y, Mittal N, et al. Anti-interferon-inducible chemokine, CXCL10, reduces colitis by impairing T helper-1 induction and recruitment in mice. Inflamm Bowel Dis. 2005;11:799–805. doi: 10.1097/01.MIB.0000178263.34099.89. [DOI] [PubMed] [Google Scholar]
- 20.Singh UP, Singh S, Singh R, Cong Y, Taub DD, Lillard JW., Jr. CXCL10-producing mucosal CD4+ T cells, NK cells, and NKT cells are associated with chronic colitis in IL-10(-/-) mice, which can be abrogated by anti-CXCL10 antibody inhibition. J Interferon Cytokine Res. 2008;28:31–43. doi: 10.1089/jir.2007.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Schillde MA, Hörmannsperger G, Weiher M, Alpert CA, Hahne H, Bäuerl C, et al. Lactocepin secreted by Lactobacillus exerts anti-inflammatory effects by selectively degrading proinflammatory chemokines. Cell Host Microbe. 2012;11:387–96. doi: 10.1016/j.chom.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Vandenbroucke K, de Haard H, Beirnaert E, Dreier T, Lauwereys M, Huyck L, et al. Orally administered L. lactis secreting an anti-TNF Nanobody demonstrate efficacy in chronic colitis. Mucosal Immunol. 2010;3:49–56. doi: 10.1038/mi.2009.116. [DOI] [PubMed] [Google Scholar]
- 23.Treuting PM, Dintzis SM. Lower gastrointestinal tract, In: Comparative Anatomy and Histology: A Mouse and Human Atlas. London: Academic Press, 2012. [Google Scholar]
- 24.Van Huynegem K, Steidler L. Clinical development of lactocepin: a novel bacterial biologic? Expert Rev Clin Immunol. 2012;8:597–9. doi: 10.1586/eci.12.55. [DOI] [PubMed] [Google Scholar]
- 25.Siezen RJ. Multi-domain, cell-envelope proteinases of lactic acid bacteria. Antonie Van Leeuwenhoek. 1999;76:139–55. doi: 10.1023/A:1002036906922. [DOI] [PubMed] [Google Scholar]
- 26.Sadat-Mekmene L, Genay M, Atlan D, Lortal S, Gagnaire V. Original features of cell-envelope proteinases of Lactobacillus helveticus. A review. Int J Food Microbiol. 2011;146:1–13. doi: 10.1016/j.ijfoodmicro.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 27.Broadbent JR, Barnes M, Brennand C, Strickland M, Houck K, Johnson ME, et al. Contribution of Lactococcus lactis cell envelope proteinase specificity to peptide accumulation and bitterness in reduced-fat Cheddar cheese. Appl Environ Microbiol. 2002;68:1778–85. doi: 10.1128/AEM.68.4.1778-1785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marugg JD, van Kranenburg R, Laverman P, Rutten GA, de Vos WM. Identical transcriptional control of the divergently transcribed prtP and prtM genes that are required for proteinase production in lactococcus lactis SK11. J Bacteriol. 1996;178:1525–31. doi: 10.1128/jb.178.6.1525-1531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikolić M, Tolinacki M, Fira D, Golić N, Topisirović L. Variation in specificity of the PrtP extracellular proteinases in Lactococcus lactis and Lactobacillus paracasei subsp. paracasei. Folia Microbiol (Praha) 2009;54:188–94. doi: 10.1007/s12223-009-0029-2. [DOI] [PubMed] [Google Scholar]
- 30.Ahrné S, Lönnermark E, Wold AE, Aberg N, Hesselmar B, Saalman R, et al. Lactobacilli in the intestinal microbiota of Swedish infants. Microbes Infect. 2005;7:1256–62. doi: 10.1016/j.micinf.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Štšepetova J, Sepp E, Kolk H, Lõivukene K, Songisepp E, Mikelsaar M. Diversity and metabolic impact of intestinal Lactobacillus species in healthy adults and the elderly. Br J Nutr. 2011;105:1235–44. doi: 10.1017/S0007114510004770. [DOI] [PubMed] [Google Scholar]
- 32.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. MetaHIT Consortium A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee EY, Lee ZH, Song YW. The interaction between CXCL10 and cytokines in chronic inflammatory arthritis. Autoimmun Rev. 2012 doi: 10.1016/j.autrev.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Flier J, Boorsma DM, van Beek PJ, Nieboer C, Stoof TJ, Willemze R, et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398–405. doi: 10.1002/1096-9896(200108)194:4<397::AID-PATH899>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 35.Chen SC, de Groot M, Kinsley D, Laverty M, McClanahan T, Arreaza M, et al. Expression of chemokine receptor CXCR3 by lymphocytes and plasmacytoid dendritic cells in human psoriatic lesions. Arch Dermatol Res. 2010;302:113–23. doi: 10.1007/s00403-009-0966-2. [DOI] [PubMed] [Google Scholar]
- 36.Lacotte S, Brun S, Muller S, Dumortier H. CXCR3, inflammation, and autoimmune diseases. Ann N Y Acad Sci. 2009;1173:310–7. doi: 10.1111/j.1749-6632.2009.04813.x. [DOI] [PubMed] [Google Scholar]
- 37.Lee EY, Lee ZH, Song YW. CXCL10 and autoimmune diseases. Autoimmun Rev. 2009;8:379–83. doi: 10.1016/j.autrev.2008.12.002. [DOI] [PubMed] [Google Scholar]


