Abstract
There is convincing evidence from recent human and animal studies that suggests the intestinal microbiota plays an important role in regulating immune responses associated with the development of allergic asthma, particularly during early infancy. Although identifying the mechanistic link between host-microbe interactions in the gut and lung mucosal tissues has proved challenging, several very recent studies are now providing significant insights. We have shown that administering vancomycin to mice early in life shifts resident gut flora and enhances future susceptibility to allergic asthma. This effect was not observed in mice given another antibiotic, streptomycin, nor when either antibiotic was administered to adult mice. In this addendum, we further analyze the link between early life administration of vancomycin and future susceptibility to asthma and describe how specific immune cell populations, which have been implicated in other asthma-related microbiota studies, are affected. We propose that shifts in gut microbiota exacerbate asthma-related immune responses when they occur shortly after birth and before weaning (perinatal period), and suggest that these effects may be mediated, at least in the case of vancomycin, by elevated serum IgE and reduced regulatory T cell populations.
Keywords: antibiotics, asthma, gut, microbiota, early life, perinatal programming, immune mechanisms
Intestinal Microbiota Impact Allergic Asthma
Allergic asthma rates have been steadily increasing in developed countries around the world.1 The disparity between asthma rates in developing countries and industrialized nations highlights the importance of environmental factors in this disease. Recent epidemiological data emphasize the significance of microbial exposures early in childhood and identify mode of birth delivery,2 farm habitation3 and antibiotic use4,5 as mitigating factors conferring protection or increasing risk of asthma later in life. These studies underscore the importance of the indigenous microbiota, and suggest that failure to establish a healthy gut flora early in life alters maturation of the immune system, promoting altered allergic responses in adulthood.6,7
Recent human studies have documented differences in the gut flora of allergic and non-allergic infants,8 and suggest that pre- and postnatal antibiotic exposures can increase the risk of allergic asthma4,5. Likewise, in a murine model of allergic airways disease, germ-free mice exhibit more severe disease than conventionally housed controls, an effect that could be ameliorated by re-colonization with conventional flora.9 A similar phenotype has been observed in mice receiving antibiotics in their drinking water10 or an antibiotic/fungal microbiota combination.11 Antibiotics create global changes in microbial communities, a characteristic which has made them incredibly useful as tools for studying how shifts in the microbiota can affect different disease states.12 We recently showed that antibiotics alter the outcome of allergic asthma when administered at clinically relevant doses; vancomycin causes an exacerbated allergic response, whereas streptomycin does not suggesting that microbial composition, and not simply bacterial numbers, can influence allergic sensitization.13
Microbiota Composition during the Perinatal Period Affects Allergic Responses
There is now substantial evidence in human and animal studies that gut colonization early in life plays an important role in guiding immune development and maintaining mucosal homeostasis throughout life.14 It has been hypothesized that there may be a “critical window” of time during which a developmental program is initiated, and if this program is disrupted by an altered pattern of microbial colonization (as a result of antibiotics, diet, mode and time of birth delivery or other environmental factors) the host may be predisposed to a number of chronic diseases initiated by aberrant mucosal immune responses.15
The importance of establishing healthy gut flora during the perinatal period has been confirmed repeatedly in a number of murine models of allergic asthma. Olszak et al.16 demonstrated that colonization of neonatal, but not adult germ-free mice, reduced the severity of lung inflammation typically observed in germ-free mice. This attenuation coincided with decreased invariant natural killer T (iNKT) cell accumulation in gut and lung mucosa, suggesting that colonization early in life establishes mucosal iNKT cell tolerance that can impact immune responses at mucosal sites into adulthood. Similarly, our lab has shown that early life exposure to vancomycin enhances the severity of allergic asthma in mice, while the same antibiotic given during adulthood does not.13 The antibiotic treatment regime compared the effects of continuous, lifelong vancomycin administration (which we termed “neonatal exposure”) to adult administration (from 7 weeks onward, which we termed “adult exposure”). Because our initial “neonatal” antibiotic regimen spanned several critical time points (prenatal, perinatal, after weaning), we have now further narrowed the window of antibiotic administration to better pinpoint the discrete time points that most significantly affect disease outcome.
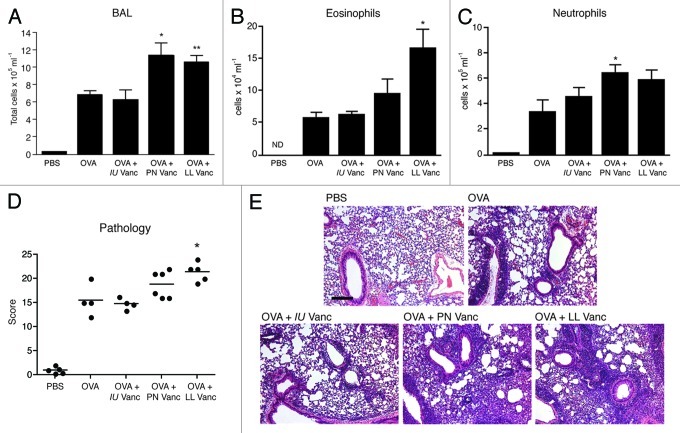
To determine whether prenatal or perinatal vancomycin treatment results in exacerbated asthma to the same extent as lifelong vancomycin treatment in our previous study, we repeated asthma induction as before, but this time included a cohort of mice treated exclusively prenatally (throughout the 21 d of in utero development) or pre- and perinatally (in utero and through the first three weeks of postnatal life up until weaning). Similar to our previous study with lifelong administration of vancomycin, ovalbumin (OVA)-challenge of mice treated perinatally with vancomycin led to increased total inflammatory cell infiltrates in the bronchoalveolar lavage (BAL) relative to the OVA-challenged controls (Fig. 1A). Animals treated with vancomycin strictly prenatally (in utero) exhibited comparable disease to the controls. Although perinatal vancomycin treatment only moderately increased eosinophil numbers in the BAL compared with controls (Fig. 1B) the bulk of the increase in BAL counts we observed appeared to be due to an increase in neutrophil infiltrates (Fig. 1C). Neutrophilia is typically observed in more severe, chronic forms of asthma.17 Pathology scores for perinatally treated mice were intermediate between OVA-challenged controls and lifelong vancomycin-treated animals (Fig. 1D). Together, these data suggest that the “critical window” for vancomycin-driven shifts in microbiota to alter immune homeostatic mechanisms to enhance future asthma susceptibility occurs between birth and 3 weeks of age.
Figure 1. Vancomycin treatment in early life but not exclusively in utero exacerbates allergic asthma. (A) Total cellular infiltrates from bronchoalveolar lavage (BAL) of control or vancomycin-treated mice challenged with ovalbumin (OVA) or PBS. (B) Eosinophil and (C) neutrophil numbers in the BAL quantified by cytospin. (D) Total pathological scores and representative hematoxylin and eosin stained lung sections. Scale bar, 300 μm. All assessments were made on day 26. The data are shown as means of 4−6 mice per group ± SEM. Statistics shown are based on comparisons to OVA-challenged controls. IU, in utero; PN, perinatal; LL, lifelong; Vanc, vancomycin; BALF, bronchoalveolar lavage fluid. *p < 0.05, **p < 0.01, n.d. = none detected.
Mechanisms Linking Gut Microbes and Lung Inflammation: The Gut-Lung Axis
Our study supports the existence of a common mucosal immune system, where mucosal tissues like the gut and lung function as a system-wide organ,18 linked by immunological mechanisms that remain largely undefined. In elegant studies, Hill et al.10 have shown that mice treated with a cocktail of antibiotics have elevated IgE levels and higher numbers of circulating basophils in their blood compared with conventionally reared mice. These mice develop more severe airway inflammation due to increased basophil-mediated Th2 responses. Mechanistically, their data suggest that intestinal bacteria downregulate the level of IgE production by B cells through a MyD88-dependent pathway. In the absence of either these bacteria or a functional MyD88 signaling cascade in B cells, IgE production increases and is captured by the FcεRI on basophil precursors in the bone marrow, leading to the upregulation of IL-3 receptor subunit, CD123, which then drives basophilopoiesis.
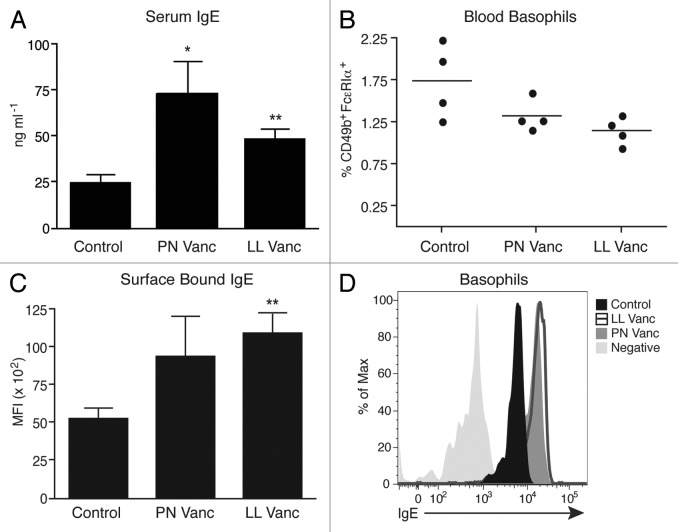
To determine whether a similar mechanism drives vancomycin-induced exacerbated asthma, we quantified serum IgE concentrations in control, perinatal vancomycin or lifelong vancomycin-treated animals under steady-state conditions. Serum IgE levels were significantly elevated in mice that had been treated perinatally or with lifelong vancomycin relative to untreated controls (Fig. 2A). Next, we quantified the number of circulating basophils in control, perinatal and lifelong vancomycin-treated animals. Although antibiotic treatment did not alter the frequency of blood basophils (identified as non-B, non-T, CD117- CD49b+ FcεRIα+, Fig. 2B), basophils isolated from mice treated with lifelong vancomycin did exhibit increased levels of surface-bound IgE, and there was a similar increasing trend in the perinatally treated mice compared with control animals (Fig. 2C and D). These data suggest that basophil-bound IgE may indeed be driving exacerbated airway inflammation in these vancomycin-treated animals.
Figure 2. Vancomycin treatment elevates steady-state serum IgE levels and enhances expression of surface-bound IgE on circulating basophils. (A) Serum IgE from naïve control or vancomycin-treated mice, as measured by ELISA. (B) Flow cytometric analysis of blood basophils from naïve control or vancomycin-treated mice. Basophils identified as CD3-CD4-CD8-B220-CD117-FcεRIα+CD49b+ cells. (C) Surface-bound IgE levels on blood basophils from untreated control or vancomycin-treated mice, as determined by flow cytometry. (D) Representative histogram of surface-bound IgE on basophils from control and vancomycin-treated mice, as determined by flow cytometry. The data are shown as means of 4–5 mice per group ± SEM. Statistics shown are based on comparisons to untreated controls. PN, perinatal; LL, lifelong; Vanc, vancomycin. *p < 0.05, **p < 0.01.
In another study, germ-free mice have been used to demonstrate that intestinal bacteria play a role in the epigenetic regulation of immune cells involved in allergic asthma. Olszak et al.16 have shown that early microbial colonization reduces methylation of the gene encoding chemokine ligand 16 (CXCL16), a chemokine required for iNKT cell homing to mucosal tissues. In the absence of colonization, Cxcl16 is hypermethylated in mucosal tissues, leading to the accumulation of iNKT cell populations at mucosal sites and increasing host susceptibility to allergic inflammation.16
To determine whether CXCL16, and thus iNKT cells, play a role in the enhanced disease susceptibility we observe in vancomycin-treated mice, we quantified Cxcl16 expression levels in control, perinatal and lifelong vancomycin-treated mice. Interestingly, we found no differences in colon or lung Cxcl16 levels in the antibiotic treated mice relative to the control animals (Fig. 3A and B). In fact, Cxcl16 expression tended to decrease after vancomycin treatment. Thus, the mechanism involved in vancomycin-induced exacerbated asthma does not appear to function through CXCL16 and iNKT cell-dependent mechanisms observed by others in germ free mice.
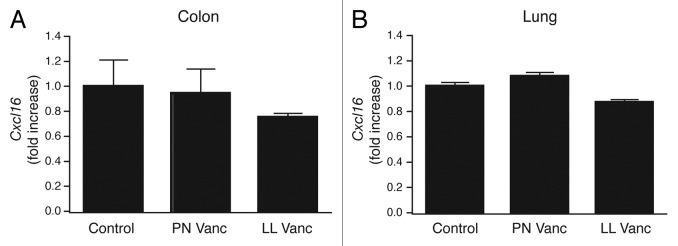
Figure 3. Vancomycin treatment has no effect on Cxcl16 transcript levels. (A) Cxcl16 expression in colon and (B) lung tissue samples harvested from age matched naïve control and vancomycin-treated mice. The data are shown as means of 4−5 mice per group ± SEM. Statistics shown are based on comparisons to untreated controls. PN, perinatal; LL, lifelong; Vanc, vancomycin.
Regulatory T cells (Tregs) may also be involved in mediating crosstalk at mucosal sites. Induction of immunological tolerance is essential for the control of allergic asthma.19 Previously, we found colonic Tregs to be significantly reduced in mice treated with lifelong vancomycin but not streptomycin,13 suggesting that tolerance mechanisms may be disrupted when specific microbial populations in the gut have been lost or altered. It has recently been suggested that specific members of the microbiota, such as Clostridium species, may be responsible for the induction of Tregs in mice.20 In the absence of these bacteria IgE levels in the blood increased,20 and when members of Clostridia were added back, a reduction in allergic airway inflammation was observed.21 Studies in humans reveal that atopic children have reduced Treg expression profiles22 and mothers with lower cord blood Treg populations have elevated IgE levels.23 These data suggest that there may be a link between IgE levels and Treg populations in the blood, however the mechanism has not yet been clearly defined.
To determine whether Treg numbers could be influenced by antibiotic treatment specifically during the perinatal period, we investigated whether mice treated perinatally with vancomycin had normal CD25+Foxp3+ Treg populations. Interestingly, mice that received perinatal vancomycin had reduced Treg populations relative to control mice, much like those observed after lifelong vancomycin treatment (Fig. 4). Thus, our data support the notion that Tregs may be responsible for restraining aberrant allergic-type (Th2) inflammation in mucosal tissues. These data support the model proposed previously by Josefowicz et al.,24 who showed that mice deficient in Tregs (specifically inducible Tregs) spontaneously develop Th2-type pathologies at gut and lung mucosal sites.
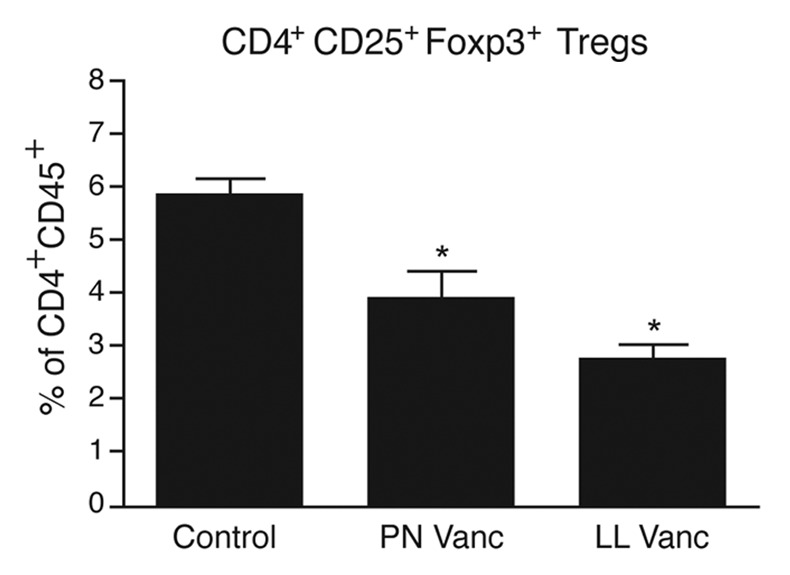
Figure 4. Perinatal vancomycin treatment reduces Treg accumulation in the colon. At 7 weeks of age, the percentage of CD25+Foxp3+ cells within the CD45+CD4+ cell population in the colon of naïve mice left untreated or treated with vancomycin was analyzed. The data shown are means of four mice per group ± SEM. Statistics shown are based on comparisons to untreated controls. PN, perinatal; LL, lifelong; Vanc, vancomycin. *p < 0.05.
To avoid chronic inflammatory disorders like asthma, the host requires a system of tightly regulated homeostatic mechanisms to maintain optimal balance between tolerogenic and Th2-type inflammatory responses. Recently published data suggests that if this balance is disrupted early in life by environmental insults and shifting microbial populations, aberrant immune responses to innocuous antigens run rampant, initiating cascades of allergy-related pathologies. It has recently been established that mucosal B cells residing in gut-associated lymphoid tissue can control Treg suppression of Th2 cell responses. Tregs induced by these B cells were capable of alleviating symptoms of allergic asthma.25 Perhaps, in the presence of a healthy microbiota, mucosal B cells promote Treg induction in a MyD88-dependent manner. Correspondingly, in the presence of an altered or absent microbiota, B cell-intrinsic programming is altered, resulting in excessive secretion of IgE and uncontrolled production of allergic mediators like basophils and Th2 inflammatory responses at the site of allergen exposure.
Alternatively, rather than changing the abundance of particular immune cell lineages, antibiotic treatment could change the phenotypic behavior of immune cell populations. There is evidence that Treg cells have phenotypic plasticity in different inflammatory environments. Respiratory syncytial virus (RSV) infection early in life has been shown to promote an inflammatory milieu in the lung, which caused local Tregs to lose their suppressive capacity, rendering the host more susceptible to allergic asthma later in life.26 It will be interesting to investigate whether Tregs in the gut or lung of mice treated with vancomycin express Th2-type inflammatory markers like GATA3 or IL-13 as demonstrated by Krishnamoorthy et al. in the RSV infection model.
What About the Lung Microbiota?
While many asthma studies have focused on contributions made by intestinal bacteria, there is new evidence suggesting that the airway microbiome may also play a role in allergic asthma.27,28 This warrants investigating whether the inflammation associated with vancomycin treatment could be the result of a change in the local lung environment rather than in the gut. Naik et al.29 suggest that in the context of skin, there is little influence from mucosal tissues like the gut. They show that while oral vancomycin significantly altered gut flora, it had no effect on the skin microbiota or cutaneous immune homeostasis.29 Vancomycin is poorly absorbed when given orally,30 which is one of the reasons why we chose to use it in our studies, so the fact that it had no effect on the skin microbiota is not surprising. This is relevant to our work because it suggests that (1) vancomycin has no effect on the skin, and this may also be true of airway microbiota, and (2) perhaps skin is a more immunologically distinct organ and is less influenced by external signals from the gut than the lung, which could be more closely linked by common inter-mucosal traffic. Regardless, knowing that skin immunity is shaped by resident bacteria in the local environment and not directly influenced gut microbes could direct our research in new ways, perhaps shifting the focus toward the immunological impact of microbes residing in the airways.
Until recently, the lung was believed to be a sterile environment, devoid of bacteria. With more sensitive sequencing methods becoming the norm, sparsely populated communities like those in the lung are easier to study. To investigate whether we could isolate any airway microbiota, we analyzed cell-free supernatants from murine BAL fluid. Despite our best efforts, we were unable to consistently amplify bacterial DNA from any of the treatment groups, thus we could not detect any vancomycin-induced effects on the lung microbiota. We also attempted to culture airway bacteria by plating BAL as well as lung homogenates incubated both aerobically and anaerobically, but obtained no growth in either condition. Perhaps alterations to our media could be made to better support growth of lung microbiota. We may need to further adapt our PCR protocol in order to detect a less abundant community of microbes; however, at face value, the small amount of bacteria detected in the lung begs the question of whether true colonization occurs in the healthy mouse lung.
Existing Challenges and New Insights
Despite the many challenges presented by the complex etiology of asthma, significant progress has been made in recent years to understand the environmental factors involved in disease development, with particular emphasis on the role of intestinal bacteria. We are beginning to understand the importance of colonization early in life and that altering microbial exposures during the perinatal period may promote dysregulated immune responses for a lifetime. It has been hypothesized that mucosal tissues like the gut and lung may function as a system-wide organ; however, we are only now beginning to understand the complex cellular and molecular mechanisms that mediate these interactions.
Summary
In this study, we used the antibiotic vancomycin as a tool to induce defined shifts in the intestinal microbiota and model an altered colonization state in early life. We have demonstrated a microbiota-driven, specific increase in susceptibility to experimental murine allergic asthma and have provided data that suggests these effects are mediated by increases in IgE as well as decreases in a regulatory T cell subset. Although the mechanisms involved in vancomycin-mediated exacerbation of murine allergic asthma remain to be directly elucidated, the data presented in this addendum provide new insights and offer avenues for future work. This new knowledge has extended our understanding of asthma development and could provide the framework for novel therapeutic or preventative approaches in the future.
Methods
Mice
C57BL/6J mice (Jackson Laboratories) were bred and maintained in a specific pathogen-free facility at The Biomedical Research Center. All experiments were in accordance with the UBC Animal Care Committee guidelines.
Antibiotic treatment
C57BL/6J breeding pairs were given vancomycin (Sigma-Aldrich) at 200 mg/liter in drinking water. In utero vancomycin-treated mice: breeding pairs were administered antibiotic-treated water for the duration of the pregnancy. When the pups were born, antibiotic-treated water was removed and replaced with regular water. Perinatal vancomycin-treated mice: breeding pairs were administered antibiotic-treated water for the duration of the pregnancy, and pups born from respective breeding pairs were reared on antibiotic-treated water until they were weaned (three weeks old). Once weaned, the pups were administered regular water. Lifelong vancomycin-treated mice: pups born from respective breeding pairs were reared on antibiotic-treated water with their littermates for the duration of the experiment.
Ovalbumin model of allergic asthma
Asthma was induced as previously described.13
Histology
Lungs were collected, sectioned, stained and scored as previously described.13
Determination of serum IgE
Total IgE in serum was measured by ELISA (BD Biosciences).
Isolation of immune cells and flow cytometry
Basophils were isolated from peripheral blood of 7-week-old naïve mice. Cells were stained with fluorochrome-conjugated antibodies against CD3, CD4, CD8, B220, CD117, FcεRIα and CD49b (BD Biosciences). Flow cytometry was performed using an LSR II (BD Biosciences) and data were analyzed with FlowJo 8.7 software (TreeStar). Colon cells were isolated and analyzed by flow cytometry as previously described.13
Quantification of Cxcl16
RNA samples were prepared using an RNeasy Mini Kit and cDNA was synthesized using the Quantitect RT Kit (both Qiagen). Real-time RT-PCR was performed using a SYBR Green I Master Mix (Roche) and a 7500 Fast Real-Time System (Applied Biosystems). Values were normalized to the expression of GAPDH for each sample. We used the same Cxcl16 primer sets as Olszak et al.16
Statistics
Differences between control and experimental groups were compared using Kruskal-Wallis one-way analysis of variance (ANOVA) to calculate statistical significance (GraphPad Prism software).
Acknowledgments
We thank members of the Biomedical Research Center Animal Facility for their expert animal care. K.M.M. is a Michael Smith Foundation for Health Research Senior Scholar. This work was funded by a Canadian Institutes of Health Research (CIHR) Catalyst Grant and a CIHR Emerging Team Grant in partnership with Genome BC and the AllerGen National Center for Excellence.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/23567
References
- 1.Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat Rev Immunol. 2001;1:69–75. doi: 10.1038/35095579. [DOI] [PubMed] [Google Scholar]
- 2.Bager P, Melbye M, Rostgaard K, Benn CS, Westergaard T. Mode of delivery and risk of allergic rhinitis and asthma. J Allergy Clin Immunol. 2003;111:51–6. doi: 10.1067/mai.2003.34. [DOI] [PubMed] [Google Scholar]
- 3.Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrländer C, et al. GABRIELA Transregio 22 Study Group Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364:701–9. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 4.Marra F, Marra CA, Richardson K, Lynd LD, Kozyrskyj A, Patrick DM, et al. Antibiotic use in children is associated with increased risk of asthma. Pediatrics. 2009;123:1003–10. doi: 10.1542/peds.2008-1146. [DOI] [PubMed] [Google Scholar]
- 5.Murk W, Risnes KR, Bracken MB. Prenatal or early-life exposure to antibiotics and risk of childhood asthma: a systematic review. Pediatrics. 2011;127:1125–38. doi: 10.1542/peds.2010-2092. [DOI] [PubMed] [Google Scholar]
- 6.Noverr MC, Huffnagle GB. The ‘microflora hypothesis’ of allergic diseases. Clin Exp Allergy. 2005;35:1511–20. doi: 10.1111/j.1365-2222.2005.02379.x. [DOI] [PubMed] [Google Scholar]
- 7.Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol. 2009;7:887–94. doi: 10.1038/nrmicro2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Nimwegen FA, Penders J, Stobberingh EE, Postma DS, Koppelman GH, Kerkhof M, et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J Allergy Clin Immunol. 2011;128:948–55, e1-3. doi: 10.1016/j.jaci.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 9.Herbst T, Sichelstiel A, Schär C, Yadava K, Bürki K, Cahenzli J, et al. Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am J Respir Crit Care Med. 2011;184:198–205. doi: 10.1164/rccm.201010-1574OC. [DOI] [PubMed] [Google Scholar]
- 10.Hill DA, Siracusa MC, Abt MC, Kim BS, Kobuley D, Kubo M, et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nat Med. 2012;18:538–46. doi: 10.1038/nm.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noverr MC, Noggle RM, Toews GB, Huffnagle GB. Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect Immun. 2004;72:4996–5003. doi: 10.1128/IAI.72.9.4996-5003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willing BP, Russell SL, Finlay BB. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat Rev Microbiol. 2011;9:233–43. doi: 10.1038/nrmicro2536. [DOI] [PubMed] [Google Scholar]
- 13.Russell SL, Gold MJ, Hartmann M, Willing BP, Thorson L, Wlodarska M, et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 2012;13:440–7. doi: 10.1038/embor.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renz H, Brandtzaeg P, Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat Rev Immunol. 2012;12:9–23. doi: 10.1038/nri3112. [DOI] [PubMed] [Google Scholar]
- 15.Penders J, Stobberingh EE, van den Brandt PA, Thijs C. The role of the intestinal microbiota in the development of atopic disorders. Allergy. 2007;62:1223–36. doi: 10.1111/j.1398-9995.2007.01462.x. [DOI] [PubMed] [Google Scholar]
- 16.Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–93. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fahy JV. Eosinophilic and neutrophilic inflammation in asthma: insights from clinical studies. Proc Am Thorac Soc. 2009;6:256–9. doi: 10.1513/pats.200808-087RM. [DOI] [PubMed] [Google Scholar]
- 18.Gill N, Wlodarska M, Finlay BB. The future of mucosal immunology: studying an integrated system-wide organ. Nat Immunol. 2010;11:558–60. doi: 10.1038/ni0710-558. [DOI] [PubMed] [Google Scholar]
- 19.Herrick CA, Bottomly K. To respond or not to respond: T cells in allergic asthma. Nat Rev Immunol. 2003;3:405–12. doi: 10.1038/nri1084. [DOI] [PubMed] [Google Scholar]
- 20.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–41. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li YN, Huang F, Liu L, Qiao HM, Li Y, Cheng HJ. Effect of oral feeding with Clostridium leptum on regulatory T-cell responses and allergic airway inflammation in mice. Ann Allergy Asthma Immunol. 2012;109:201–7. doi: 10.1016/j.anai.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Savilahti EM, Karinen S, Salo HM, Klemetti P, Saarinen KM, Klemola T, et al. Combined T regulatory cell and Th2 expression profile identifies children with cow’s milk allergy. Clin Immunol. 2010;136:16–20. doi: 10.1016/j.clim.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Hinz D, Simon JC, Maier-Simon C, Milkova L, Röder S, Sack U, et al. Reduced maternal regulatory T cell numbers and increased T helper type 2 cytokine production are associated with elevated levels of immunoglobulin E in cord blood. Clin Exp Allergy. 2010;40:419–26. doi: 10.1111/j.1365-2222.2009.03434.x. [DOI] [PubMed] [Google Scholar]
- 24.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–9. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu KH, Chiang BL. Regulatory T cells induced by mucosal B cells alleviate allergic airway hypersensitivity. Am J Respir Cell Mol Biol. 2012;46:651–9. doi: 10.1165/rcmb.2011-0246OC. [DOI] [PubMed] [Google Scholar]
- 26.Krishnamoorthy N, Khare A, Oriss TB, Raundhal M, Morse C, Yarlagadda M, et al. Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma. Nat Med. 2012;18:1525–30. doi: 10.1038/nm.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang YJ, Nelson CE, Brodie EL, Desantis TZ, Baek MS, Liu J, et al. National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372–81, e1-3. doi: 10.1016/j.jaci.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, et al. Compartmentalized control of skin immunity by resident commensals. Science. 2012;337:1115–9. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armstrong CJ, Wilson TS. Systemic absorption of vancomycin. J Clin Pathol. 1995;48:689. doi: 10.1136/jcp.48.7.689-b. [DOI] [PMC free article] [PubMed] [Google Scholar]