Abstract
In addition to their recognized roles in intra- and inter-species signaling, bacterial quorum-sensing molecules have been implicated in inter-kingdom signaling. A new study in Pseudomonas aeruginosa suggests that mammalian bitter taste receptors may recognize bacterial quorum sensing molecules, and widens the scope of such inter-kingdom communication. Intestinal cells also harbor these receptors, but whether they eavesdrop on bacterial conversations remains an open question.
Keywords: quorum sensing, Acyl homoserine lactone, taste receptor, bitter, T2R38
The field of taste perception has made significant advances in the past 15 y. Sweet, umami and bitter taste recognition occurs when corresponding molecules engage specific homo- or hetero-dimeric G-protein coupled receptors (T1Rs or T2Rs), followed by activation of the heterotrimeric G-protein subunit α-gustducin (Gαi) (Salty and sour tastes are recognized by a different system).1 While sweet and umami tastes each have a single receptor, humans harbor 25 receptors (mice have 35) for sensing bitter compounds, consistent with the notion that the ability to taste diverse bitter compounds prevents the consumption of potentially toxic substances. T1R and T2R receptors, however, are widely distributed in the body, and have been implicated in various non-gustatory roles.2
The bitter taste sensor T2R38 is a receptor for the molecule phenylthiocarbamide (PTC), and genetic polymorphisms in the corresponding chromosomal locus segregates the population into "tasters" and “non-tasters.”3 Tasters have a functional allele that includes a proline-valine-alanine (PAV) sequence, while the corresponding sequence in non-tasters is alanine-valine-isoleucine (AVI). A new study implicates T2R38 in a non-gustatory role, that of sensing bacterial quorum-sensing (QS) molecules and stimulating innate immune responses (Fig. 1).
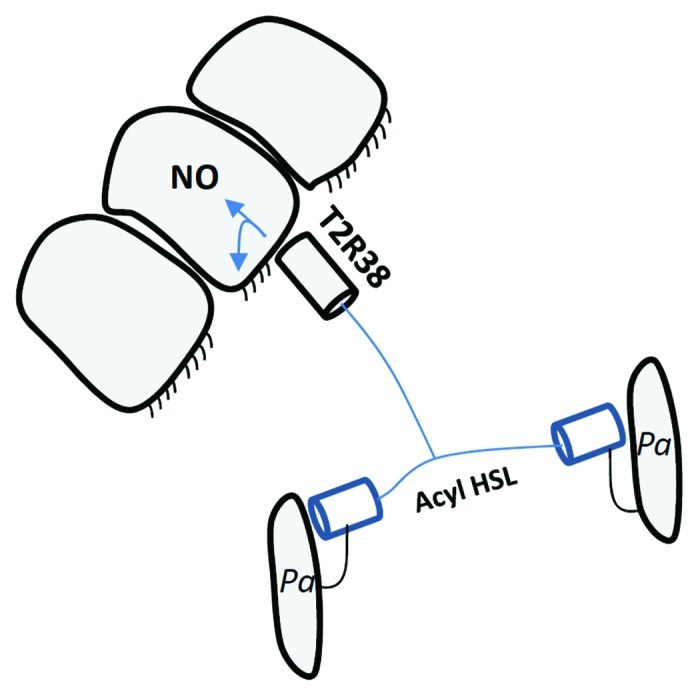
Figure 1. Epithelial cells co-opt the T2R38 receptor to eavesdrop on P. aeruginosa conversations. Consequent innate immune responses help clear the bacteria.
Bacteria use QS systems to coordinate gene expression in a population-dependent manner.4 At threshold population densities, small molecules produced by the bacteria (“autoinducers”) interact with specific proteins and activate signaling pathways that eventually alter bacterial gene expression. Pseudomonas aeruginosa produces two quorum-sensing molecules, C12HSL and C4HSL, that belong to the broad class of autoinducers known as acylated homoserine lactones (HSLs).
In this new study,3 Lee et al. confirmed T2R38 receptor expression in the apical membrane and cilia of upper airway sino-nasal respiratory epithelial cells from surgical explants, and demonstrated PTC- and P. aeruginosa acyl-HSL-dependent calcium signaling, but only in cells harboring the functional receptor (PAV/PAV). Elevated calcium levels were also observed in response to conditioned P. aeruginosa culture medium. T2R38 signaling was dependent on PLCβ2 and TRPM5 (as in gustation responses), but was independent of TLR signaling. Acyl HSL-stimulation of T2R38 resulted in nitric oxide (NO) production and increased ciliary beat frequency, processes that play a key role in mucociliary clearance of pathogens. Both responses were abrogated by inhibitors of PLCβ2 and nitric oxide synthase. Significantly, P. aeruginosa exposed to PAV/PAV cell cultures, but not to PAV/AVI or AVI/AVI cell cultures, were killed in high numbers in a NO-dependent fashion.
To determine the relevance of T2R38-dependent innate immune responses in clinical settings, the investigators examined the correlation between patients’ T2R38 alleles and the corresponding microbiological reports for bacterial growth, including P. aeruginosa. Gram-negative bacteria, including P. aeruginosa, were present in sino-nasal swabs from patients harboring the non-functional T2R38 alleles (PAV/AVI or AVI/AVI), but not in those encoding the functional allele (PAV/PAV).
Despite the relatively small numbers of patient samples tested, and the limited information about normal flora, this result is of considerable interest. As suggested by the authors, T2R38 polymorphism-related innate immune defects in people could be tested and correlated with a simple T2R38-dependent taste test. PTC itself may not be useful for this test since heterozygotes (PAV/AVI) display varying abilities to taste this compound, whereas the sino-nasal cells from these individuals do not display acyl HSL-dependent innate immune responses, and are more similar to the non-functional homozygotes (AVI/AVI) in this respect. The study does not indicate if the QS molecules C12HSL or C4HSL themselves have a bitter taste, and if there is an allele-dependent heterogeneity in perception of this taste.
The observation that bacterial HSLs stimulate host responses, in itself, is not novel. Numerous studies have demonstrated responses to acyl HSLs, including apoptotic and inflammatory signaling, in a range of host cells.4 P. aeruginosa acyl HSLs have even been demonstrated to directly bind to the lipid-sensing PPAR (peroxisome proliferator activated receptor) transcription factors and impair their activity. More recently, P. aeruginosa acyl HSLs were shown to interact with IQ-motif containing GTPase-activating protein (IQGAP), and impact Rac1 and Cdc42-dependent cell migration.5 The T2R38-acyl HSL interaction, however, suggests that the host is specifically tuning in to a conversation between bacteria. Notably, no endogenous ligands of T2R38 have been identified to date. Is it possible that acyl HSLs are the “natural” ligands for these receptors?
Could T2R38-dependent innate immune responses play a similar role in the inside passage? T2R38 is expressed on intestinal enteroendocrine cells, as well as in Caco-2 cell lines, and is responsive to PTC-dependent signaling.6,7 The presence and prevalence of acyl-HSLs in the gut, however, is unclear. In a recent review, Swearingen et al. comprehensively analyze published studies and state that “there has been no direct detection of acyl-HSLs within the normal mammalian intestinal tract” to date, except during Yersinia enterocolitica infections.8 Yet, they emphasize the caveats to all the negative results, including the lack of sensitivity of the biosensors used to detect acyl-HSLs. It is, therefore, conceivable that T2R38 plays a role in intestinal innate immunity, at least in the context of specific infections. More broadly, the effects of T2R38 polymorphisms on the endogenous microbiota in the gut, and elsewhere, remains an open question.
A relatively better established system of inter-kingdom signaling relates to the AI-3 quorum sensing system in various strains of E. coli and S. Typhimurium.9 In this instance, the bacteria not only respond to AI-3 in a density dependent manner, but also to host epinephrine and norepinephrine (E/NE), and this signaling can be blocked by α-adrenergic antagonists. It is likely that AI-3, which remains to be structurally characterized, also modulates host adrenergic signaling. Inter-kingdom signaling appears to have a long evolutionary history, and the main point of novelty here is that we human beings are finally privy to some of these conversations.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/23776
References
- 1.Chandrashekar J, Hoon MA, Ryba NJP, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–94. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 2.Trivedi BP. Neuroscience: hardwired for taste. Nature. 2012;486:S7–9. doi: 10.1038/486S7a. [DOI] [PubMed] [Google Scholar]
- 3.Lee RJ, Xiong G, Kofonow JM, Chen B, Lysenko A, Jiang P, et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest. 2012;122:4145–59. doi: 10.1172/JCI64240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker CT, Sperandio V. Cell-to-cell signalling during pathogenesis. Cell Microbiol. 2009;11:363–9. doi: 10.1111/j.1462-5822.2008.01272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karlsson T, Turkina MV, Yakymenko O, Magnusson K-E, Vikström E. The Pseudomonas aeruginosa N-acylhomoserine lactone quorum sensing molecules target IQGAP1 and modulate epithelial cell migration. PLoS Pathog. 2012;8:e1002953. doi: 10.1371/journal.ppat.1002953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Negri R, Morini G, Greco L. From the tongue to the gut. J Pediatr Gastroenterol Nutr. 2011;53:601–5. doi: 10.1097/MPG.0b013e3182309641. [DOI] [PubMed] [Google Scholar]
- 7.Jeon TI, Seo Y-K, Osborne TF. Gut bitter taste receptor signalling induces ABCB1 through a mechanism involving CCK. Biochem J. 2011;438:33–7. doi: 10.1042/BJ20110009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swearingen MC, Sabag-Daigle A, Ahmer BM. Are There Acyl-Homoserine Lactones within Mammalian Intestines? J Bacteriol. 2013;195:173–9. doi: 10.1128/JB.01341-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pacheco AR, Sperandio V. Inter-kingdom signaling: chemical language between bacteria and host. Curr Opin Microbiol. 2009;12:192–8. doi: 10.1016/j.mib.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


