Abstract
In the last decade, the role of magnetic resonance imaging (MRI) in neonatal care for prematurely-born infants has rapidly expanded and evolved. Recent investigations addressed many of the practical issues pertaining to image acquisition and interpretation, enabling high-quality MR images to be obtained without sedating medications in preterm infants at any institution. Expanded application has demonstrated MRI provides superior ability to assess cerebral development and identify and define cerebral injury in comparison to other imaging modalities. Term equivalent MRI results have been shown to correlate with neurodevelopmental outcomes, providing improved predictive ability over other neuroimaging, clinical, or physical examination measures. Regular utilization of MRI in this population is fundamental to gaining the knowledge and expertise necessary for rational, accurate application. Ongoing experiences will continue to shape the nature and type of information available to clinicians and families using MRI, further refining its role as a routine element of neonatal care.
Keywords: premature infant, magnetic resonance imaging, neurodevelopmental outcome
Introduction
In recent decades, survival rates for very preterm infants (born less than 30 weeks gestation) have improved dramatically due to advances in perinatal and neonatal care. In contrast to this improvement in mortality, long-term neurodevelopmental outcomes have not improved and remain problematic, with significant associated costs to individuals, families, and society (1–5). In recent years, significant investigation has been undertaken correlating varied demographic, perinatal, medical, and physical examination findings with long-term neurodevelopmental outcomes in attempt to identify the infants at greatest risk. Despite these efforts, clinicians and researchers continue to possess limited ability to definitively predict and meaningfully improve neurodevelopmental outcomes (6), with only significant abnormalities on cranial ultrasound (US) strongly predictive of poor neurodevelopmental outcome (7, 8). Application of magnetic resonance imaging (MRI) in this population has provided an improved ability to assess cerebral development, providing clinicians with a novel mechanism for identifying at-risk infants.
In recent years, MRI scanners have become increasingly available. Currently, the majority of high-level care facilities containing Neonatal Intensive Care Units (NICUs) also possess MRI scanners suitable for studying neonates. Increased utilization of these scanners has demonstrated that image acquisition is typically well tolerated by even the youngest and smallest patients. Additionally, scans can be performed successfully without the use of sedating medications, eliminating the risk associated with this procedure. Increased application in this group has permitted improvements in pulse sequences used to acquire images and enabled development of infant-specific head coils, improving the quality of the images obtained. Further, growing experience has provided neuroradiologists with necessary knowledge and tools for image interpretation, as population-specific norms for sequences such as diffusion weighted imaging (DWI) and magnetic resonance spectroscopy (MRS) have been established (9–12). As a result, most institutions now possess the ability to obtain high-quality MRI scans on prematurely-born infants at term equivalent (TE) age.
In the last 15 years, the role of MRI in neonatal care has been increasingly investigated by clinicians and researchers. As practical issues pertaining to image acquisition and interpretation have been more clearly determined, investigators have transitioned to defining the role of MRI in the clinical domain. Recent inquiries have demonstrated the potency of MRI as a diagnostic tool used to assess brain development and injury in this population (13–16). Subsequent investigations have demonstrated MRI findings obtained on a routine clinical scan can also provide invaluable information regarding neurodevelopmental outcomes that can be utilized for dictating individualized care plans, counseling families, and ultimately improving outcomes in this high-risk group. As a result of these inquiries, a growing body of evidence supports routine use of MRI as a component of NICU clinical care for prematurely-born infants.
Practical Considerations Pertinent to MRI Acquisition in Preterm Infants
Several practical issues must be considered when performing MRI scans in prematurely-born infants. At almost every institution, obtaining an MRI scan on an infant requires transport out of the NICU to the neuroradiology suite, often located on another floor of the hospital. By TE, the preponderance of prematurely-born infants is medically stable enough for safe transport to the scanner and tolerates the procedure well. The time required to prepare each infant for the scan is approximately 30–60 minutes, and can be readily coordinated with individualized care and feeding plans for each infant by the NICU staff. Establishment of imaging protocols at several institutions has demonstrated a ready ability to obtain high-quality images in non-sedated infants, eliminating the risk and cost associated with this procedure. The equipment required to perform these studies is relatively limited, inexpensive, and commercially available, consisting of items such as ear protection, head stabilizing devices, and MRI-compatible cardiopulmonary monitoring equipment. Isolettes that can be taken into the MR suite can also be obtained, though these are not typically required for scanning TE prematurely-born infants.
At our institution, guidelines published by Mathur have now been successfully utilized to safely obtain MRI scans without sedation on greater than 1000 prematurely born infants (17) (Figure 1). These practices are readily transferrable between institutions. The procedures detailed by Mathur were successfully implemented to perform non-sedated MRI scans in preterm infants at TE at another nearby institution (18). This group was able to complete non-sedated MRI studies at a 94% success rate, with satisfactory image quality realized in more than 97% of attempts. Further, complication rates and time away from the NICU for scan acquisition were both significantly reduced when sedation was not used. Other institutions have also published results obtained from successfully scanning infants without sedation (19, 20). Most recently, Neubauer demonstrated the feasibility of performing MRI scans in non-sedated infants at an “inexperienced center” (21). Using self-developed guidelines, the group performed non-sedated scans on prematurely-born infants at TE at a 93% success rate. A subset of these scans was also completed in the outpatient setting. Time for scan acquisition was again significantly decreased when sedation was not used. These reports demonstrate high-quality, non-sedated MRI scans can be obtained on this population at any institution, requiring cursory amounts of specialized equipment, technical infrastructure, and expertise.
Figure 1. Procedures for preparing infant for non-sedated MRI scan.
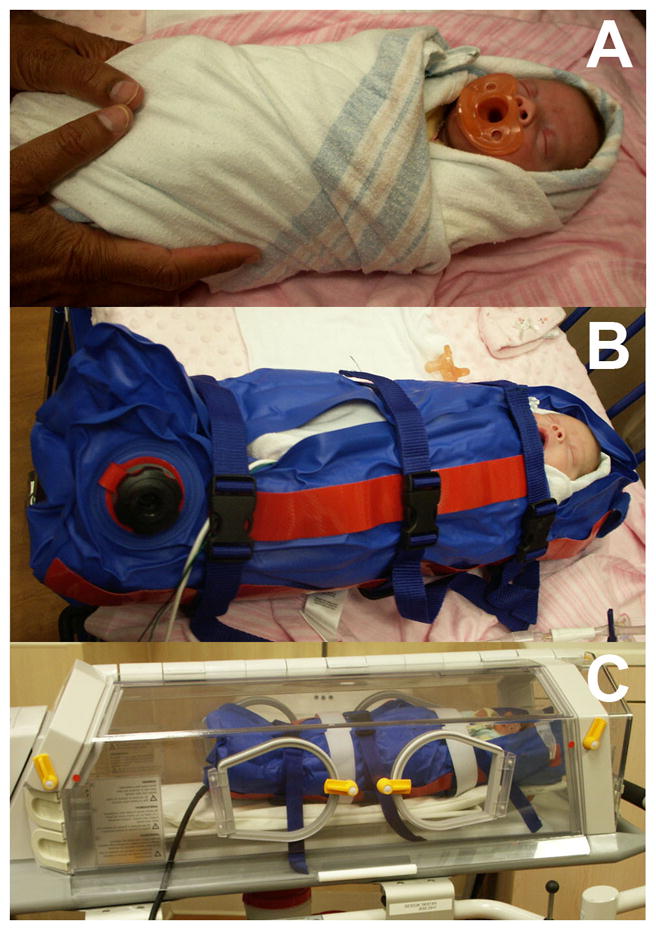
Procedures for preparing infants prior to placement in the MRI scanner includes A) snuggly wrapping the infant prior to placement in the vacuum bag; B) securing the infant in the stabilizing vacuum bag; C) placing the stabilized infant in the MR-compatible isolette for movement into the scanner.
Assessment of Cerebral Development and Injury Using MRI in Preterm Infants
When performed successfully, MRI provides non-invasive, high-resolution images of the entirety of cerebral anatomy obtainable in less than an hour. These images are superior in quality and more inclusive than those available using other commonly available imaging modalities, including US and computerized tomography (CT). Additionally, MRI does not present the same long-term risks to the developing brain associated with radiation exposure found with CT scans. Further, these scans can be performed serially throughout early development making longitudinal assessment feasible. The result is an improved ability to perform comprehensive in vivo assessments of cerebral anatomy, detail the marked changes associated with normative development, and detect and characterize brain injury.
Throughout the neonatal period, the brain becomes increasingly folded with the onset of cortical sulcation and gyration in a stereotyped, regionally-specific distribution. In recent years, MRI has been increasingly applied to characterize the patterns and timing of these changes in prematurely-born infants in a manner previously available only through neuropathological investigations (13, 14). This work has produced detailed descriptions of normative folding patterns for infants, enabling quantification of these configurations (Figure 2). Accurate assessment of cortical folding on TE studies provides an important marker for structural brain growth and maturation during this critical developmental period. MRI also enables non-invasive assessment and characterization of myelination, another critical component of normative cerebral development. In typically developing brains, myelination involving the posterior limb of the internal capsule (PLIC) is first apparent on MRI scans at around 36 to 38 weeks gestation, before expanding to incorporate other regions in a stereotyped pattern throughout early development (15, 16). It is often most readily identifiable on T1-weighted scans, but can also be identified on T2-weighted images (Figure 3). Evaluation of its presence and symmetry on TE MRI scans also provides insight into ongoing cerebral development. MRI is currently the only clinically available imaging modality that enables accurate assessment of these important developmental benchmarks.
Figure 2. Development of cortical folding in the premature human brain.
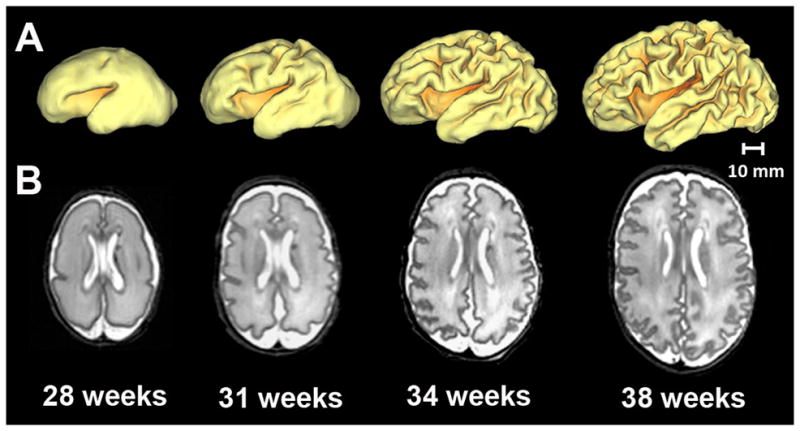
Representative A) 3-dimensional surfaces and B) axial T2-weighted images illustrating regionally-specific cortical folding occurring in the premature brain secondary to sulcation and gyration throughout early development. Provided are images obtained from a single preterm infant from MRI scans performed at 28, 31, 34, and 38 weeks post-mentrual age. Note the marked increase in brain size and folding complexity between each set of images.
Figure 3. Myelination of the posterior limb of the internal capsule.
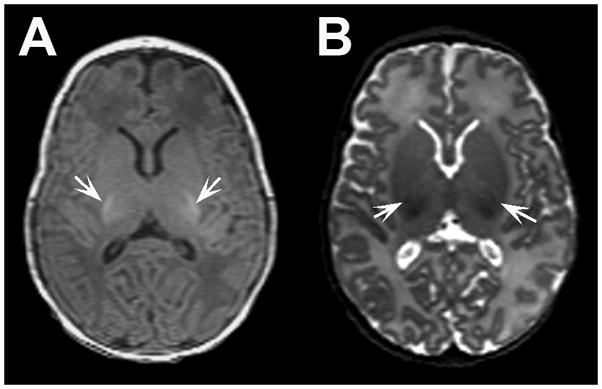
Representative axial A) T1- and B) T2-weighted MR images from a very preterm infant at term equivalent age demonstrating myelination evident in the the posterior limb of the internal capsule bilaterally. Note myelinated white matter appears hyperintense on T1-weighted images and hypointense on T2-weighted images (arrows).
In addition to information on cerebral development, MRI also enables comprehensive assessment of brain injury, providing detailed information regarding injury type, location, extent, and timing. Recent application has served to re-establish normative values regarding frequency and severity for specific injury types in this population. In a large, multicenter trial, Kidokoro recently found that 33% of infants demonstrate cerebral injury on TE MRI scan, including 12% of infants with periventricular leukomalacia, 19% of infants with intraventricular hemorrhage, and 10% of infants with cerebellar hemorrhage (Figure 4) (22). Further, high-grade injury and multiple injury types were identified in multiple infants. In comparison to US, MRI possesses improved ability for detecting cerebral injury. This difference is most consistent with less white matter injury (WMI), an injury type common in neonates. In one of the early works investigating this issue, Maalouf performed a comparison between US and MRI, demonstrating an improved ability to detect injury and accurately characterize WMI using MRI (23). Inder also performed a comparison between US and MRI, demonstrating significant advantages in detecting non-cystic WMI with MRI (24). Additionally, Miller demonstrated improved sensitivity for identifying WMI using MRI compared to US, most notably for less severe forms of injury (25). Finally, similar patterns have been reported on MRI performed prior to TE, with an improved ability to detect less severe forms of injury (26). Secondary to its expanded field of view, MRI also provides an improved ability to identify posterior fossa and cerebellar injury. Miall reported an improved ability to identify cerebellar injury using MRI in comparison to US (27). Finally, MRI can also be used to identify impaired brain growth. Kidokoro demonstrated characteristic patterns of impaired brain growth in preterm infants at TE, identifying self-defined “small brain” (biparietal width z-score < −0.5) and “hypoplastic brain” (interhemispheric distance ≥ 4.0 mm) models. For each of these injury patterns, MRI provides not only the most comprehensive ability to assess and define injury severity, but also a mechanism to investigate its subsequent impact on neurodevelopmental outcome.
Figure 4. Classification of periventricular leukomalacia and cerebellar hemorrhage.
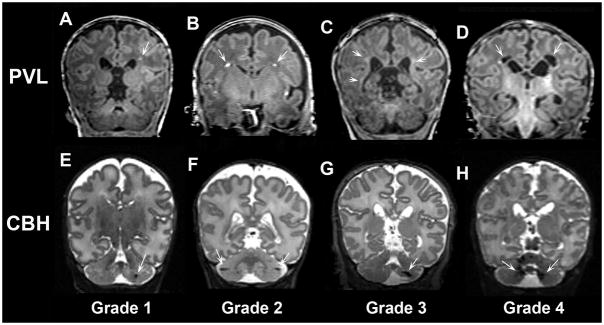
Coronal T1- and T2-weighted MR images demonstrating representative examples of periventricular leukomalacia (PVL, upper panel) and cerebellar hemorrhage (CBH, lower panel) of progressive severity. A) Grade 1 and B) Grade 2 PVL defined by punctate lesions; C) Grade 3 PVL defined by high signal along the wall of lateral ventricles; D) Grade 4 PVL defined by cysts in the periventricular white matter. CBH was also classified into 4 grades including: E) Grade 1 CBH defined by unilateral punctate lesions ≤3 mm in size; F) Grade 2 CBH defined by bilateral punctate lesions; G) Grade 3 CBH defined by a unilateral lesion >3 mm in size; H) Grade 4 CBH defined by extensive lesions bilaterally.
Correlation of MRI Findings with Neurodevelopmental Outcome using TE Scans
Over the course of the last decade, multiple studies have demonstrated the utility of MRI scans as a tool for neurodevelopmental outcome prediction in prematurely-born infants. The predominance of these investigations correlated results from a TE MRI scan with neurodevelopmental outcomes in the first several years of life, though some groups have investigated more long-term outcomes. Importantly, these inquiries have demonstrated that MRI outperforms other neuroimaging, clinical, or physical examination measures for outcome prediction in this high-risk group. MRI’s predictive ability has most commonly been contrasted with US, which has been demonstrated to have high specificity but low sensitivity (7, 28, 29). In recent years, improvements in image quality, growing experience with image interpretation and development and institution of new tools for image assessment (Supplement 1) have continued to widen this gap, increasing the predictive potency of these scans.
Early application of MRI as an outcome prediction tool correlated qualitative assessments of scan results with neurodevelopmental outcomes. In one of the earliest investigations of this type, Valkama correlated results from a TE scan with 18 month outcomes, demonstrating 100% sensitivity (improved from 67% with US for the same cohort) and 79% specificity for subsequent motor impairment or diagnosis of cerebral palsy (CP) based upon qualitative assessment of MRI scans (30). Mirmiran also used qualitative assessment of TE MRI scans correlated with 20 and 31 month outcomes to demonstrate MRI possesses 86% sensitivity (versus 43% with US) and 89% specificity (versus 82% with US) for prediction of CP at 31 months (29). Following these early studies, novel tools were developed to systematically assess the presence and severity of white and gray matter injury and correlate these findings with neurodevelopmental outcomes. Woodward used a WMI scoring system to correlate findings from TE scans with 2 year outcomes (28). The group demonstrated moderate to severe WMI was highly predictive of poor outcome, associated with poor performance on standardized assessment of motor and cognitive performance and greater frequency of CP. Similar assessment of gray matter injury in the same cohort demonstrated that it was also significantly associated with cognitive and motor delay and a diagnosis of cerebral palsy. Recently, using the same standardized injury scoring system, Skiöld demonstrated a relationship between moderate to severe WMI and a diagnosis of CP at 30 months (31).
Recent investigations have correlated other specific imaging findings of varied types, including patterns of injury, on TE MRI scans with outcome. De Vries investigated the association between myelination in the PLIC with developmental outcome at ≥12 months in infants with grade IV intraventricular hemorrhage (32). Their group demonstrated absence of myelin in the PLIC was strongly predictive of subsequent development of hemiplegia and asymmetric PLIC signal was suggestive of increased risk for motor asymmetry. Tam investigated the relationship between cerebellar hemorrhage on TE scan and 3–6 year outcomes in small cohort of infants (33). They demonstrated cerebellar hemorrhage detectable only on MRI scan was associated with increased likelihood of abnormality on neurological examination, with larger hemorrhage associated with worse outcome. Finally, Nguyen correlated simple brain growth metrics on TE scans with neurodevelopmental outcomes at 2 years of age (34). They demonstrated measures of motor and cognitive performance were positively associated with a number of brain metrics, with biparietal diameter the strongest predictor of outcome. In addition to these positive findings, MRI has also been used to demonstrate specific patterns of injury are not suggestive of poor outcome. Hart demonstrated no significant differences in outcome at 18 months of age in infants with diffuse excessive high signal intensity (DEHSI) on TE scan (35). This finding was corroborated by Skiöld and Kidokoro, who demonstrated no correlation between DEHSI and outcomes at 2 years and 30 months of age (31, 36).
These investigations illustrate the variety of neuroimaging findings available from a single MRI scanning session that can be utilized for neurodevelopmental outcome prediction in this population. In addition, the practical nature of many of the measures illustrates the feasibility of routine implementation at any institution by individuals with limited initial training. Regular application of these techniques by a consistent group of users serves to provide the invaluable experience necessary for robust identification of infants at greatest risk. Recent work has begun to correlate longer-term neurodevelopmental assessments with TE MRI scan results, suggesting the technique maintains its predictive power throughout middle childhood (37). While additional longitudinal study remains necessary for continuing to define the role of MRI for outcome prediction in this population, these inquiries underscore its vast potential for this purpose in the clinical domain.
Utility of Preterm Scans in Outcome Prediction
MRI scans performed on prematurely-born infants prior to TE can also provide invaluable information regarding neurodevelopmental outcome. Miller correlated MRI scan results performed at 31–33 weeks gestation and TE with 18 month developmental outcomes (38). They demonstrated that the presence of moderate to severe WMI on MRI significantly increased the risk of abnormal outcome. Further, the severity of adverse outcome was significantly associated with worsened WMI, ventriculomegaly, and intraventricular hemorrhage. Importantly, abnormalities on the earlier scans were comparably predictive of aberrant neurodevelopmental outcome as those identified on the TE scan. This work suggests a clinical role for preterm scans as well, particularly for infants where there are logistical obstacles to obtaining a TE scan. Additionally, these findings indicate a clinical scan performed earlier during the neonatal period is preferable to not scanning a patient.
Conclusion
Recent practices have demonstrated that high-quality MRI scans can be safely and routinely performed in prematurely-born infants at TE without sedating medications at any institution. Performing these scans affords an improved ability to assess brain development and injury. Importantly, results of these imaging studies also provide invaluable prognostic information regarding neurodevelopmental outcomes superior to those obtained using other neuroimaging modalities, providing a critical adjunct to information available through history and physical findings. While fewer investigations have been performed correlating results from early MRI scans with neurodevelopmental outcomes, this practice also holds promise moving forward. Additionally, increasing application of more advanced MR modalities such as diffusion tensor imaging (DTI), surface based morphometry (SBM), volumetry and/or functional connectivity magnetic resonance imaging (fcMRI), may further expand the developmental and prognostic information obtainable using this modality in the future.
Over the last decade, the role of MRI in NICUs has rapidly expanded. Growing experience applying the technique to study this population has provided invaluable lessons for clinicians, most prominently that MRI is highly effective with routine implementation. The knowledge gained through regular use of MRI translates to enhanced skill necessary for appropriate acquisition and interpretation of the imaging studies by a multidisciplinary group of health care providers. Ultimately, this information can be translated to identify high-risk infants, allowing implementation of interventions designed to improve neurodevelopmental outcomes via development of targeted, cost-effective health care plans initiated during the NICU course and continued following discharge. In the coming years, ongoing experiences will continue to shape the nature and type of clinical information available to clinicians and families from a single MR scanning session, further solidifying its role as a routine and necessary component of NICU care.
Supplementary Material
Key Points.
High-quality magnetic resonance imaging (MRI) studies can be performed without sedating medications in term equivalent prematurely-born infants at any institution.
Term equivalent MRI scans provide invaluable information regarding brain injury and development. Results can also be utilized as an effective tool for neurodevelopmental outcome prediction.
Term equivalent MRI scans should be considered a routine component of NICU care for prematurely-born infants. Regular utilization is fundamental to gaining the knowledge and expertise necessary for rational, accurate application.
Acknowledgments
This work was supported by the Child Neurology Foundation [to C.D.S.], National Institutes of Health [grant numbers K12 NS001690 to C.D.S., UL1 RR024992 to C.D.S, R01 HD05709801 to T.E.I., P30 HD062171 to T.E.I], and the Doris Duke Foundation [to T.E.I. and H.K.]. The funders had no role in decision to publish or preparation of the manuscript.
Abbreviations
- US
ultrasound
- MRI
magnetic resonance imaging
- NICU
neonatal intensive care unit
- DWI
diffusion weighted imaging
- MRS
magnetic resonance spectroscopy
- TE
term equivalent
- CT
computerized tomography
- PLIC
posterior limb of the internal capsule
- WMI
white matter injury
- CP
cerebral palsy
- DEHSI
diffuse excessive high signal intensity
- DTI
diffusion tensor imaging
- SBM
surface based morphometry
- fcMRI
functional connectivity magnetic resonance imaging
Footnotes
The authors declare that they have no competing financial interests or conflicts of interest.
Contributor Information
Christopher D. Smyser, Email: smyserc@neuro.wustl.edu, 660 South Euclid Avenue, Campus Box 8111, Saint Louis, Missouri 63110-1093, Phone: 314.454.6120, Fax: 314.454.2523.
Hiroyuki Kidokoro, Email: kidokoro_h@kids.wustl.edu, 660 South Euclid Avenue, Campus Box 8116, Saint Louis, Missouri 63110-1093.
Terrie E. Inder, Email: inder_t@kids.wustl.edu, 660 South Euclid Avenue, Campus Box 8116, Saint Louis, Missouri 63110-1093.
References
- 1.Holsti L, Grunau RV, Whitfield MF. Developmental coordination disorder in extremely low birth weight children at nine years. Journal of developmental and behavioral pediatrics : JDBP. 2002;23(1):9–15. doi: 10.1097/00004703-200202000-00002. Epub 2002/03/13. [DOI] [PubMed] [Google Scholar]
- 2.Taylor HG, Minich NM, Klein N, Hack M. Longitudinal outcomes of very low birth weight: neuropsychological findings. Journal of the International Neuropsychological Society : JINS. 2004;10(2):149–63. doi: 10.1017/S1355617704102038. Epub 2004/03/12. [DOI] [PubMed] [Google Scholar]
- 3.Barre N, Morgan A, Doyle LW, Anderson PJ. Language abilities in children who were very preterm and/or very low birth weight: a meta-analysis. J Pediatr. 2011;158(5):766–74. e1. doi: 10.1016/j.jpeds.2010.10.032. Epub 2010/12/15. [DOI] [PubMed] [Google Scholar]
- 4.Anderson PJ, Doyle LW. Cognitive and educational deficits in children born extremely preterm. Semin Perinatol. 2008;32(1):51–8. doi: 10.1053/j.semperi.2007.12.009. Epub 2008/02/06. [DOI] [PubMed] [Google Scholar]
- 5.Hintz SR, Kendrick DE, Wilson-Costello DE, Das A, Bell EF, Vohr BR, et al. Early-childhood neurodevelopmental outcomes are not improving for infants born at <25 weeks’ gestational age. Pediatrics. 2011;127(1):62–70. doi: 10.1542/peds.2010-1150. Epub 2010/12/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laptook AR, O’Shea TM, Shankaran S, Bhaskar B. Adverse neurodevelopmental outcomes among extremely low birth weight infants with a normal head ultrasound: prevalence and antecedents. Pediatrics. 2005;115(3):673–80. doi: 10.1542/peds.2004-0667. Epub 2005/03/03. [DOI] [PubMed] [Google Scholar]
- 7.De Vries LS, Van Haastert IL, Rademaker KJ, Koopman C, Groenendaal F. Ultrasound abnormalities preceding cerebral palsy in high-risk preterm infants. J Pediatr. 2004;144(6):815–20. doi: 10.1016/j.jpeds.2004.03.034. Epub 2004/06/12. [DOI] [PubMed] [Google Scholar]
- 8.Pinto-Martin JA, Whitaker AH, Feldman JF, Van Rossem R, Paneth N. Relation of cranial ultrasound abnormalities in low-birthweight infants to motor or cognitive performance at ages 2, 6, and 9 years. Developmental medicine and child neurology. 1999;41(12):826–33. doi: 10.1017/s0012162299001644. Epub 2000/01/05. [DOI] [PubMed] [Google Scholar]
- 9.Neil JJ, Shiran SI, McKinstry RC, Schefft GL, Snyder AZ, Almli CR, et al. Normal brain in human newborns: apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology. 1998;209(1):57–66. doi: 10.1148/radiology.209.1.9769812. Epub 1998/10/14. [DOI] [PubMed] [Google Scholar]
- 10.McKinstry RC, Mathur A, Miller JH, Ozcan A, Snyder AZ, Schefft GL, et al. Radial organization of developing preterm human cerebral cortex revealed by non-invasive water diffusion anisotropy MRI. Cereb Cortex. 2002;12(12):1237–43. doi: 10.1093/cercor/12.12.1237. Epub 2002/11/13. [DOI] [PubMed] [Google Scholar]
- 11.Kreis R, Hofmann L, Kuhlmann B, Boesch C, Bossi E, Huppi PS. Brain metabolite composition during early human brain development as measured by quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med. 2002;48(6):949–58. doi: 10.1002/mrm.10304. Epub 2002/12/05. [DOI] [PubMed] [Google Scholar]
- 12.Huppi PS, Fusch C, Boesch C, Burri R, Bossi E, Amato M, et al. Regional metabolic assessment of human brain during development by proton magnetic resonance spectroscopy in vivo and by high-performance liquid chromatography/gas chromatography in autopsy tissue. Pediatr Res. 1995;37(2):145–50. doi: 10.1203/00006450-199502000-00003. Epub 1995/02/01. [DOI] [PubMed] [Google Scholar]
- 13.Battin MR, Maalouf EF, Counsell SJ, Herlihy AH, Rutherford MA, Azzopardi D, et al. Magnetic resonance imaging of the brain in very preterm infants: visualization of the germinal matrix, early myelination, and cortical folding. Pediatrics. 1998;101(6):957–62. doi: 10.1542/peds.101.6.957. Epub 1998/06/02. [DOI] [PubMed] [Google Scholar]
- 14.Dubois J, Benders M, Cachia A, Lazeyras F, Ha-Vinh Leuchter R, Sizonenko SV, et al. Mapping the early cortical folding process in the preterm newborn brain. Cereb Cortex. 2008;18(6):1444–54. doi: 10.1093/cercor/bhm180. Epub 2007/10/16. [DOI] [PubMed] [Google Scholar]
- 15.Cowan FM, de Vries LS. The internal capsule in neonatal imaging. Seminars in fetal & neonatal medicine. 2005;10(5):461–74. doi: 10.1016/j.siny.2005.05.007. Epub 2005/07/09. [DOI] [PubMed] [Google Scholar]
- 16.Counsell SJ, Maalouf EF, Fletcher AM, Duggan P, Battin M, Lewis HJ, et al. MR imaging assessment of myelination in the very preterm brain. AJNR Am J Neuroradiol. 2002;23(5):872–81. Epub 2002/05/15. [PMC free article] [PubMed] [Google Scholar]
- 17.Mathur AM, Neil JJ, McKinstry RC, Inder TE. Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatric radiology. 2008;38(3):260–4. doi: 10.1007/s00247-007-0705-9. Epub 2008/01/05. [DOI] [PubMed] [Google Scholar]
- 18.Haney B, Reavey D, Atchison L, Poull J, Dryer L, Anderson B, et al. Magnetic resonance imaging studies without sedation in the neonatal intensive care unit: safe and efficient. The Journal of perinatal & neonatal nursing. 2010;24(3):256–66. doi: 10.1097/JPN.0b013e3181e8d566. Epub 2010/08/11. [DOI] [PubMed] [Google Scholar]
- 19.Vigneron DB, Barkovich AJ, Noworolski SM, von dem Bussche M, Henry RG, Lu Y, et al. Three-dimensional proton MR spectroscopic imaging of premature and term neonates. AJNR Am J Neuroradiol. 2001;22(7):1424–33. Epub 2001/08/11. [PMC free article] [PubMed] [Google Scholar]
- 20.Huppi PS, Warfield S, Kikinis R, Barnes PD, Zientara GP, Jolesz FA, et al. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Annals of neurology. 1998;43(2):224–35. doi: 10.1002/ana.410430213. Epub 1998/03/04. [DOI] [PubMed] [Google Scholar]
- 21.Neubauer V, Griesmaier E, Baumgartner K, Mallouhi A, Keller M, Kiechl-Kohlendorfer U. Feasibility of cerebral MRI in non-sedated preterm-born infants at term-equivalent age: report of a single centre. Acta Paediatr. 2011;100(12):1544–7. doi: 10.1111/j.1651-2227.2011.02388.x. Epub 2011/06/23. [DOI] [PubMed] [Google Scholar]
- 22.Kidokoro H, Anderson PJ, Doyle LW, Woodward LJ, Neil JJ, Inder TE. The Nature of Brain Injury and Altered Brain Growth in Preterm Infants - Predictors and Prognosis. Pediatrics. 2012 doi: 10.1542/peds.2013-2336. (under review) [DOI] [PubMed] [Google Scholar]
- 23.Maalouf EF, Duggan PJ, Counsell SJ, Rutherford MA, Cowan F, Azzopardi D, et al. Comparison of findings on cranial ultrasound and magnetic resonance imaging in preterm infants. Pediatrics. 2001;107(4):719–27. doi: 10.1542/peds.107.4.719. Epub 2001/05/23. [DOI] [PubMed] [Google Scholar]
- 24.Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr. 2003;143(2):171–9. doi: 10.1067/S0022-3476(03)00357-3. Epub 2003/09/13. [DOI] [PubMed] [Google Scholar]
- 25.Miller SP, Cozzio CC, Goldstein RB, Ferriero DM, Partridge JC, Vigneron DB, et al. Comparing the diagnosis of white matter injury in premature newborns with serial MR imaging and transfontanel ultrasonography findings. AJNR Am J Neuroradiol. 2003;24(8):1661–9. Epub 2003/09/19. [PMC free article] [PubMed] [Google Scholar]
- 26.Debillon T, N’Guyen S, Muet A, Quere MP, Moussaly F, Roze JC. Limitations of ultrasonography for diagnosing white matter damage in preterm infants. Archives of disease in childhood Fetal and neonatal edition. 2003;88(4):F275–9. doi: 10.1136/fn.88.4.F275. Epub 2003/06/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miall LS, Cornette LG, Tanner SF, Arthur RJ, Levene MI. Posterior fossa abnormalities seen on magnetic resonance brain imaging in a cohort of newborn infants. Journal of perinatology : official journal of the California Perinatal Association. 2003;23(5):396–403. doi: 10.1038/sj.jp.7210941. Epub 2003/07/09. [DOI] [PubMed] [Google Scholar]
- 28.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. The New England journal of medicine. 2006;355(7):685–94. doi: 10.1056/NEJMoa053792. Epub 2006/08/18. [DOI] [PubMed] [Google Scholar]
- 29.Mirmiran M, Barnes PD, Keller K, Constantinou JC, Fleisher BE, Hintz SR, et al. Neonatal brain magnetic resonance imaging before discharge is better than serial cranial ultrasound in predicting cerebral palsy in very low birth weight preterm infants. Pediatrics. 2004;114(4):992–8. doi: 10.1542/peds.2003-0772-L. Epub 2004/10/07. [DOI] [PubMed] [Google Scholar]
- 30.Valkama AM, Paakko EL, Vainionpaa LK, Lanning FP, Ilkko EA, Koivisto ME. Magnetic resonance imaging at term and neuromotor outcome in preterm infants. Acta Paediatr. 2000;89(3):348–55. Epub 2000/04/20. [PubMed] [Google Scholar]
- 31.Skiold B, Vollmer B, Bohm B, Hallberg B, Horsch S, Mosskin M, et al. Neonatal magnetic resonance imaging and outcome at age 30 months in extremely preterm infants. J Pediatr. 2012;160(4):559–66. e1. doi: 10.1016/j.jpeds.2011.09.053. Epub 2011/11/08. [DOI] [PubMed] [Google Scholar]
- 32.De Vries LS, Groenendaal F, van Haastert IC, Eken P, Rademaker KJ, Meiners LC. Asymmetrical myelination of the posterior limb of the internal capsule in infants with periventricular haemorrhagic infarction: an early predictor of hemiplegia. Neuropediatrics. 1999;30(6):314–9. doi: 10.1055/s-2007-973511. Epub 2000/03/08. [DOI] [PubMed] [Google Scholar]
- 33.Tam EW, Rosenbluth G, Rogers EE, Ferriero DM, Glidden D, Goldstein RB, et al. Cerebellar hemorrhage on magnetic resonance imaging in preterm newborns associated with abnormal neurologic outcome. J Pediatr. 2011;158(2):245–50. doi: 10.1016/j.jpeds.2010.07.049. Epub 2010/09/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tich SN, Anderson PJ, Hunt RW, Lee KJ, Doyle LW, Inder TE. Neurodevelopmental and perinatal correlates of simple brain metrics in very preterm infants. Archives of pediatrics & adolescent medicine. 2011;165(3):216–22. doi: 10.1001/archpediatrics.2011.9. Epub 2011/03/09. [DOI] [PubMed] [Google Scholar]
- 35.Hart A, Whitby E, Wilkinson S, Alladi S, Paley M, Smith M. Neuro-developmental outcome at 18 months in premature infants with diffuse excessive high signal intensity on MR imaging of the brain. Pediatric radiology. 2011;41(10):1284–92. doi: 10.1007/s00247-011-2155-7. Epub 2011/06/18. [DOI] [PubMed] [Google Scholar]
- 36.Kidokoro H, Anderson PJ, Doyle LW, Neil JJ, Inder TE. High signal intensity on T2-weighted MR imaging at term-equivalent age in preterm infants does not predict 2-year neurodevelopmental outcomes. AJNR Am J Neuroradiol. 2011;32(11):2005–10. doi: 10.3174/ajnr.A2703. Epub 2011/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwata S, Nakamura T, Hizume E, Kihara H, Takashima S, Matsuishi T, et al. Qualitative brain MRI at term and cognitive outcomes at 9 years after very preterm birth. Pediatrics. 2012;129(5):e1138–47. doi: 10.1542/peds.2011-1735. Epub 2012/04/25. [DOI] [PubMed] [Google Scholar]
- 38.Miller SP, Ferriero DM, Leonard C, Piecuch R, Glidden DV, Partridge JC, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005;147(5):609–16. doi: 10.1016/j.jpeds.2005.06.033. Epub 2005/11/18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


