Abstract
Aquaporin (AQP) water channels are expressed primarily in cell plasma membranes. In this paper, we review recent evidence that AQPs facilitate cell migration. AQP-dependent cell migration has been found in a variety of cell types in vitro and in mice in vivo. AQP1 deletion reduces endothelial cell migration, limiting tumor angiogenesis and growth. AQP4 deletion slows the migration of reactive astrocytes, impairing glial scarring after brain stab injury. AQP1-expressing tumor cells have enhanced metastatic potential and local infiltration. Impaired cell migration has also been seen in AQP1-deficient proximal tubule epithelial cells, and AQP3-deficient corneal epithelial cells, enterocytes, and skin keratinocytes. The mechanisms by which AQPs enhance cell migration are under investigation. We propose that, as a consequence of actin polymerization/depolymerization and transmembrane ionic fluxes, the cytoplasm adjacent to the leading edge of migrating cells undergoes rapid changes in osmolality. AQPs could thus facilitate osmotic water flow across the plasma membrane in cell protrusions that form during migration. AQP-dependent cell migration has potentially broad implications in angiogenesis, tumor metastasis, wound healing, glial scarring, and other events requiring rapid, directed cell movement. AQP inhibitors may thus have therapeutic potential in modulating these events, such as slowing tumor growth and spread, and reducing glial scarring after injury to allow neuronal regeneration.
Keywords: Angiogenesis, AQP, Astrocyte, Cancer, Cell membrane, Cell motility, Lamellipodium, Water channel
Introduction
The aquaporins (AQPs) are a family of small integral membrane proteins (monomers ~30 kDa) that transport water alone, or water and small solute(s) such as glycerol. AQPs increase cell plasma membrane water permeability 5–50 times compared with that in membranes where water moves primarily through the lipid bilayer. There are at least 13 AQPs in mammals expressed in many epithelia, endothelia, and other types of cells. Phenotype analysis has revealed a variety of important, and in some cases unanticipated physiological roles of AQPs in the urinary concentrating mechanism, glandular fluid secretion, brain swelling, neural excitability, fat metabolism, and skin hydration (reviewed in [39]). This review will focus on a recently discovered, unanticipated role for AQPs in facilitating cell migration.
Overview of the cell migration process
Migration is a fundamental property of cells that occurs during many physiological and pathological processes including organogenesis in the embryo, repair of damaged tissue after injury, the inflammatory response, formation of new blood vessels, and the spread of cancer. Here, we provide a brief overview of the migration process; for more details, the reader is referred to [28, 38, 41]. Based on observations of migrating cells in culture, cell migration has been divided into four processes: polarization, protrusion, traction, and retraction.
Initially, cells detect a chemotactic gradient and polarize into a predominantly front part and a retracting rear part, defined by distinct signaling events. Polarization is initiated by Cdc42, PAR proteins, and atypical protein kinase. Plasma membrane protrusions form by actin reorganization, consisting of spike-like filopodia, which sense and explore the local environment, and broad lamellipodia, which provide a foundation for the cell to move forward. Several proteins control actin dynamics including Ena/VASP, fascin, the Arp2/3 complex, Wasp/Wave family members, profilin, capping proteins, the ADF/cofilin family, cortactin, filamin A, and beta-actinin. The newly extended protrusions adhere to the extracellular matrix through integrins, with traction forces being generated at these adhesion sites by myosin II interaction with actin.
Protein kinase C or Rap1 promote integrin activation (increasing integrin affinity causing adhesion), whereas Raf-1 suppresses integrin activation (favoring detachment). To extend protrusions, adhesions transiently disassemble and, once the protrusion has extended, adhesions reassemble allowing traction for the cell to pull forward on the substratum. Myosin II is crucial for retraction of the cell rear and the development of tension between adhesions at the rear and the retraction machinery. This tension opens stretch-activated Ca2+ channels, activating calpain, which contributes to adhesion disassembly at the cell rear by cleaving focal adhesion proteins.
A role for AQPs in cell migration
It is evident from the above description that during migration in culture, cells undergo rapid changes in shape primarily because of the rapid formation and retraction of cell membrane protrusions such as lamellipodia and filopodia. In Chinese Hamster Ovary (CHO) cells, changes in the shapes of plasma membrane protrusions are apparent over seconds to minutes by light microscopy [31], comparable to the time course for osmotic cell swelling seen after a rapid decrease in extracellular osmolality [31, 32, 36]. Cell-shape changes are even more pronounced in vivo, where the entire cell needs to squeeze through the extracellular space, which in brain can be as small as ~50 nm. These rapid cell shape changes are likely accompanied by changes in cell volume, which require water flow into and out of the cell. The importance of cell volume changes and transmembrane water flow during cell migration is poorly understood compared with the biochemical events described above.
Changes in cell volume may not only facilitate these cell-shape changes, but may also help to propel the cell, in effect augmenting the contribution of actin-mediated mechanisms to forward cell motion. Theoretical calculations suggest that unequal rates of water entry into the front vs back portions of a cell (produced by placing a cell in an osmotic gradient) can generate enough force to propel a cell forward toward hypo-osmolality without the need for actin involvement [14]. Based on these observations, we suggest that AQP-mediated transmembrane water movements may play two important roles in the migration process: facilitate cell shape changes and help propel the cell forward. We will first review the evidence for AQP involvement in cell migration and then discuss further the possible molecular mechanisms.
AQP1 and endothelial cell migration
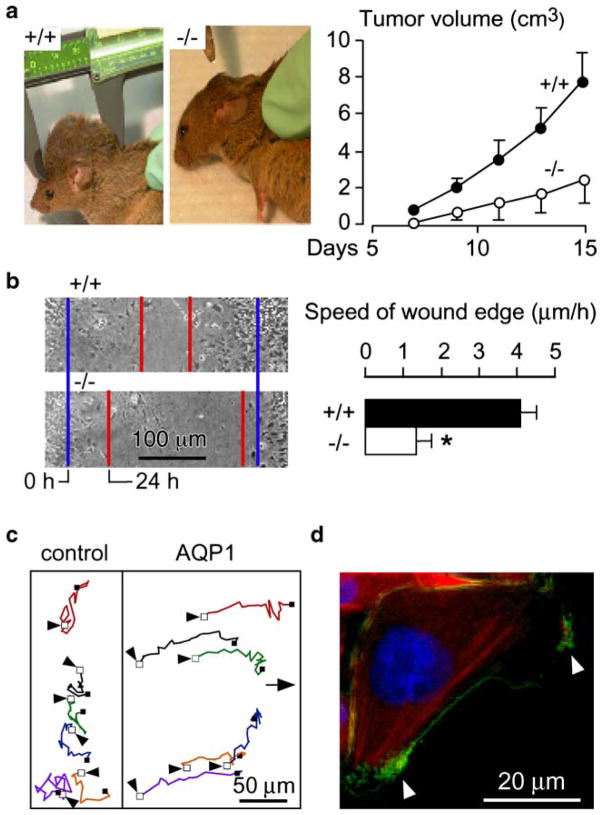
AQP1 is expressed in vascular endothelial cells throughout the body, except in the central nervous system [8, 40]. Our initial observation was that AQP1 deletion in mice reduces tumor growth after subcutaneous injection of melanoma cells in mice (Fig. 1a), which was associated with increased tumor necrosis and reduced blood vessel formation within the tumor bed [31]. In experiments designed to elucidate the mechanism of defective tumor angiogenesis in AQP1 deficiency, we discovered that cultured aortic endothelial cells from AQP1 null mice migrate more slowly toward a chemotactic stimulus compared with AQP1-expressing endothelial cells. Further experiments were done using AQP1-, AQP4-, and green fluorescent protein (GFP)-transfected cell lines. In two standard cell migration assays, in vitro wound healing (Fig. 1b,c) and transwell migration, we again observed faster migration in the AQP-expressing cell lines, but not in the GFP-expressing cells.
Fig. 1.
Impaired tumor growth and endothelial cell migration in AQP1 null mice. a Left. Tumor in an AQP1+/+ vs an AQP1−/− mouse, 2 weeks after subcutaneous injection of 106 B16F10 melanoma cells. Right. Tumor growth data (ten mice per group, mean ± SEM, P<0.001). b Left. Wound healing of cultured endothelial cells (initial wound edge, blue; after 24 h, red). Right. Wound edge speed (n=4 per group, mean ± SEM, *P<0.01). c Tracks of six migrating CHO cells expressing AQP1 vs six non-AQP expressing control CHO cells, tracked for 4 h. Initial cell position indicated by arrow. d AQP1 protein (green) polarization to lamellipodia (arrows) in a migrating CHO cell. For more details, see [31]
These findings imply that AQP-dependent cell migration may be a general phenomenon, independent of AQP and cell type. Interestingly, in migrating cells, AQP1 becomes polarized to the front end of cells (Fig. 1d) and is associated with increased turnover of cell membrane protrusions, suggesting an important role for AQPs at the leading edge of migrating cells.
AQP4 and astrocyte migration
In normal brain, AQP4 is expressed strongly in astrocytes, where it plays a major role in the formation and absorption phases of brain edema, by controlling water flow into and out of the brain [19, 24, 25]. After injury to the brain, AQP4 becomes upregulated in reactive astrocytes, which form throughout the central nervous system and migrate toward the site of injury to form a glial scar [16]. Similar to the observation that AQP1 deletion slows endothelial cell migration, we found that AQP4 deletion slows astrocyte migration in vitro in the transwell (Fig. 2a) and in vitro wound assays, which was associated with delayed glial scar formation in vivo [32]. In that study, we again noted more cell membrane protrusions (i.e., increased cell membrane irregularity, quantified using fractals) at the leading edge of migrating AQP4-expressing compared with non-expressing astrocytes (Fig. 2b), as well as polarization of AQP4 to the leading edge of migrating cells (Fig. 2c). Astrocyte migration speed in vitro was altered by exposing cells to osmotic gradients with enhanced migration toward hypo-osmolality and reduced migration toward hyper-osmolality, suggesting that cell migration involves water flow into the front end of the cell. Even more pronounced differences in migration between AQP4-expressing and non-expressing astrocytes were found in a novel in vivo assay [1].
Fig. 2.
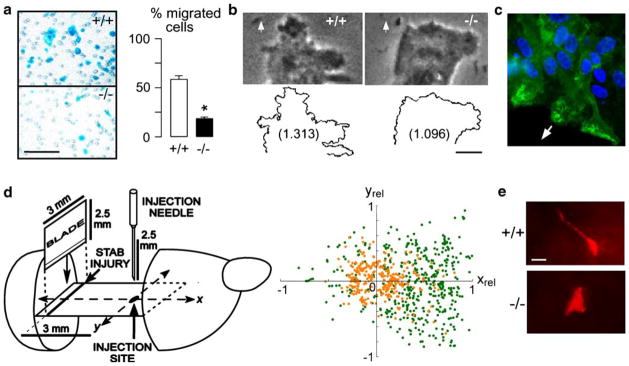
Slowed migration of AQP4 null astrocytes. a Left. Representative photos of Boyden chamber migration assay showing AQP4+/+ and AQP4−/− astrocytes (blue) after scraping off the non-migrated cells. In these experiments, astrocytes were plated on the top chamber of poly-L-lysine coated transwells and were allowed to migrate through 8-μm pores using 10% FBS as chemoattractant. Bar = 100 μm. Right. Summary of migration experiments (15 AQP4+/+ vs 13 AQP4−/− transwells, mean ± SEM, *P<0.001). b Phase contrast micrographs (top) and outline (bottom) of the leading end of a migrating AQP4+/+ and AQP4−/− astrocyte in the in vitro wound assay. Arrows show direction of migration. Numbers are fractal dimensions (larger number denotes more irregular cell membrane). Bar = 10 μm. c AQP4 protein (green) polarization to the front end of migrating astrocytes in the in vitro wound assay. Arrow shows direction of migration. d Left. Stab injury/cell injection model of astrocyte migration in mouse brain. Two days before cell injection, a stab was created as shown. Cultured AQP4+/+ and AQP4−/− astrocytes were fluorescently labeled and injected as indicated. Right. Locations of migrating fluorescently stained AQP4+/+ cells (green) and AQP4−/− cells (orange). The x-axis is shown as percentage of distance between injection and stab injury sites (xrel). Data obtained from 15 different mice. e High-magnification fluorescence micrographs of a migrating AQP4+/+ and AQP4−/− cell. Bar = 5 μm. For further details, see [1, 32]
In these experiments, mouse brain was injured by a stab wound 2 days before injection of fluorescently labeled, AQP4-expressing and non-expressing astrocytes 3 mm away from the stab (Fig. 2d, left). Two days after injecting the astrocytes, the brains were fixed and sectioned, and the coordinates of fluorescent astrocytes in relation to the injection and stab injury sites were determined. Remarkably greater migration toward the injury site was found for the AQP4-expressing astrocytes (Fig. 2d, right) associated with more elongation in the AQP4 expressing vs non-expressing migrating astrocytes (Fig. 2e). Together, these observations suggest that AQP4 deficiency slows glial scar formation after injury. Because glial scarring is the major impediment to neuronal regeneration in the central nervous system after injury [35], AQP4 inhibition might provide a new approach to augment axonal sprouting and synaptogenesis in the brain.
AQP1 and tumor spread
Migration is a key component of tumor spread, including local tumor cell infiltration into surrounding tissue as well as distant metastases. In a proof-of-concept study to test whether AQPs might be involved in tumor spread and metastasis, AQP1-expressing and non-expressing tumor cells (B16F10 melanoma and 4T1 breast tumor) were injected intravenously into mice [13]. The AQP1-expressing tumor cells migrated faster than the non-expressing in in vitro, as predicted, and their extravasation into lung tissue at 6 h after intravenous injection was significantly enhanced. The mice injected with AQP1-expressing melanoma cells developed more lung metastases at 3 weeks after injection (Fig. 3a, b). Histological examination of metastatic tumors (Fig. 3b) as well as subcutaneously implanted tumors revealed that the AQP1-expressing tumors were more infiltrative compared with AQP1-null tumors.
Fig. 3.
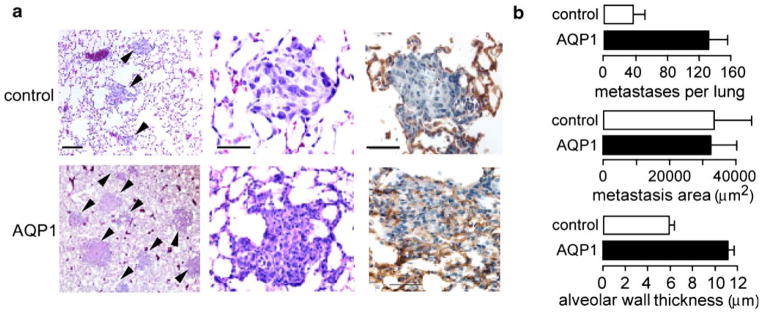
AQP1 expression increases lung metastasis after tail vein injection of tumor cells. a Left and middle. Hematoxylin and eosin staining of paraformaldehye-fixed paraffin-embedded sections of mouse lung tissue at 14 days after tail vein injection of 106 control or AQP1-expressing 4T1 breast tumor cells. Tumor metastases indicated by arrows. Right. AQP1 immunohistochemistry showing labeling (brown) of alveoli/vessels in both micrographs, with AQP1 also in tumor cells in the lower micrograph. b Data summary showing number of metastases per lung, area of tumor colonies, and alveolar wall thickness within 50 μm of metastases (five mice per group, mean ± SEM, *P<0.02). See [13] for further details
These results provide evidence for AQP involvement in cancer spread. AQPs are upregulated in a variety of human cancers [12, 17, 21, 30, 42], and where studied, AQP expression correlates with higher tumor grade. For example, AQP4 expression is greatly upregulated in diffuse astrocytomas, with more prominent AQP4 expression in the most malignant grade IV astrocytoma (glioblastoma) [30, 42].
Other reports of AQP-dependent cell migration
Since the original observations of delayed migration in AQP1-deficient endothelial cells and astrocytes, several other studies have confirmed AQP-dependent cell migration. Renal proximal tubule epithelial cells expressing AQP1 migrate faster than AQP1-deficient cells, resulting in an exaggerated response of the proximal tubule to ischemic injury in AQP1 deficiency [10]. In the skin, AQP3 expression increases keratinocyte migration and proliferation, with impaired wound healing in AQP3-deficient mice [11]. In the eye, AQP3 expression increases migration and proliferation in corneal epithelial cells, with impaired corneal resurfacing after a corneal wound [15]. AQP3 deletion in mice also impairs enterocyte regeneration in the colon by a mechanism that in part involves impaired migration, with AQP3 null mice having greatly worse outcomes than wild-type mice in models of chemically induced colitis [37].
AQP3 has also been found to be expressed in cultured fibroblasts, with AQP3 knockdown by RNA inhibition reducing fibroblast migration [3]. Transfection of different cell lines with wild-type AQP9 (but not mock or serine-substituted AQP9 mutants) induced the formation of plasma membrane protrusions (filopodia) and increased plasma membrane water permeability [18]. These findings again emphasize that increased water flow through AQP channels is critically involved in the formation of cell membrane protrusions during cell migration. In a further study, AQP expression in glioma cell lines enhanced cell migration in vitro [20] raising the possibility that the strong expression of AQP4 reported in human malignant gliomas, which are highly infiltrative tumors, may facilitate the infiltration of tumor cells into normal brain [30, 42]. Together, these findings provide further evidence that AQP-facilitated cell migration is a general phenomenon independent of AQP or cell type.
Proposed molecular mechanisms
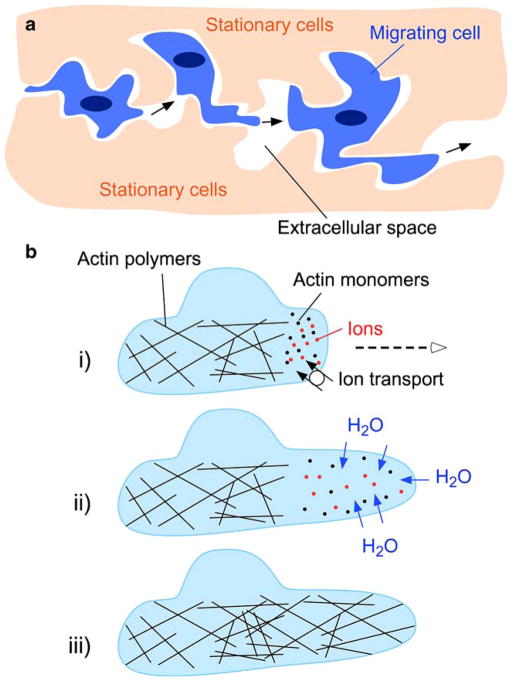
The mechanisms by which AQPs enhance cell migration are the subject of the ongoing investigation. The enhanced cell migration found for multiple structurally different AQPs (AQP1, AQP3, AQP4, AQP9) independent of their modulation method (transfection, knock-out, RNA inhibition) suggests that AQP-facilitated transmembrane water transport is the responsible mechanism. One way by which AQPs may accelerate cell migration is by facilitating the rapid changes in cell volume, which accompany the changes in cell shape that occur as the migrating cell squeezes through the irregularly shaped extracellular space (Fig. 4a). Water flow across the cell membrane may also allow the migrating cell to generate hydrostatic forces that “push apart” adjacent stationary cells. This mechanism, however, does not account for the polarization of AQPs to the front end of migrating cells or for the association between AQP expression and the increased cell shape irregularity anteriorly.
Fig. 4.
Proposed mechanisms of AQP involvement in cell migration. a Schematic showing changes in cell shape, which take place as cells migrate through the extracellular space. AQP expression may facilitate the transmembrane water movements that mediate rapid changes in cell volume. b i) Actin depolymerization and ionic influx increase osmolality at the front end of the cell. ii) Water influx across the cell membrane increases local hydrostatic pressure causing cell membrane expansion, which forms a protrusion. AQP polarization to the front end of the cell facilitates water flow into the cell. iii) Actin re-polymerizes to stabilize the emerging protrusion. See text for further explanations
These observations support another role for water movement into and out of the leading edge during cell migration. AQP1 was found recently by single particle imaging methods to be rapidly mobile throughout the cell plasma membrane [5], allowing its rapid polarization to the leading edge of migrating cells. AQP4 expression in intact astrocytes in vivo is normally polarized to the astrocyte foot processes [23, 27], although non-polarized expression is seen in cultured astrocytes [36]; therefore, the molecular machinery to polarize and unpolarize AQP4 is already present in astrocytes. The precise molecular mechanisms responsible for AQP polarization in cells are not known, but for AQP4 there is evidence that a complex of intracellular proteins including α-syntrophin plays a crucial role [22].
A proposed mechanism by which AQPs may facilitate cell migration, which takes into consideration AQP polarization, is shown in Fig. 4b. According to this hypothesis, actin de-polymerization and ion influx increase cytoplasmic osmolality at the front end of the migrating cell. Changes in actin polymerization [6, 7] and transmembrane ion movements (mediated by the ion exchangers Na+/H+ and ) [34] at the front end of migrating cells are well-documented. The Na+/H+ ion exchanger polarizes to the leading edge of migrating cells. It has been suggested that ion transporters mediate changes in cell volume during migration such that water enters through the plasma membrane at the leading end, producing localized cell swelling, and exits the plasma membrane from the rear, producing localized cell shrinkage [34]. Consistent with the idea that water flows into and out of migrating cells are reports that migration can be inhibited or accelerated by changing the osmolality of the extracellular medium [9, 26, 32].
We propose that water influx then causes expansion of the adjacent plasma membrane by increased local hydrostatic pressure. This is followed by rapid actin re-polymerization to stabilize the cell membrane protrusion. Recent evidence shows that regional hydrostatic pressure changes within cells do not equilibrate throughout the cytoplasm on scales of 10 μm and 10 s [4], and could thus contribute to the formation of localized cell membrane protrusions. It has been suggested that there may be different modes of cell migration, including a primarily actin-mediated mode as well as an amoeboid mode [33]. The latter may involve the formation of plasma membrane blebs by changes in intracellular hydrostatic pressure, and is reminiscent of the migration of leukocytes, cancer cells, and the prokaryote Dictyostelium (amoeba) [2]. We speculate that AQPs may play a role in amoeboid cell migration, which is also sensitive to changes in extracellular osmolality. Although the mechanism in Fig. 4b could explain the involvement of polarized AQP in the migration process, direct measurements of water flow across the leading edge of migrating cells are needed for validation.
Conclusions
A growing body of evidence suggests that AQPs facilitate cell migration. Despite the involvement of AQP1 in endothelial cell migration and AQP4 in astrocyte migration, AQP1- and AQP4-null mice develop normally, indicating that AQPs are not an absolute requirement for cell migration. Rather, AQPs are conducive to migration by facilitating the rapid cell volume changes and augmenting cell propulsion as discussed above. Even in the absence of AQPs, water still crosses the cell membrane through the lipid bilayer, albeit more slowly than through AQP pores, so that cell migration still occurs at a lower speed. For example, we found recently that retinal angiogenesis in a neonatal hyperoxia model is AQP1-independent [29], indicating that AQPs are not essential for cell migration.
The emerging roles of water movement in cell migration are not only important in our mechanistic understanding of the migration process, but may also have a wide range of therapeutic implications including augmentation of wound healing (AQP3 activator), reduction of glial scarring and glioma infiltration (AQP4 inhibitor), and reduction of tumor growth (AQP1 inhibitor). Currently, non-toxic AQP-modulating drugs are not available, but their search is the subject of considerable interest.
Acknowledgments
Supported by grants EB00415, DK35124, EY13574, HL59198, DK72517, and HL73856 from the National Institutes of Health, and Research Development Program and Drug Discovery grants from the Cystic Fibrosis Foundation (to ASV), and by a Wellcome Trust Clinician-Scientist Fellowship (to MCP).
Contributor Information
M. C. Papadopoulos, Department of Medicine and Physiology, University of California, San Francisco, CA, USA, Academic Neurosurgery Unit, St. George’s, University of London, London, UK
S. Saadoun, Department of Medicine and Physiology, University of California, San Francisco, CA, USA, Academic Neurosurgery Unit, St. George’s, University of London, London, UK
A. S. Verkman, Email: Alan.Verkman@ucsf.edu, Department of Medicine and Physiology, University of California, San Francisco, CA, USA, University of California at San Francisco, 1246 Health Sciences East Tower, San Francisco, CA, USA, URL: http://www.ucsf.edu/verklab
References
- 1.Auguste KI, Jin S, Uchida K, Yan D, Manley GT, Papadopoulos MC, Verkman AS. Greatly impaired migration of implanted aquaporin-4-deficient astroglial cells in mouse brain toward a site of injury. FASEB J. 2007;21:108–116. doi: 10.1096/fj.06-6848com. [DOI] [PubMed] [Google Scholar]
- 2.Barber MA, Welch HC. PI3K and RAC signalling in leukocyte and cancer cell migration. Bull Cancer. 2006;93:E44–E52. [PubMed] [Google Scholar]
- 3.Cao C, Sun Y, Healey S, Bi Z, Hu G, Wan S, Kouttab N, Chu W, Wan Y. EGFR-mediated expression of aquaporin-3 is involved in human skin fibroblast migration. Biochem J. 2006;400:225–234. doi: 10.1042/BJ20060816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charras GT, Yarrow JC, Horton MA, Mahadevan L, Mitchison TJ. Non-equilibration of hydrostatic pressure in blebbing cells. Nature. 2005;435:365–369. doi: 10.1038/nature03550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crane J, Verkman AS. Long-range non-anomalous diffusion of quantum dot-labeled aquaporin-1 water channels in the cell plasma membrane. Biophys J. 2007 doi: 10.1529/biophysj.107.115121. http://dx.doi.org/10.1529/biophysj.107.115121 (in press) [DOI] [PMC free article] [PubMed]
- 6.Diez S, Gerisch G, Anderson K, Muller-Taubenberger A, Bretschneider T. Subsecond reorganization of the actin network in cell motility and chemotaxis. Proc Natl Acad Sci U S A. 2005;102:7601–7606. doi: 10.1073/pnas.0408546102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Disanza A, Steffen A, Hertzog M, Frittoli E, Rottner K, Scita G. Actin polymerization machinery: the finish line of signaling networks, the starting point of cellular movement. Cell Mol Life Sci. 2005;62:955–970. doi: 10.1007/s00018-004-4472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dolman D, Drndarski S, Abbott NJ, Rattray M. Induction of aquaporin 1 but not aquaporin 4 messenger RNA in rat primary brain microvessel endothelial cells in culture. J Neurochem. 2005;93:825–833. doi: 10.1111/j.1471-4159.2005.03111.x. [DOI] [PubMed] [Google Scholar]
- 9.Dong C, Aznavoorian S, Liotta LA. Two phases of pseudopod protrusion in tumor cells revealed by a micropipette. Microvasc Res. 1994;47:55–67. doi: 10.1006/mvre.1994.1005. [DOI] [PubMed] [Google Scholar]
- 10.Hara-Chikuma M, Verkman AS. Aquaporin-1 facilitates epithelial cell migration in kidney proximal tubule. J Am Soc Nephrol. 2006;17:39–45. doi: 10.1681/ASN.2005080846. [DOI] [PubMed] [Google Scholar]
- 11.Hara-Chikuma M, Verkman AS. Aquaporin-3 facilitates epidermal cell migration and proliferation during wound healing. J Mol Med. 2007 doi: 10.1007/s00109-007-0272-4. (in press) [DOI] [PubMed] [Google Scholar]
- 12.Hoque MO, Soria JC, Woo J, Lee T, Lee J, Jang SJ, Upadhyay S, Trink B, Monitto C, Desmaze C, Mao L, Sidransky D, Moon C. Aquaporin 1 is overexpressed in lung cancer and stimulates NIH-3T3 cell proliferation and anchorage-independent growth. Am J Pathol. 2006;168:1345–1353. doi: 10.2353/ajpath.2006.050596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu J, Verkman AS. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. FASEB J. 2006;20:1892–1894. doi: 10.1096/fj.06-5930fje. [DOI] [PubMed] [Google Scholar]
- 14.Jaeger M, Carin M, Medale M, Tryggvason G. The osmotic migration of cells in a solute gradient. Biophys J. 1999;77:1257–1267. doi: 10.1016/S0006-3495(99)76977-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin MH, Verkman AS. Aquaporin-3-dependent cell migration and proliferation during corneal re-epithelialization. Invest Ophthalmol Vis Sci. 2006;47:4365–4372. doi: 10.1167/iovs.06-0335. [DOI] [PubMed] [Google Scholar]
- 16.Lim JH, Gibbons HM, O’Carroll SJ, Narayan PJ, Faull RL, Dragunow M. Extracellular signal-regulated kinase involvement in human astrocyte migration. Brain Res. 2007;1164C:1–13. doi: 10.1016/j.brainres.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 17.Liu YL, Matsuzaki T, Nakazawa T, Murata S, Nakamura N, Kondo T, Iwashina M, Mochizuki K, Yamane T, Takata K, Katoh R. Expression of aquaporin 3 (AQP3) in normal and neoplastic lung tissues. Hum Pathol. 2007;38:171–178. doi: 10.1016/j.humpath.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Loitto VM, Huang C, Sigal YJ, Jacobson K. Filopodia are induced by aquaporin-9 expression. Exp Cell Res. 2007;313:1295–1306. doi: 10.1016/j.yexcr.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 19.Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- 20.McCoy E, Sontheimer H. Expression and function of water channels (aquaporins) in migrating malignant astrocytes. Glia. 2007;55:1034–1043. doi: 10.1002/glia.20524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon C, Soria JC, Jang SJ, Lee J, Obaidul Hoque M, Sibony M, Trink B, Chang YS, Sidransky D, Mao L. Involvement of aquaporins in colorectal carcinogenesis. Oncogene. 2003;22:6699–6703. doi: 10.1038/sj.onc.1206762. [DOI] [PubMed] [Google Scholar]
- 22.Neely JD, Amiry-Moghaddam M, Ottersen OP, Froehner SC, Agre P, Adams ME. Syntrophin-dependent expression and localization of Aquaporin-4 water channel protein. Proc Natl Acad Sci U S A. 2001;98:14108–14113. doi: 10.1073/pnas.241508198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papadopoulos MC, Manley GT, Krishna S, Verkman AS. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004;18:1291–1293. doi: 10.1096/fj.04-1723fje. [DOI] [PubMed] [Google Scholar]
- 25.Papadopoulos MC, Verkman AS. Aquaporin-4 and brain edema. Pediatr Nephrol. 2007;22:778–784. doi: 10.1007/s00467-006-0411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabinovitch M, DeStefano MJ. Spontaneous migration of normal human polymorphonuclear neutrophils under agarose: enhancement by media of lowered pH or osmolality. J Reticuloendothel Soc. 1981;29:329–339. [PubMed] [Google Scholar]
- 27.Rash JE, Yasumura T, Hudson CS, Agre P, Nielsen S. Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membranes in rat brain and spinal cord. Proc Natl Acad Sci U S A. 1998;95:11981–11986. doi: 10.1073/pnas.95.20.11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz-Ederra J, Verkman AS. Aquaporin-1 independent microvessel proliferation in a neonatal mouse model of oxygen induced retinopathy. Invest Ophthalmol Vis Sci. 2007;48:4802–4810. doi: 10.1167/iovs.07-0537. [DOI] [PubMed] [Google Scholar]
- 30.Saadoun S, Papadopoulos MC, Davies DC, Krishna S, Bell BA. Aquaporin-4 expression is increased in oedematous human brain tumours. J Neurol Neurosurg Psychiatry. 2002;72:262–265. doi: 10.1136/jnnp.72.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–792. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- 32.Saadoun S, Papadopoulos MC, Watanabe H, Yan D, Manley GT, Verkman AS. Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J Cell Sci. 2005;118:5691–5698. doi: 10.1242/jcs.02680. [DOI] [PubMed] [Google Scholar]
- 33.Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5:711–719. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]
- 34.Schwab A, Nechyporuk-Zloy V, Fabian A, Stock C. Cells move when ions and water flow. Pflugers Arch. 2007;453:421–432. doi: 10.1007/s00424-006-0138-6. [DOI] [PubMed] [Google Scholar]
- 35.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 36.Solenov E, Watanabe H, Manley GT, Verkman AS. Sevenfold-reduced osmotic water permeability in primary astrocyte cultures from AQP-4-deficient mice, measured by a fluorescence quenching method. Am J Physiol Cell Physiol. 2004;286:C426–C432. doi: 10.1152/ajpcell.00298.2003. [DOI] [PubMed] [Google Scholar]
- 37.Thiagarajah JR, Zhao D, Verkman AS. Impaired enterocyte proliferation in aquaporin-3 deficiency in mouse models of colitis. Gut. 2007 doi: 10.1136/gut.2006.10462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Haastert PJ, Devreotes PN. Chemotaxis: signalling the way forward. Nat Rev Mol Cell Biol. 2004;5:626–634. doi: 10.1038/nrm1435. [DOI] [PubMed] [Google Scholar]
- 39.Verkman AS. More than just water channels: unexpected cellular roles of aquaporins. J Cell Sci. 2005;118:3225–3232. doi: 10.1242/jcs.02519. [DOI] [PubMed] [Google Scholar]
- 40.Verkman AS. Aquaporins in endothelia. Kidney Int. 2006;69:1120–1123. doi: 10.1038/sj.ki.5000226. [DOI] [PubMed] [Google Scholar]
- 41.Vicente-Manzanares M, Webb DJ, Horwitz AR. Cell migration at a glance. J Cell Sci. 2005;118:4917–4919. doi: 10.1242/jcs.02662. [DOI] [PubMed] [Google Scholar]
- 42.Warth A, Kroger S, Wolburg H. Redistribution of aquaporin-4 in human glioblastoma correlates with loss of agrin immunore-activity from brain capillary basal laminae. Acta Neuropathol (Berl) 2004;107:311–318. doi: 10.1007/s00401-003-0812-0. [DOI] [PubMed] [Google Scholar]