Abstract
The objective was to evaluate the effect of separate interventions on antimicrobial prescribing for uncomplicated upper respiratory tract infections. The authors conducted a quasi-experimental pre-post study with concurrent control groups for each intervention. Academic detailing led to a significant reduction in unnecessary antibiotic prescribing. However, there was no significant change in antibiotic prescribing in response to educational mailings to providers or to provider involvement in patient mailings. Organizations that seek to reduce inappropriate use of antibiotics should use proven approaches, even when they are more expensive. (Population Health Management 2013;16:22–27)
Introduction
Acute bronchitis and other typically viral upper respiratory tract infections are among the most common diagnoses in ambulatory adult primary care medical practice.1 Although there may be a benefit to using antimicrobials in selected acute bronchitis patients with chronic obstructive pulmonary disease and asthma,2,3 systematic reviews have concluded that current evidence supports the existence of no, or at best limited, clinical benefit of antimicrobials in otherwise healthy patients.4
Antimicrobial use and overuse results in adverse drug reactions,5,6 unnecessary drug expenditures,7,8 and contributes to the development of antimicrobial drug resistance and risk of antimicrobial-resistant infections.9–11 Of special relevance to outpatients is the rise in penicillin-resistant Streptococcus pneumoniae and the association of prior antimicrobial use with infections with this pathogen.12 Given these concerns, practice guidelines and editorials recommend against prescribing antimicrobials for nonspecific respiratory tract infections such as acute bronchitis.13,14 Although antibiotic prescribing for acute bronchitis in adults has declined somewhat in the previous decade, the prevalence of prescribing remains over 50%, and the use of broad-spectrum antimicrobials actually has increased.15
Few studies have demonstrated the effectiveness of programs to reduce the use of antimicrobials for acute bronchitis or other upper respiratory infections. The most successful intervention has been multidimensional and included a patient mailing, in-office patient educational materials, the use of provider opinion leaders, and provider education.16 This is in keeping with theories of human behavior such as the PRECEDE model, which suggest that such multidimensional interventions are most likely to be successful.17 However, such approaches are prohibitively expensive in many settings, and it is not known which elements of the multidimensional intervention are most important and which may be unnecessary.
In late 1999, prompted by the results of national data,5 the investigators performed an internal audit of antimicrobial use practices at the Clinical Practices of the University of Pennsylvania (CPUP), and subsequently launched a remediation program as a quality improvement effort. Their objective was to evaluate the impact of 2 separate interventions on the prevalence of antimicrobial prescribing for upper respiratory tract infection: (1) intensive academic detailing of providers with high rates of antibiotic prescribing, and (2) provider involvement in patient educational mailings regarding the inappropriate use of antibiotics for upper respiratory tract infections.
Methods
Academic detailing study
Overview
The authors implemented a pre-post study with an untreated comparison group in the form of a repeated cross-sectional design to evaluate the combination of an academic detailing intervention with patient and provider education materials. The study was set in the Clinical Care Associates (CCA) and the CPUP. The CPUP practice providers are faculty at the University of Pennsylvania; CCA practices include nonfaculty providers who are affiliated with the University.
Provider subjects for an intensive intervention group and a mild intervention group were selected from the CPUP providers. The 7 CPUP providers with the highest prevalence of antibiotic prescribing for acute bronchitis in 1998 were selected for the intensive intervention group. The 7 CPUP providers with the next highest rate of antibiotic prescribing for acute bronchitis were selected for the mild intervention group. A no intervention group of 14 providers were selected from the CCA practices. Although the initial evaluation and intervention did not include the CCA practices, the authors selected 14 CCA providers based on the number of acute bronchitis visits in 1998, matching each one to the 14 total selected CPUP providers.
Patients of study providers were identified using health system electronic records. For each provider, the authors identified 15 patients with a visit for upper respiratory infection in 1998 (the baseline year) and 15 in 2000 (the year following the intervention). Individual patients were included only once. The inclusion diagnoses were: acute bronchitis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 466.0); bronchitis, not otherwise specified (ICD-9-CM code 490); cough (ICD-9-CM code 786.2); acute pharyngitis (ICD-9-CM code 462); and acute upper respiratory infection, not otherwise specified (ICD-9-CM code 465.9).
A diagnosis of chronic bronchitis (ICD-9-CM code 491.*) or emphysema (ICD-9-CM codes 492.*, 518.1, 518.2) at any time in the patient's recorded history served as an exclusion criterion, as did any of the study diagnoses within 60 days prior to the index visit, to avoid visits associated with primary treatment failure. In addition, a diagnosis of acute or chronic sinusitis (ICD-9-CM codes 461.*, 473.*) or pneumonia (ICD-9-CM codes 481.*, 482.*, 483.*, 484.*, 485.*, 486.*) within 60 days prior to the index visit also served as an exclusion criterion.
Intervention
The intensive intervention group received academic detailing. A pharmacist and an opinion leader in antibiotic use (director of the hospital Antimicrobial Stewardship Program) together met with each intensive intervention provider (6 in person, 1 by telephone). During these interventions, published literature and the provider-specific evaluation results were presented. The providers were provided with 2 sets of patient-oriented printed educational materials. The first was a “prescription pad,” developed by the Centers for Disease Control and Prevention, which contains space for the provider to indicate symptomatic treatment modalities. The second consisted of pads of patient information sheets explaining the need to refrain from antibiotic use in the setting of acute bronchitis; the patient information sheets were adapted from material used in a previous study.16 The educational materials used for the intensive intervention group were mailed to all CPUP providers in the mild intervention group in October of 1999.
Outcome
The study outcome was the proportion of visits for acute bronchitis or upper respiratory infection for which there was prescription of at least 1 antibacterial antibiotic either during an office visit or during a telephone consultation for which the visit was a follow-up. A clinical research pharmacist abstracted data from medical records at each practice site using a structured form.
Statistical analysis
First, the prevalence of antibiotic prescribing by each provider intervention group at each time point (pre vs. post) was described. The authors then sought to estimate the differences in the impact of the intervention using observations made at 2 points in time (ie, does each intervention lead to a greater reduction in antibiotic prescribing than a comparator group?). To estimate this effect, a generalized linear model was used with time and intervention groups as main effects, and a time-by-intervention interaction term. The regression model was fit using robust estimation methods that account for correlation among providers.9
Patient mailing intervention study
Overview
The authors implemented a repeated cross-sectional design with an untreated control group to evaluate the effect of a provider-approved patient educational mailing on the prevalence of antimicrobial prescribing by providers. The design repeated observations at the physician level but was cross-sectional in that at each time period the patients, who served as the units of analysis, differed.
Twenty CPUP providers were selected to be the intervention group: providers in practice during all of 2000–2003 (the study period) who had the highest number of visits for the inclusion diagnoses. These physicians also were required to be in a practice subgroup that used the health system's ambulatory electronic medical record system (EpicCare; Epic Systems Corporation, Verona, WI). The 7 providers who received academic detailing in the prior study were excluded. The 20 CCA providers with the highest number of inclusion diagnosis visits during the study period were selected for the control group. If there were not 20 providers available within CPUP or CCA who had been in the practice during the entire study period, other providers (and their patients) were included if they had data from at least 1 preintervention and 1 intervention observation period.
For each of these providers, the authors identified 15 patients—or as many as available if fewer than 15—with a visit for an upper respiratory infection excluding the period when the interventions took place (September to January) from 2000 through 2003. Thus, for each provider, they selected 15 patients for each from February 1, 2001 to August 31, 2001 and from February 1, 2000 to August 31, 2000 (the preintervention periods), and patients for each during each of the 2 post-intervention periods: from February 1, 2002 to August 31, 2002, and from February 1, 2003 to August 31, 2003. Inclusion and exclusion ICD-9-CM diagnoses were the same as described for the academic detailing study. Patients may have been selected for more than 1 point in time during the study, but were excluded if their selected visits included providers in both the intervention and control groups. An additional eligible patient replaced each patient who was excluded. If there were more than 15 eligible patient visits for a provider during one of the sampling periods, then 15 patients were randomly selected from the identified eligible patients for that provider and period.
Interventions
Between September 1, 2001 and January 1, 2002, an educational brochure, titled “Head & Chest Colds,” as well as an explanatory letter signed by the patient's provider was mailed to patients who received an upper respiratory infection diagnosis during a provider visit to a CPUP practice in the prior 2 years. A second mailing was sent between September 1, 2002 and January 1, 2003 to patients who had one of these diagnoses in the prior year. The second mailing only included patients with eligible visits to a provider in CPUP who was using the health system's ambulatory electronic medical record. For all mailings, the provider was given the option of selecting some or all patients in their practice who should not receive these materials. If the provider preferred, the director of the Antimicrobial Stewardship Program signed the letter instead of the patient's provider. In addition, intervention group providers had previously received 2 sets of patient-oriented printed educational materials (as described in the academic detailing study).
Outcome
The outcome was the proportion of visits for acute bronchitis or upper respiratory infection with prescription of an antibiotic. Among visits with antibiotic prescriptions, the authors also examined whether the antimicrobial(s) prescribed were broad or narrow spectrum. Antimicrobials considered broad spectrum were azithromycin, clarithromycin, amoxicillin-clavulanate, fluoroquinolones, and 2nd and 3rd generation cephalosporins.15 All other antimicrobials were classified as narrow spectrum. Data were abstracted from the electronic medical record for intervention group (CPUP) providers, and from on-site paper medical records for control (CCA) providers. Trained research assistants abstracted the medical records using a structured data abstraction form.
Statistical analysis
The authors first described the prevalence of the outcome (antibiotic prescribing in eligible visits) for each observation period for the intervention and control groups. They then used a piecewise generalized linear regression model.18,19 Regression models were fit using robust estimation methods that account for correlation within each provider.20 A mixed effects logistic regression21 was used for the primary method of analysis, with a random intercept for provider and a random slope for time. This model permitted estimating the key estimate (ie, the time by intervention interaction term), while allowing variation across providers in both the baseline rate of prescribing (intercept) and the degree of change over time (slope).
In secondary analyses, the authors examined the effect of the intervention on the prescription of broad- vs. narrow-spectrum antibiotics. Analyses were performed using SAS v 9.1 (SAS Institute, Inc., Cary, NC). The University of Pennsylvania Institutional Review Board approved these studies.
Results
Academic detailing study
The number of patients for each intervention group, and measure of association of the intervention with the prevalence of antibiotic prescribing are shown in Table 1. There was a significant reduction in the prevalence of antibiotic prescribing in the academic detailing (intensive intervention) group from before to after the intervention (43% to 33%). This association was consistently present when examining the change in prescribing over time for the intensive intervention group alone, when comparing the high intervention group to no intervention, and when controlling for potential confounders in the latter analysis.
Table 1.
Effect of Academic Detailing on Antibiotic Prescribing
| |
|
|
Effect of Intervention |
||
|---|---|---|---|---|---|
| |
|
|
|
Comparison to control (no intervention), ROR (CI) |
|
| Intervention | Providers (n) | Patients, pre/post (n) | Change over time within group, OR (CI) | Unadjusted | Adjusted* |
| Academic detailing | 7 | 119/103 | 0.49 (0.26–0.89) | 2.60 (1.23–5.48) | 2.80 (1.32–5.95) |
| Patient mailings | 7 | 91/91 | 0.76 (0.38–1.51) | 1.67 (0.74–3.79) | 1.66 (0.73–3.80) |
| None | 14 | 188/216 | 1.27 (0.82–1.94) | — | — |
Adjusted for sex, smoking, and antibiotic prescription.
CI, 95% confidence interval; OR, odds ratio; ROR, ratio of ORs.
In contrast, there was no significant change in antibiotic use over time in the no intervention (control) group, the mild intervention group (patient-oriented educational materials only), nor when the control and mild intervention groups were combined.
Patient mailing intervention study
In the intervention group, there were a total of 1344 patient visits over the 4 observation periods. The intervention group had 48 providers from 2 practices. The control group had 22 providers from 13 practices. The baseline (preintervention) prevalence of antibiotic prescribing was much lower in the intervention group than the control group (Table 2). Although there was a slight reduction in antibiotic prescribing over time in the intervention group, it started before the intervention and was not significantly associated with the intervention. The prescribing rates pre-post for the intervention group were 18.9% versus 14.2% (4.7% decrease) while for the control group they were 57.8% versus 58.6% (1.2% increase). These changes observed in the control and intervention groups were not significantly different from each other (P=0.133).
Table 2.
Antibiotic Prevalence by Intervention Group and Time in the Patient Mailing Study
| |
Time Period (year) |
|||
|---|---|---|---|---|
| |
|
Intervention |
||
| Group | Preintervention 2000 | 2001 | 2002 | 2003 |
| Control | ||||
| Number of visits with an antibiotic prescribed | 191 | 174 | 187 | 186 |
| Total number of visits for an upper respiratory tract infection | 320 | 312 | 317 | 320 |
| Prevalence of antibiotic use | 59.7% | 55.8% | 59.0% | 58.1% |
| Intervention | ||||
| Number of visits with an antibiotic prescribed | 60 | 47 | 61 | 50 |
| Total number of visits for an upper respiratory tract infection | 254 | 312 | 386 | 392 |
| Prevalence of antibiotic use | 23.6% | 15.1% | 15.8% | 12.8% |
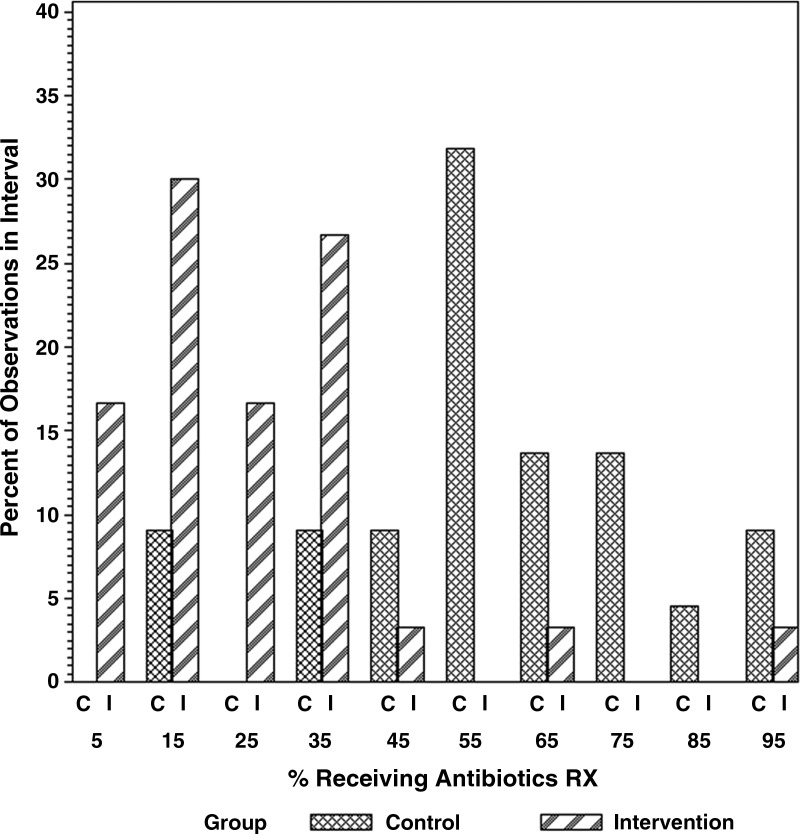
In secondary analyses, among visits during which antibiotics were prescribed, there was no change in the use of broad versus narrow-spectrum antibiotics associated with the intervention (data not shown). Interestingly, wide variation was found in the prevalence of antibiotic prescribing between individual providers and between practices (Fig. 1).
FIG. 1.
Variability in baseline prevalence of antibiotic prescribing by individual providers in the control and intervention groups.
Discussion
We found that academic detailing together with a 1-time mailing of patient-oriented educational materials to providers—but not the mailing of patient-oriented educational materials alone—was associated with a reduction in unnecessary antimicrobial prescribing for upper respiratory infections. In a separate study in the same setting, repeated provider-approved patient education mailings did not result in lower antibiotic prescribing for the providers involved. We also noted a wide variation in the prevalence of antibiotic prescribing between different provider practices and different providers.
Academic detailing22 has been found to reduce unnecessary antibiotic prescribing in the setting of a multidimensional intervention,16 led to narrower spectrum antibiotic use for upper respiratory infections,23 and reduced use of targeted broad-spectrum antibiotics in the inpatient setting.24 Our study extends these findings to demonstrate that 1-time academic detailing alone (without additional education sessions or feedback on performance) can reduce unnecessary prescribing for acute bronchitis and other upper respiratory infections for which antibiotics are not indicated.
The success of educational interventions appears to depend on their intensity, with the most success for multidimensional interventions that include academic detailing, then with mixed results for small group educational sessions and auditing with feedback.25 Use of printed provider educational materials has not been successful in reducing antibiotic use. However, the generalizability of these studies to the current prescribing environment in Western countries is not clear because 2 were performed in 1983,26,27 and 1 was performed in Sri Lanka more than 20 years ago.28 The finding from our first study that a single mailing to providers (the mild intervention) did not result in behavioral change confirms the findings from prior studies.
Our study of patient-directed mailings addressed a hypothesized factor in antibiotic overuse: patient expectation for antibiotics.29,30 Although this effort was not successful, other patient educational interventions have had success. For example, in a prior study, patients with acute bronchitis were less likely to fill an antibiotic prescription if they also were given an informational leaflet.31 However, our intervention did not provide educational materials at the time of the provider visit, which may have reduced its effectiveness.
In a post hoc analysis, we found wide variation in antibiotic prescribing for different practices and for different individuals within practices. To our knowledge, this finding has not been described. Although prior studies found that antibiotic (or broad-spectrum antibiotic) prescribing for upper respiratory infections differed by provider specialty,15,32 our findings suggest that additional unmeasured provider factors may be important.
In the patient mailing intervention study, we found much lower baseline antibiotic prescribing rates among providers in the intervention group (selected from faculty at the University of Pennsylvania) compared with the control group (nonfaculty providers affiliated with the University). These differences suggest that the intervention and control groups differed in important ways at baseline, with the intervention group much closer to following evidence-based practices regarding antibiotic use. It is notable that even with this much lower baseline antibiotic prescribing rate, there was a further decrease among the providers in the intervention group, although the reduction did not reach statistical significance when compared with the control group.
Our studies have several potential limitations. One potential limitation is that the frequency of antimicrobial use in both the exposed and control groups may have declined because of contamination of the unexposed group by aspects of the intervention. This would lead us to conclude that the intervention was less effective than it actually was. Selection bias would occur if the intervention group providers were more likely to respond to prescribing interventions (or nonstudy influences on prescribing such as media coverage of public health campaigns to decrease antimicrobial prescribing) than control group providers. However, both groups of practices are in the same metropolitan area and associated with the same academic institution. Our statistical power to detect modest differences in the study of patient mailings was limited, as reflected in the 95% confidence intervals associated with each measure of association. Finally, it is unclear how well these findings would generalize to nonacademic settings.
In summary, we found that academic detailing, but not provider educational mailings nor provider-approved patients mailings, led to decreased antibiotic prescribing for upper respiratory infections. We also found that there was marked variation in the prevalence of antibiotic prescribing likely not accounted for by provider specialty. Hospitals should continue to consider the use of academic detailing rather than less time-intensive but likely ineffective interventions such as provider and patient mailings. Further investigations are needed to identify interventions to decrease antibiotic use, which may include identifying, describing, and targeting clinicians with high prescribing rates, recognizing the wide variability in the behaviors of individual physicians.
Acknowledgments
The authors thank Cristin Palumbo, MPH, for assistance with data cleaning and analysis.
Author Disclosure Statement
Drs. Vinnard, Linkin, Localio, Leonard, Fishman, and Hennessy, and Ms. Teal disclosed no conflicts of interest with regard to the research, authorship, and/or publication of this article.
This project was supported by an Agency for Healthcare Research and Quality (AHRQ) Centers for Education and Research on Therapeutics cooperative agreement (HS10399), and supported in part by a National Institutes of Health Mentored Patient-Oriented Research Career Development Award (K23-AI-060887) from the National Institute of Allergy and Infectious Diseases (DRL).
References
- 1.Stafford RS. Saglam D. Causino N, et al. Trends in adult visits to primary care physicians in the United States. Arch Fam Med. 1999;8:26–32. doi: 10.1001/archfami.8.1.26. [DOI] [PubMed] [Google Scholar]
- 2.Saint S. Bent S. Vittinghoff E. Grady D. Antibiotics in chronic obstructive pulmonary disease exacerbations. A meta-analysis. JAMA. 1995;273:957–960. [PubMed] [Google Scholar]
- 3.National Asthma Education and Prevention Program. Expert panel report: Guidelines for the diagnosis and management of asthma update on selected topics–2002. J Allergy Clin Immunol. 2002;110:S141–S219. [PubMed] [Google Scholar]
- 4.Smucny J. Fahey T. Becker L. Glazier R. McIsaac W. Antibiotics for acute bronchitis. Cochrane Database Syst Rev. 2000;4:CD000245. doi: 10.1002/14651858.CD000245. [DOI] [PubMed] [Google Scholar]
- 5.Leape LL. Brennan TA. Laird N, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324:377–384. doi: 10.1056/NEJM199102073240605. [DOI] [PubMed] [Google Scholar]
- 6.Thomas EJ. Studdert DM. Burstin HR, et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38:261–271. doi: 10.1097/00005650-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 7.White AC., Jr Atmar RL. Wilson J. Cate TR. Stager CE. Greenberg SB. Effects of requiring prior authorization for selected antimicrobials: Expenditures, susceptibilities, and clinical outcomes. Clin Infect Dis. 1997;25:230–239. doi: 10.1086/514545. [DOI] [PubMed] [Google Scholar]
- 8.Frank MO. Batteiger BE. Sorensen SJ, et al. Decrease in expenditures and selected nosocomial infections following implementation of an antimicrobial-prescribing improvement program. Clin Perform Qual Health Care. 1997;5:180–188. [PubMed] [Google Scholar]
- 9.Shlaes DM. Gerding DN. John JF, Jr, et al. Society for Healthcare Epidemiology of America and Infectious Diseases Society of America Joint Committee on the Prevention of Antimicrobial Resistance: Guidelines for the prevention of antimicrobial resistance in hospitals. Infect Control Hosp Epidemiol. 1997;18:275–291. doi: 10.1086/647610. [DOI] [PubMed] [Google Scholar]
- 10.McGowan JE., Jr Antimicrobial resistance in hospital organisms and its relation to antibiotic use. Rev Infect Dis. 1983;5:1033–1048. doi: 10.1093/clinids/5.6.1033. [DOI] [PubMed] [Google Scholar]
- 11.Singh N. Rogers P. Atwood CW. Wagener MM. Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162:505–511. doi: 10.1164/ajrccm.162.2.9909095. [DOI] [PubMed] [Google Scholar]
- 12.Gonzales R. Bartlett JG. Besser RE, et al. Principles of appropriate antibiotic use for treatment of acute respiratory tract infections in adults: Background, specific aims, and methods. Ann Intern Med. 2001;134:479–486. doi: 10.7326/0003-4819-134-6-200103200-00013. [DOI] [PubMed] [Google Scholar]
- 13.Snow V. Mottur-Pilson C. Gonzales R. Principles of appropriate antibiotic use for treatment of nonspecific upper respiratory tract infections in adults. Ann Intern Med. 2001;134:487–489. doi: 10.7326/0003-4819-134-6-200103200-00014. [DOI] [PubMed] [Google Scholar]
- 14.Gonzales R. Sande M. What will it take to stop physicians from prescribing antibiotics in acute bronchitis? Lancet. 1995;345:665–666. doi: 10.1016/s0140-6736(95)90861-7. [DOI] [PubMed] [Google Scholar]
- 15.Steinman MA. Landefeld CS. Gonzales R. Predictors of broad-spectrum antibiotic prescribing for acute respiratory tract infections in adult primary care. JAMA. 2003;289:719–725. doi: 10.1001/jama.289.6.719. [DOI] [PubMed] [Google Scholar]
- 16.Gonzales R. Steiner JF. Lum A. Barrett PH., Jr Decreasing antibiotic use in ambulatory practice: Impact of a multidimensional intervention on the treatment of uncomplicated acute bronchitis in adults. JAMA. 1999;281:1512–1519. doi: 10.1001/jama.281.16.1512. [DOI] [PubMed] [Google Scholar]
- 17.Green LW. Health Education Planning: A Diagnostic Approach. Palo Alto, CA: Mayfield Publishing Company; 1980. [Google Scholar]
- 18.Naumova EN. Must A. Laird NM. Tutorial in biostatistics: Evaluating the impact of ‘critical periods' in longitudinal studies of growth using piecewise mixed effects models. Int J Epidemiol. 2001;30:1332–1341. doi: 10.1093/ije/30.6.1332. [DOI] [PubMed] [Google Scholar]
- 19.Fitzmaurice GM. Laird NM. Ware JH. Applied Longitudinal Analysis. New York: Wiley; 2004. [Google Scholar]
- 20.Diggle PJ. Liang KY. Zeger SL. Analysis of Longitudinal Data. New York: Oxford University Press Inc.; 1994. [Google Scholar]
- 21.McCulloch CE. Searle SR. Generalized, Linear, and Mixed Models. New York: Wiley; 2001. [Google Scholar]
- 22.Soumerai SB. Avorn J. Principles of educational outreach (‘academic detailing’) to improve clinical decision making. JAMA. 1990;263:549–556. [PubMed] [Google Scholar]
- 23.Ilett KF. Johnson S. Greenhill G, et al. Modification of general practitioner prescribing of antibiotics by use of a therapeutics adviser (academic detailer) Br J Clin Pharmacol. 2000;49:168–173. doi: 10.1046/j.1365-2125.2000.00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solomon DH. Van Houten L. Glynn RJ, et al. Academic detailing to improve use of broad-spectrum antibiotics at an academic medical center. Arch Intern Med. 2001;161:1897–1902. doi: 10.1001/archinte.161.15.1897. [DOI] [PubMed] [Google Scholar]
- 25.Arnold SR. Straus SE. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst Rev. 2005;4:CD003539. doi: 10.1002/14651858.CD003539.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avorn J. Soumerai SB. Improving drug-therapy decisions through educational outreach. A randomized controlled trial of academically based “detailing.”. N Engl J Med. 1983;308:1457–1463. doi: 10.1056/NEJM198306163082406. [DOI] [PubMed] [Google Scholar]
- 27.Schaffner W. Ray WA. Federspiel CF. Miller WO. Improving antibiotic prescribing in office practice. A controlled trial of three educational methods. JAMA. 1983;250:1728–1732. [PubMed] [Google Scholar]
- 28.Angunawela II. Diwan VK. Tomson G. Experimental evaluation of the effects of drug information on antibiotic prescribing: A study in outpatient care in an area of Sri Lanka. Int J Epidemiol. 1991;20:558–564. doi: 10.1093/ije/20.2.558. [DOI] [PubMed] [Google Scholar]
- 29.Avorn J. Solomon DH. Cultural and economic factors that (mis)shape antibiotic use: The nonpharmacologic basis of therapeutics. Ann Intern Med. 2000;133:128–135. doi: 10.7326/0003-4819-133-2-200007180-00012. [DOI] [PubMed] [Google Scholar]
- 30.Hamm RM. Hicks RJ. Bemben DA. Antibiotics and respiratory infections: Are patients more satisfied when expectations are met? J Fam Pract. 1996;43:56–62. [PubMed] [Google Scholar]
- 31.Macfarlane J. Holmes W. Gard P. Thornhill D. Macfarlane R. Hubbard R. Reducing antibiotic use for acute bronchitis in primary care: Blinded, randomised controlled trial of patient information leaflet. BMJ. 2002;324:91–94. doi: 10.1136/bmj.324.7329.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linder JA. Stafford RS. Antibiotic treatment of adults with sore throat by community primary care physicians: A national survey, 1989–1999. JAMA. 2001;286:1181–1186. doi: 10.1001/jama.286.10.1181. [DOI] [PubMed] [Google Scholar]