Abstract
Objective
Vascular remodeling during liver damage involves loss of healthy liver sinusoidal endothelial cell (LSEC) phenotype via capillarisation. Hedgehog (Hh) signaling regulates vascular development and increases during liver injury. Therefore, we examined its role in capillarisation.
Design
Primary LSEC were cultured for 5 days to induce capillarisation. Pharmacologic, antibody-mediated, and genetic approaches were used to manipulate Hh signaling. Effects on mRNA and protein expression of Hh-regulated genes and capillarisation markers were evaluated by qRT-PCR and immunoblot. Changes in LSEC function were assessed by migration and tube forming assay, and gain/loss of fenestrae was examined by electron microscopy. Mice with acute or chronic liver injury were treated with Hh inhibitors; effects on capillarisation were assessed by immunohistochemistry.
Results
Freshly isolated LSEC expressed Hh ligands, Hh receptors, and Hh ligand antagonist Hhip. Capillarisation was accompanied by repression of Hhip and increased expression of Hh-regulated genes. Treatment with Hh agonist further induced expression of Hh ligands and Hh-regulated genes, and up-regulated capillarisation-associated genes; whereas Hh signaling antagonist or Hh ligand neutralizing antibody each repressed expression of Hh target genes and capillarisation markers. LSEC isolated from SmoloxP/loxP transgenic mice that had been infected with adenovirus expressing Cre-recombinase to delete Smoothened showed over 75% knockdown of Smoothened. During culture, Smoothened-deficient LSEC had inhibited Hh signaling, less induction of capillarisation-associated genes, and retention of fenestrae. In mice with injured livers, inhibiting Hh signaling prevented capillarisation.
Conclusions
LSEC produce and respond to Hh ligands, and use Hh signaling to regulate complex phenotypic changes that occur during capillarisation.
Keywords: CELL BIOLOGY, CELL SIGNALLING
Introduction
The liver sinusoidal endothelial cell (LSEC) has a unique phenotype among all mammalian endothelial cells, i.e. LSEC have open fenestrae grouped into sieve plates and lack an organized basement membrane. LSEC lose this highly specialized morphology during a process called capillarisation. Capillarisation occurs in vivo during many different kinds of liver injury (e.g., fibrosis, 1, 2 hepatitis, 3, 4 alcoholic liver injury, 5 arsenic poisoning 6) and increases naturally with age (called pseudo-capillarisation).7 The process can be modeled in vitro by culturing LSEC on plastic dishes in serum-containing medium. 8, 9 However, the molecular mechanisms driving capillarisation have not been fully elucidated. Improved understanding of the latter will clarify how LSEC maintain their unique phenotype and identify therapeutic targets to retain and/or restore their healthy phenotype during liver injury or aging.
During fetal development, angiogenesis and vasculogenesis are regulated, in part, by the Hedgehog (Hh) pathway. 10, 11 Therapeutic activation of this pathway has also been reported to improve vascularization of injured tissues in adults. 12, 13 These findings demonstrate that certain types of endothelial cells are Hh-responsive, and prompted us to investigate the hypothesis that Hh is one of the factors that controls capillarisation. Hh is a conserved morphogenic signaling pathway that modulates the fates of various types of cells, including endothelial progenitors. 14, 15 In Hh-responsive cells, the canonical Hh signaling pathway is activated when any of the three Hh ligands (Sonic hedgehog (Shh), Indian hedgehog (Ihh) or Desert hedgehog (Dhh)) engage the plasma membrane spanning receptor, Patched (Ptc). This interaction prevents Ptc from repressing Smoothened (Smo), a co-receptor-like molecule that transduces Hh-initiated signaling intra-cellularly. The latter eventually result in the stabilization and nuclear localization of Gli-family transcription factors (Gli1, Gli2, and Gli3). Gli-binding, in turn, regulates the transcriptional activity of Hh target genes that influence cell viability, proliferation, and differentiation. 16, 17
Canonical Hh pathway activity is very low in healthy adult livers because Hh ligands are scarce. However, liver injury increases local production of Hh ligands. This promotes Hh pathway activation and thus, Hh signaling becomes dramatically activated in damaged livers. 18-20 Several cell types that accumulate in injured livers, including myofibroblastic hepatic stellate cells, various immune cells, and different types of liver progenitor cells, produce and respond to Hh ligands. Genetic and pharmacologic approaches that modulate Hh pathway activity have been shown to influence liver fibrosis, regeneration and cancer. Thus, there is growing evidence that Hh signaling plays a significant role in the adult liver repair. 21, 22
A few reports suggest that Hh signaling may regulate vascular remodeling responses to liver injury. 18, 23 For example, similar to cultured human vascular endothelial cells, 24 cultured LSEC respond to exogenous Hh ligands by increasing expression of Hh-target genes. The Hh-regulated transcription factor, Gli2, has also been demonstrated in nuclei of LSEC within injured liver tissue using immunohistochemistry.18, 23 However, knowledge about how Hh pathway activation influences the phenotype of LSEC is quite limited. Improved understanding of this issue will clarify whether or not Hh pathway activation promotes capillarisation of hepatic sinusoids during liver injury and aging. Therefore, we examined LSEC at different time points during culture-provoked capillarisation to profile changes that might have resulted from Hh pathway activation, and then used genetic, antibody-mediated, and pharmacologic approaches to manipulate Hh signaling to determine if, and how, this modified the capillarisation process.
Materials and Methods
Reagents
Chemicals were obtained from Sigma-Aldrich Corporation (St. Louis, MO) unless stated otherwise. Reagents used include VEGF (Peprotech, Rocky Hill, NJ); osmium tetroxide (OsO4), glutaraldehyde, cacodylate buffer, tannic acid (Electron Microscopy Sciences, Hatfield, PA); hexamethyldisilazane (Ted Pella, Redding, CA); cyclopamine and tomatidine (Toronto Research Chemicals, Toronto, Canada); SAG (Enzo Life Sciences, Plymouth Meeting, PA); 5E1-neutralizing antibody (Iowa Hybridoma bank, University of Iowa); GDC-0449 (Selleck Chemicals, Houston, TX); mouse IgG1 isotype control antibody (R&D Systems, Minneapolis, MN); Dil-Ac-LDL (Biomedical Technologies, Stoughton, MA); iodixanol (Accurate Chemical and Scientific, Westbury, NY); phycoerythrin (PE)-conjugated mouse anti-rat CD31 (BD Pharmingen, San Diego, CA); Rabbit polyclonal antibody to Desmin (Abcam, Cambridge, MA); CD105 and F4/80 antibody (BioLegend, San Diego, CA), Endothelin-1 antibody (Thermo Fisher Scientific, Rockford, IL), iNOS antibody (Santa Cruz Biotechnology, Santa Cruz, CA). FITC-labeled formaldehyde-treated serum albumin (FITC-FSA) was prepared as previously described. 25
Animals
200g male Sprague-Dawley rats were obtained from Charles River Laboratories (Wilmington, MA). SmoloxP/loxP transgenic mice 26 and C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Mdr2−/− mice were a gift from Dr. Detlef Schuppan (Beth Israel Deaconess Medical Center, Boston, MA). GDC-0449 treatment 27, 70% partial hepatectomy (PHx) and cyclopamine treatment 21 were performed as previously described. Animal experiments fulfilled National Institutes of Health and Duke University-Institutional Animal Care and Use Committee requirements for humane animal care.
LSEC Isolation
LSEC were isolated from normal Sprague-Dawley rats or SmoloxP/loxP transgenic mice by collagenase perfusion, iodixanol density gradient centrifugation, and centrifugal elutriation as previously described 28 with modification. Yields of LSEC are on average 80 million sinusoidal endothelial cell per 10 g liver with viability of >95% and purity of >96%, as determined by scanning electron microscopy, FACS for CD31 and CD105, and positive staining for fluorescent acetylated low-density lipoprotein (Dil-Ac-LDL, Biomedical Technologies, Stoughton, MA) and FITC-FSA. FACS on F4/80 and desmin were used to exclude contamination by macrophage/Kupffer cells and hepatic stellate cells (HSC). Cells were cultured on rat-tail collagen-coated plates (400,000 cells/cm2) in Dulbecco’s minimal essential medium (DMEM)-low glucose with 10% fetal bovine serum (FBS). Cells usually attached to the culture dish within 3 h of plating.
Pharmacological Manipulation of Hh Signaling
LSEC were treated with Smo antagonist, Cyclopamine (3μM), or tomatidine, a catalytically inactive analog (3μM), for 48 hours. 19 In separate experiments, Hh-neutralizing antibody (5E1, 10 μg/ml) or control immunoglobulin G were added to the LSEC culture. 29 SAG, an Hh agonist (0.3μM) was used to activate Hh pathway. 30
Adenoviral Transduction of LSEC
Primary LSEC isolated from SmoloxP/loxP transgenic mice (n=10) were cultured in the presence of adenoviral vectors carrying green fluorescent protein (Ad-GFP) or Cre recombinanse (Ad-Cre) (multiplicity of infection (MOI) 25). 31 Cultures were analyzed at 48 h. In separate experiments, SmoloxP/loxP mice were infected with Ad-GFP (50μl) or Ad-Cre by tail vein injection. LSEC were isolated 2 days later and cultured on collagen-coated plates for additional 2 days before further analysis.
Scanning Electron Microscopy and Quantitative Imaging
Sample preparation was performed as previously described. 32 Briefly, LSEC and livers were fixed with glutaraldehyde, postfixed with OsO4, dehydrated with graded alcohols, dried with hexamethyldisilazane, sputter-coated with gold and examined using a FEI XL30 ESEM scanning electron microscope (FEI, Hillsboro, OR). Porosity (percentage of LSEC surface occupied by fenestrae) was measured in scanning electron microscopy (SEM) micrographs of cells; 32 in brief, total LSEC surface area and the open area of individual fenestrae were quantified. Open areas were summed, divided by total surface area, expressed as a percentage of open area, and averaged. Each average was taken from 15 images and analysis was done using MetaMorph software (Molecular Devices, Downingtown, PA).
mRNA Quantification by Real-Time Reverse-Transcription Polymerase Chain Reaction
Total RNA was prepared using RNeasy Mini Kit (Qiagen, Valencia, CA) according to manufacturer’s instruction. 1.5 μg of RNA was reverse-transcribed using random primers and Superscript RNase H-reverse transcriptase (Invitrogen, Carlsbad, CA). Samples were incubated at 25°C for 15 min, 42°C for 55 min; reverse transcriptase was inactivated by heating at 70°C for 15 min followed by cooling at 4°C for 10 min.. cDNA samples were used for quantitative reverse-transcription polymerase chain reaction (qRT-PCR) using iQ-SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA). 19 β-actin was used as internal control. For qRT-PCR parameters were as follows: denaturating at 95°C for 3 min, followed by 40 cycles of denaturing at 95°C for 10 s and annealing-extension at the optimal primers temperatures for 60 s. Threshold cycles (Ct) were automatically calculated by the iCycler iQ Real-Time Detection System. Target gene levels in the cells are presented as a ratio to levels detected in the corresponding control cells according to the ΔΔCt method. Primer sequences are listed in Supplementary table 1 and 2.
Western blotting
Western blotting was performed as previously described; 19 results were normalized to β-actin expression.
Migration Assay
Migration was measured by using a modified Boyden chamber assay. LSEC, 400,000 cells/cm2, were plated on collagen-coated Transwell inserts with 8-μm pore size (Costar, Corning, NY) in DMEM low glucose supplemented with 2% FBS. Inserts were placed on top of 2 cm2 wells containing medium plus 2% FBS with different treatment. Cells migrated were counted after 20 h in 10 random 20x fields for each insert.
Tube Formation Assay
LSEC were seeded on growth factor-reduced Matrigel (BD Bioscience, Bedford, MA) with/without treatment as described. 18 The length of capillary-like tube formation was quantified in 10 randomly chosen optical fields using light microscopy after 6 hours.
Immunohistochemsitry
Formalin-fixed, paraffin-embedded samples were deparaffinized and rehydrated. Antigen retrieval was performed by heating in 10 mM sodium citrate buffer. Sections were blocked (Dako Envision, Carpinteria, CA) and incubated with Gli2 antibody (Genway, San Diego, CA) overnight at 4°C and developed with diaminobenzidine (DAB), followed by incubation with CD31 antibody (Santa Cruz Biotechnology) overnight at 4°C and developed with Ferangi Blue (Biocare Medical, Concord, CA). The number of Gli2/CD31 double positive cells were counted in 5 representative 63x fields/section from each sample (n=3).
Statistics
All data, expressed as mean ± standard error of the mean (SE), were from at least three separate experiments. Groups were compared by analysis of variance (ANOVA) with à posteriori contrast by least significant difference; or by Student t test using the Microsoft Excel Analysis ToolPak (Microsoft, Redmond, WA). Results with p < 0.05 were considered significant.
Results
Characterization of Elutriated Primary LSEC
LSEC isolated using cell elutriation methodology demonstrate typical morphology under light microscopy (Figure 1A). Scanning electron microscopy (SEM) confirms that they possess fenestrae grouped into sieve plates (Figure 1B), the defining feature of the liver sinusoidal endothelium. 28 By flow cytometry, ~96% of these cells are double-positive for two endothelial markers, CD31 and CD105 (endoglin),33, 34 whereas less than 2% express the macrophage marker, F4/80, and none are positive for desmin, a marker of quiescent and myofibroblastic HSC (Figure 1C). The aggregate findings, therefore, indicate that our primary LSEC preparations are a highly pure population. The vast majority of these cells were able to take up fluorescent acetylated low-density lipoprotein (Dil-Ac-LDL, Figure 1D) and FITC-FSA (Figure 1E), demonstrating that they remain endocytically active in vitro. Furthermore, on day 5, most of the cells were still able to take up Dil-Ac-LDL (Supplementary figure 1A), but not FITC-FSA (Supplementary figure 1B), consistent with previous reports 9, 35, suggesting that LSEC lose their endocytic activity during culture.
Figure 1. Characterization of liver sinusoidal endothelial cells (LSEC) isolated by elutriation method.
(A) LSEC cultured for 1 day on collagen coated plate demonstrate typical “cobblestone” morphology under phase contrast microscopy. Scale bar: 100 μm. (B) Scanning electron microscopy shows LSEC have fenestrae grouped into sieve plates (arrow), the defining morphology of LSEC. Scale bar: 5 μm. (C) FACS analysis of freshly isolated LSEC. ~96% of LSEC were double positive for CD31 and CD105(endoglin). Only 2% of LSEC were F4/80 positive (Kupffer cell marker) and none expressed desmin (hepatic stellate cell marker). Uptake of Ac-Di-LDL (D) and FITC-FSA (E) by day 1 LSEC demonstrate the typical endocytic activity of the cells. Scale bar: 50 μm for left images, 20 μm for right images. All experiments were performed at least three times.
Primary LSEC Undergo Capillarisation in vitro
It is well established that LSEC lose their healthy, quiescent phenotype (fenestrae grouped into sieve plates) rapidly during culture. 8, 36 During the first day of culture, our LSEC maintained normal fenestrae and sieve plates, but both of these features were dramatically reduced after 2 or 3 days of culture. No sieve plates were observed, and only occasional fenestrae remained, by culture day 4 or 5 (Figure 2A). The loss of macroscopically-evident fenestrae was further confirmed by porosity measurement (Figure 2A). As others have reported, the morphological changes in LSEC that occurred during culture induced-capillarisation were accompanied by the down-regulation of mRNA for endothelial nitric oxide synthase (eNOS), 37 VEGF receptor (VEGFR) 1/2, 38 and up-regulation of mRNA and protein for inducible nitric oxide synthase (iNOS), 23 endothelin-1(ET-1) 39 (Figure 2B and C). Interestingly, mRNA and protein expression of CD31 (Figure 2B and C), a commonly-used marker for LSEC capillarisation, 6, 8 was easily demonstrated in our freshly-isolated LSEC. CD31 mRNA levels fell by ~80% during the initial day of culture, but eventually recovered to ~50% of basal levels by day 3. Earlier studies typically compared CD31 expression between day 1 and day 3 LSEC, and noted an increase in CD31 protein/mRNAs during this time interval. 8, 40 Thus, our findings are consistent with those reports. Our Western blot analysis demonstrated that whole cell levels of CD31 protein remained relatively constant during LSEC culture, mirroring similar data from Géraud. 38 Because LSEC expression of CD31 remains relatively constant in this model of capillarisation, we used changes in mRNA and protein levels of iNOS, another capillarisation marker (Figure 2B and C), to track capillarisation in subsequent experiments.
Figure 2. LSEC undergo capillarisation spontaneously in vitro.
(A) Rat LSEC were cultured on collagen coated plates, processed and examined by SEM at X10,000 magnification. Day 1 LSEC show numerous fenestrae grouped into sieve plates (arrow). Day 2 and 3 LSEC have fewer fenestrae and sieve plates (arrow). Day 4 and 5 LSEC have only occasional fenestrae (arrowhead). Porosity measurement was used to quantify the percentage of fenestrae per surface area. Scale bar: 5 μm. **: p<0.01 day 1 cells, n=3. (B) qRT-PCR analysis of endothelial cell associated gene expression changes during culture induced capillarisation. * p<0.05, ** p<0.01, *** p<0.001 vs day 0 cells, n=3. (C) Western blot analysis of protein harvested from rat LSEC with densitometry to confirm the gene expression change described in B. Results are representative of triplicate experiments. * p<0.05, ** p<0.01 vs day 0 cells, # non-detectable.
Primary LSEC Produce and Express Hh-target Genes
Messenger RNA and protein expression of Shh ligand, Hh signaling components, and various Hh-target genes was assessed in freshly isolated LSEC and at different time points during culture-induced capillarisation. Freshly isolated LSEC express Shh ligand, components of the canonical Hh signaling pathway (e.g., the receptor Ptc, co-receptor Smo, and Hh-regulated transcription factors Gli2 and Gli3), and several Hh-target genes (e.g., Hhip, Ptc, and sFRP1) (Figure 3A and B). During the first day of culture, Hh interacting protein (Hhip, a Hh ligand antagonist) expression virtually disappears at both the mRNA and protein level and there is increased accumulation of Shh protein (Figure 3A and B). This is accompanied by increased mRNA expression of the Hh-regulated transcription factors, Gli2 and Gli3 (Figure 3A). Day 1 LSEC maintain normal fenestrae (Figure 1B and 2A), thus capillarisation is not evident in day 1 LSEC. Therefore, activation of Hh signaling occurs before the first overt evidence of capillarisation (loss of fenestrae), which emerges on day 2 (Figure 2A). As capillarisation progresses, there is further up-regulation of both Gli2 and Gli3 during culture days 3-5 (Figure 3A and B), indicating increased Hh pathway activity in capillarised LSEC compared to freshly-isolated LSEC. Increased mRNA expression of several genes that are known to be transcriptionally activated by Gli2 (e.g., Ptc, soluble frizzled related peptide 1 (sFRP1), 41 osteopontin (OPN) 30, and the mesenchymal marker, Twist, Figure 3A and B) occurred when LSEC exhibited increased accumulation of Gli2 and Gli3 proteins.
Figure 3. Hedgehog (Hh) pathway is activated during LSEC capillarisation in vitro.
(A) Rat LSEC were cultured, mRNA were harvested and qRT-PCR were used to analysis the expression change of Hh target genes (Gli2, Gli3, Sonic Hh (Shh), Hedgehog-interacting protein (Hhip), Patched (Ptc), Smoothened, Twist2, secreted frizzled-related protein 1 (sFRP1), osteopontin (OPN)). * p<0.05, ** p<0.01, *** p<0.001 vs day 0 cells, n=3. (B) Western blot analysis of protein harvested from rat LSEC with densitometry to confirm the gene expression change described in B. β-Actin was used as loading control. Results are representative of triplicate experiments. * p<0.05, ** p<0.01, *** p<0.001 vs day 0 cells, # non-detectable.
LSEC are Hh-responsive Cells during Culture
To examine the significance of Hh signaling during capillarisation, Hh pathway activity was manipulated in day 3 LSEC (which are capillarised, Figure 2A). 48 hours following further activation of Hh signaling by treatment with a Hh agonist (SAG, 0.3μM), we noted a 2-fold or greater increase in mRNAs encoding Hh ligands (Shh, Ihh), Hh-sensitive transcription factors (Gli2, Gli3), and Hh-target genes (sFRP1, OPN). Augmenting Hh pathway activity also caused LSECs to further up-regulate their expression of activation markers (e.g., iNOS) and mesenchymal genes (e.g., snail, twist) (Figure 4A). Conversely, inhibition of endogenous Hh signaling by treating LSEC with a Smoothened antagonist (cyclopamine, 3 μM) resulted in >50% repression of Hh ligands, Hh signaling components, Hh target genes, and LSEC activation markers (Figure 4B). Treating LSECs with Hh ligand neutralizing antibody (5E1, 10 μg/ml) resulted in similar changes in target gene expression (Figure 4C), suggesting an autocrine effect of Hh ligands in activating Hh signaling in capillarised LSEC.
Figure 4. LSEC are Hh responsive.
(A) Rat LSEC were cultured for 3 days and treated with either DMSO or SAG (0.3 μM, an Hh agonist) for 2 more days. mRNA were isolated and gene expression changes were examined by qRT-PCR. (B) Day 3 rat LSEC were treated with either cyclopamine (Cyc, 3μM, a pharmacological inhibitor of Hh signaling) or tomatidine (Tom, an inert cyclopamine analog) for 2 days. mRNA was harvested, and changes in gene expression were determined by qRT-PCR. (C) Rat LSEC were cultured for 3 days and treated with either 5E1(10 μg/ml, Hh ligand neutralizing antibody) or IgG for 2 more days. mRNA was isolated and gene expression change were monitored by qRT-PCR. *: p<0.05, **: p<0.01, ***: p<0.001. n= 3.
To exclude the possibility that these gene expression changes were due to potential off-target effects of cyclopamine, 42 LSEC were isolated from SmoloxP/loxP transgenic mice, cultured for 3 days, infected with adenovirus expressing Cre-recombinase (Ad-Cre, for conditional deletion of Smo), and harvested 48 h later (on day 5 of culture). This approach knocked down Smo gene expression by 76.59±3.60%, and caused 40-50% repression of Shh and various Hh target genes (e.g. Gli1, Gli2), as assessed by qRT-PCR and/or western blot (Figure 5A and B). Targeted disruption of Smo gene expression also down-regulated expression of iNOS, CD31 and ET-1, supporting a role for the Hh pathway in maintaining the phenotype of capillarised LSEC.
Figure 5. Effect of knocking down LSEC Smo gene expression in vitro on Hh pathway.
(A) Primary LSEC were isolated from SmoloxP/loxP transgenic mice, placed in monocultures for 3 days, and infected with either adenovirus expressing GFP (Ad-GFP, control) or Cre-recombinase (Ad-Cre, for conditional deletion of the Hh signaling intermediate, Smo). After 48 h, cells were harvested to obtain mRNA for qRT-PCR analysis. *:p<0.05, **:p<0.01, ***:p<0.001 vs control. n =3. (B) Western blot analysis of protein harvested from mouse LSEC described in A with densitometry to confirm the gene expression change. β-Actin was used as loading control. *:p<0.05, **:p<0.01 vs control. Results are representative of triplicate experiments.
Hh Signaling Promotes LSEC Migration and Vascular Tube Formation
It is known that Hh pathway activation can induce migration and capillary tube formation by different types of cultured endothelial cells,11, 24, 43-45 including LSEC. 18 To further explore the role of Hh signaling on migration and vascular tube formation by LSEC, Hh pathway activity was manipulated in isolated LSEC cultured on collagen coated inserts or matrigel. Inhibiting Hh signaling with cyclopamine impaired the ability of LSEC to migrate (Figure 6C and F) and form tubes in vitro (Figure 7C and F), whereas activation of the Hh pathway by SAG promoted both migration (Figure 6E and F) and vascular tube formation (Figure 7E and F). Cyclopamine also blocked the ability of VEGF to induce LSEC migration (Figure 6D and F) and tube formation (Figure 7D and F), possibly by inhibiting VEGFR1 and VEGFR2 expression by LSEC (Supplementary figure 2). Collectively, these data confirmed that Hh signaling promotes complex phenotypic changes in LSEC that are necessary for them to migrate and form vascular tubes in vitro.
Figure 6. Hh pathway promotes LSEC migration in vitro.
Primary rat LSEC were seeded on collagen coated insert and treated with (A) normal growth medium (ctrl), (B) 40ng/ml VEGF, (C) 3μM Cyclopamine (Cyc), (D) VEGF with Cyclopamine (VEGF + Cyc), or (E) 0.3 μM SAG for 20 hours. (F) Effects on migration were assessed by counting the number of cells in the bottom of the inserts in triplicate experiments. Representative micrographs and statistical summary are shown. *: p<0.05, **: p<0.01 vs control. #: p<0.05 vs VEGF. Scale bar: 50 μm.
Figure 7. Hh pathway promotes LSEC tube formation in vitro.
Primary rat LSEC were seeded on growth factor reduced Matrigel and treated with (A) normal growth medium (ctrl), (B) 40ng/ml VEGF, (C) 3μM Cyclopamine (Cyc), (D) VEGF with Cyclopamine (VEGF + Cyc), or (E) 0.3 μM SAG for 6 hours. (F) Effects on vascular tube formation were assessed by quantifying the length of capillary like-tube in triplicate experiments. Representative micrographs and statistical summary are shown. *: p<0.05 vs control. #: p<0.05 vs VEGF. Scale bar: 100 μm.
Hh Signaling Regulates LSEC Capillarisation
LSEC become capillarised after 2 days culture (Figure 2A), and this process is accompanied by activation of the Hh pathway (Figure 3A and B). Inhibiting Hh signaling in capillarised LSEC by treating them with cyclopamine or anti-Hh neutralizing antibody significantly repressed expression of capillarisation-associated genes, such as iNOS, CD31 and ET-1 (Figure 4B and C). Similar effects were achieved by knocking down Smo gene expression in culture-capillarised LSEC (Figure 5A and B). However, SEM examination demonstrated that none of these approaches restored LSEC fenestrae (data not shown). These data suggest, therefore, that once LSEC become capillarised, inhibiting Hh signaling only partially reverses their capillarised phenotype.
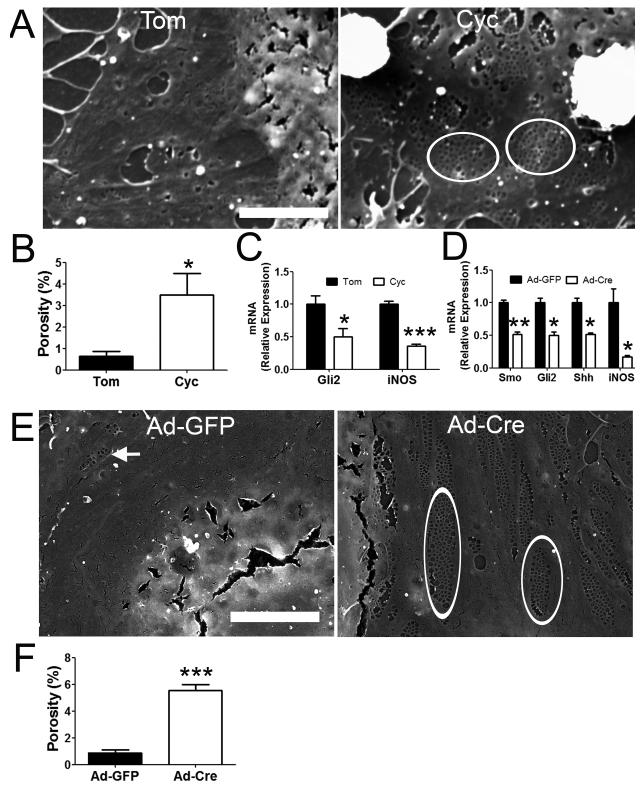
To determine whether capillarisation might be prevented by blocking induction of Hh signaling in LSEC before capillarisation begins, freshly isolated, primary LSEC were immediately incubated with cyclopamine or an inactive cyclopamine analog (control) after attachment to culture plates. LSEC treated with the control agent underwent capillarisation, completely losing fenestrae within 2 days after plating. In contrast, LSEC that were treated with cyclopamine immediately after plating maintained porosity (Figure 8A and B). LSEC cultures that retained the fenestrated phenotype exhibited less induction of mRNAs that mark Hh signaling (Gli2) and capillarisation (iNOS) (Figure 8C). Because the process of LSEC isolation per se might initiate the cascade of events that culminates in capillarisation, we did further studies in which we abrogated Hh signaling in LSEC before the cells were isolated. This was accomplished by injecting adenoviral vectors for Cre recombinase (Ad-Cre) into SmoloxP/loxP mice 48 h prior to liver perfusion and LSEC harvest. Results were compared to SmoloxP/loxP mice that were similarly treated with an irrelevant adenovirus encoding green fluorescent protein (Ad-GFP). LSEC from the mice that were injected with Ad-Cre virus in vivo exhibited knock-down of smo, and smo-depleted LSECs failed to up-regulate expression of Shh ligand, Gli2, or iNOS after 2 days in culture (Fig 8D). The inhibited induction of Hh signaling and capillarisation-associated genes in the smo-deficient LSEC was accompanied by maintainenance of fenestrae (Figure 8E and F), providing further evidence that inhibition of Hh signaling prevents LSEC from undergoing capillarisation.
Figure 8. Inhibition of Hh pathway prevents LSEC capillarisation in vitro.
Primary rat LSEC were plated on collagen coated coverslip. After 3 hours, LSEC attached and were then incubated with either tomatidine (Tom) or cyclopamine (Cyc, 3μM). Cells were fixed and processed for SEM on day 2. Representative (A) SEM micrograph and (B) porosity measurement were shown. LSEC treated with cyclopamine maintained fenestrae grouped into sieve plates (circle), while LSEC treated with tomatidine lose fenestrae. (C) mRNA were also isolated from the treated cells described in (A) for qRT-PCR analysis on Gli2 and iNOS gene expression. SmoloxP/loxP transgenic mice were pretreated with adenovirus expressing GFP (Ad-GFP) or Cre (Ad-Cre) by tail vein injection. Primary LSEC were isolated 2 days after treatment and plated on collagen-coated dish for another 2 days. (D) mRNA expression of Smo, Gli2, Shh and iNOS were examined by qRT-PCR. Cells were also fixed for (E) SEM micrograph and (F) porosity measurement was shown. Ad-Cre infected LSEC maintain normal fenestrae grouped into sieve plates (circle), while Ad-GFP infected LSEC lose fenestrae. Note that occasional fenestrae and sieve plates (arrow) can still be observed in Ad-GFP infected LSEC, indicating that the loss of fenestrae was not due to the contamination of other kinds of endothelial cells. *: p<0.05, **:p<0.01, ***p<0.001. n=3-6. Scale bar: 5 μm.
To validate that Hh signaling regulates LSEC capillarisation in vivo, Hh pathway activity was manipulated in different liver injury models: mdr2−/− mice 27 (a model of chronic liver injury and repair that results in progressive biliary-type fibrosis) and wild type mice following acute partial hepatectomy (a model of acute liver cell loss and repair). Mdr2−/− mice consistently expressed Hh ligands and progressively accumulated Hh-responsive liver myofibroblasts 27. Treatment of Mdr2−/− mice with GDC-0449 (Hh signaling antagonist) significantly reduced the number Gli2 and CD31 (a commonly used capillarisation marker in vivo) double positive cells in the liver (Figure 9A and B). Similar results were observed in PHx mice treated with cyclopamine (Figure 9C). Thus, Hh inhibition prevents LSEC capillarisation in both chronic and acute liver injury.
Figure 9. Inhibition of Hh pathway prevents LSEC capillarisation in vivo.
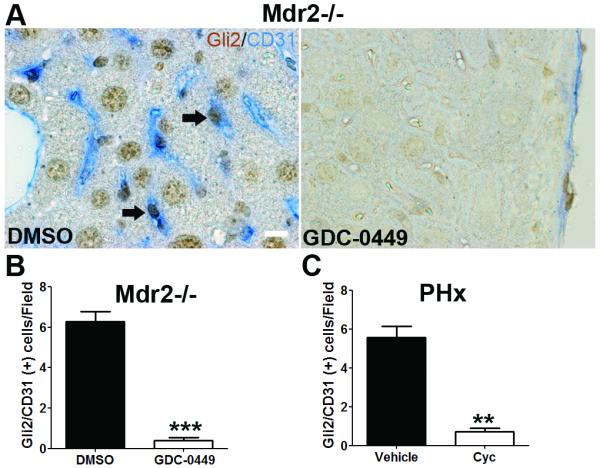
(A) Liver sections from DMSO and GDC-0449 treated Mdr2−/− mice were double stained for Gli2 (brown, Hh target gene) and CD31 (blue, capillarisation marker). Note that LSEC co-express Gli2 and CD31 (arrow). Scale bar: 10μm. Number of Gli2/CD31 double positive cells per field (B) was counted in 5 random fields per mice, ***p<0.001. n=3. (C) Liver sections from vehicle and cyclopamine treated PHx mice were stained for Gli2 and CD31, and the number of Gli2/CD31 double positive cells was counted. **p<0.01. n=3.
Discussion
The current study demonstrates that Hh signaling is activated during LSEC capillarisation and plays a major role in controlling this process in cultured LSEC. Freshly isolated primary LSEC were shown to express both Hh ligands and receptor. We also found that LSEC expression of Hh ligands, signaling components, and target genes increased significantly during culture-induced capillarisation. Manipulating Hh signaling via pharmacologic, antibody-mediated, or genetic approaches altered the phenotype of cultured LSEC, proving that LSEC are Hh-responsive cells. Moreover, cultured LSEC required Hh signaling to orchestrate complex biologic responses, such as the genesis of vascular tubes, because both cell migration and tube formation was blocked by Hh pathway inhibitors, but enhanced by Hh pathway agonists. Indeed, capillarisation itself proved to be a Hh-dependent process because Hh pathway inhibitors partially reverted capillarised LSEC to their healthy differentiated phenotype, and completely prevented LSEC from becoming capillarised both in vitro and in vivo.
Several types of resident liver cells are capable of producing Hh ligands and/or responding to Hh ligands including hepatocytes,31 HSC, 19 cholangiocytes,22 and immune cells. 46 Here we provide the first direct evidence that freshly isolated, primary LSECs produce Hh ligands (both Shh and Ihh). Capillarisation in vitro does not further increase LSEC expression of Hh ligands. Rather, the LSECs repress their production of the Hh ligand antagonist, Hhip, and this appears to be permissive for up-regulating pathway activity because mRNAs of Hh-regulated genes are induced. The importance of autocrine activation of Hh signaling is further supported by evidence that neutralizing anti-Hh antibodies suppress Hh pathway activity in cultured LSECs. Both canonical 47and non-canonical (Smo independent) 48 Hh signaling have been reported in other types of endothelial cells. Non-canonical pathways that are capable of activating Hh-regulated transcription factors may also be deployed as LSEC undergo capillarisation in vitro, because treating cultured LSEC with recombinant Shh fails to further activate Hh signaling (data not shown). It remains unclear if sinusoidal capillarisation in injured livers is also controlled mainly via autocrine mechanisms, or if Hh ligands that are released from neighboring liver cells act in a paracrine fashion to enhance Hh signaling in LSECs. It is also conceivable that mechanical forces that occur during liver damage, such as changes in blood flow, might influence LSEC Hh signaling by altering primary cilia. 49, 50 The genetic approach that we used to knock down Smo expression in primary LSEC repressed Hh signaling, however, showing unequivocally that Hh activation in LSEC occurs at least partly through the canonical Hh pathway.
Inhibition of Hh signaling blocks VEGF-induced LSEC migration and tube formation. Hh signaling has been reported to interact with VEGF signaling by inducing VEGF 51, Neuropilin 52 (co-receptor for VEGF) expression. Our data here also suggest that Hh signaling may regulate VEGFR1 and VEGFR2 expression. This is very intriguing since VEGF signaling is an important regulator for angiogenesis, which is an essential process during development and many kinds of adult injury repair. Further study is needed to elucidate the link between these pathways.
The most intrigue finding of this study is that Hh signaling plays a key role in capillarisation. Although fenestrae did not reappear in cultured LSEC after blocking Hh signaling, the cells did regain a gene expression profile that is more typical of the fully differentiated LSEC in healthy livers. Moreover, loss of fenestrae during culture and two different types of liver injury was prevented by abrogating Hh signaling. There are several clinical implications for these novel findings. First, differentiated (non-capillarised) LSEC prevent HSC activation and cause activated, myofibroblastic HSC to revert to quiescence, 53 while capillarised LSEC lose these actions. 53, 54 Thus, our new data suggest that inhibiting Hh signaling in fibrotic livers may reduce liver fibrosis by promoting restitution of fenestrated LSEC which antagonize HSC activation, as well as by directly acting on the HSC themselves to prevent/reverse the myofibroblastic phenotype, as was suggested previously. 19 Second, fenestrated LSEC have been proposed to play an important role in clearing chylomicron remnants. 55 Because accumulation of these remnants is believed to initiate atherosclerosis, 56 evidence that Hh pathway inhibitors maintain LSEC fenestra suggests a novel approach to treat atherosclerosis. Lastly, age-induced pseudo-capillarisation and injury-related capillarisation produce similar phenotypic changes in LSECs. Hence, it will be intriguing to examine whether there is increased Hh pathway activity during aging.
Supplementary Material
Significance of this study.
What is already known about this subject?
-
✓
Liver injury induces capillarisation of liver sinusoidal endothelial cells (LSEC) and results in remodeling of the hepatic vasculature.
-
✓
Hedgehog (Hh) pathway regulates vascular development during embryogenesis.
-
✓
Liver injury triggers activation of the Hh pathway.
What are the new findings?
-
✓
Freshly isolated LSEC express both Hh ligands and receptors, and activation of Hh signaling is accompanied by LSEC capillarisation in vitro.
-
✓
Cultured LSEC respond to Hh agonist, antagonist, and Hh ligand neutralizing antibody, thus are Hh responsive cells.
-
✓
LSEC capillarisation can be prevented by the inhibition of Hh pathway both in vitro and in vivo.
How might it impact on clinical practice in the foreseeable future?
-
✓
Our data suggest a novel way to prevent and/or reverse LSEC capillarisation, a process involved in liver fibrosis progression.
Acknowledgments
Grant Support: This work was supported by National Institutes of Health grant R01 DK077794 (A.M.D.).
Abbreviations
- Gli
glioblastoma
- Hh
Hedgehog
- iNOS
inducible nitric oxide synthase
- LSEC
liver sinusoidal endothelial cell
- OsO4
osmium tetroxide
- Ptc
Patched
- SEM
scanning electron microscopy
- Shh
sonic Hedgehog
- Smo
smoothened
Footnotes
Disclosures: None of the authors have a financial conflict of interest with the work in this manuscript.
Author Contributions: G.X. designed and performed experiments, analyzed data, and wrote the manuscript; S.S.C., W.K.S., and G.A.M, designed and performed experiments; M.Z., G.K., I.S.C. and Y.C. performed experiments; A.M.D. designed experiments, supervised research, analyzed data, and wrote the manuscript.
References
- 1.Schaffner F, Poper H. Capillarisation of hepatic sinusoids in man. Gastroenterology. 1963;44:239–42. [PubMed] [Google Scholar]
- 2.DeLeve LD, Wang X, Kanel GC, et al. Prevention of hepatic fibrosis in a murine model of metabolic syndrome with nonalcoholic steatohepatitis. Am J Pathol. 2008;173:993–1001. doi: 10.2353/ajpath.2008.070720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu B, Broome U, Uzunel M, et al. Capillarisation of hepatic sinusoid by liver endothelial cell-reactive autoantibodies in patients with cirrhosis and chronic hepatitis. Am J Pathol. 2003;163:1275–89. doi: 10.1016/S0002-9440(10)63487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warren A, Bertolino P, Benseler V, et al. Marked changes of the hepatic sinusoid in a transgenic mouse model of acute immune-mediated hepatitis. J Hepatol. 2007;46:239–46. doi: 10.1016/j.jhep.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 5.Horn T, Junge J, Christoffersen P. Early alcoholic liver injury: changes of the Disse space in acinar zone 3. Liver. 1985;5:301–10. doi: 10.1111/j.1600-0676.1985.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 6.Straub AC, Clark KA, Ross MA, et al. Arsenic-stimulated liver sinusoidal capillarisation in mice requires NADPH oxidase-generated superoxide. J Clin Invest. 2008;118:3980–9. doi: 10.1172/JCI35092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hilmer SN, Cogger VC, Fraser R, et al. Age-related changes in the hepatic sinusoidal endothelium impede lipoprotein transfer in the rat. Hepatology. 2005;42:1349–54. doi: 10.1002/hep.20937. [DOI] [PubMed] [Google Scholar]
- 8.DeLeve LD, Wang X, Hu L, et al. Rat liver sinusoidal endothelial cell phenotype is maintained by paracrine and autocrine regulation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G757–63. doi: 10.1152/ajpgi.00017.2004. [DOI] [PubMed] [Google Scholar]
- 9.Elvevold K, Smedsrod B, Martinez I. The liver sinusoidal endothelial cell: a cell type of controversial and confusing identity. Am J Physiol Gastrointest Liver Physiol. 2008;294:G391–400. doi: 10.1152/ajpgi.00167.2007. [DOI] [PubMed] [Google Scholar]
- 10.Dyer MA, Farrington SM, Mohn D, et al. Indian hedgehog activates hematopoiesis and vasculogenesis and can respecify prospective neurectodermal cell fate in the mouse embryo. Development. 2001;128:1717–30. doi: 10.1242/dev.128.10.1717. [DOI] [PubMed] [Google Scholar]
- 11.Vokes SA, Yatskievych TA, Heimark RL, et al. Hedgehog signaling is essential for endothelial tube formation during vasculogenesis. Development. 2004;131:4371–80. doi: 10.1242/dev.01304. [DOI] [PubMed] [Google Scholar]
- 12.Kusano KF, Pola R, Murayama T, et al. Sonic hedgehog myocardial gene therapy: tissue repair through transient reconstitution of embryonic signaling. Nat Med. 2005;11:1197–204. doi: 10.1038/nm1313. [DOI] [PubMed] [Google Scholar]
- 13.Pola R, Ling LE, Silver M, et al. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7:706–11. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- 14.Ingham PW, Placzek M. Orchestrating ontogenesis: variations on a theme by sonic hedgehog. Nat Rev Genet. 2006;7:841–50. doi: 10.1038/nrg1969. [DOI] [PubMed] [Google Scholar]
- 15.Ingham PW, Nakano Y, Seger C. Mechanisms and functions of Hedgehog signalling across the metazoa. Nat Rev Genet. 2011;12:393–406. doi: 10.1038/nrg2984. [DOI] [PubMed] [Google Scholar]
- 16.Wilson CW, Chuang PT. Mechanism and evolution of cytosolic Hedgehog signal transduction. Development. 2010;137:2079–94. doi: 10.1242/dev.045021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omenetti A, Choi S, Michelotti G, et al. Hedgehog signaling in the liver. J Hepatol. 2011;54:366–73. doi: 10.1016/j.jhep.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pereira Tde A, Witek RP, Syn WK, et al. Viral factors induce Hedgehog pathway activation in humans with viral hepatitis, cirrhosis, and hepatocellular carcinoma. Lab Invest. 2010;90:1690–703. doi: 10.1038/labinvest.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi SS, Omenetti A, Witek RP, et al. Hedgehog pathway activation and epithelial-to-mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1093–106. doi: 10.1152/ajpgi.00292.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Syn WK, Jung Y, Omenetti A, et al. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology. 2009;137:1478–1488. e8. doi: 10.1053/j.gastro.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ochoa B, Syn WK, Delgado I, et al. Hedgehog signaling is critical for normal liver regeneration after partial hepatectomy in mice. Hepatology. 2010;51:1712–23. doi: 10.1002/hep.23525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omenetti A, Porrello A, Jung Y, et al. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest. 2008;118:3331–42. doi: 10.1172/JCI35875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witek RP, Yang L, Liu R, et al. Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology. 2009;136:320–330. e2. doi: 10.1053/j.gastro.2008.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanda S, Mochizuki Y, Suematsu T, et al. Sonic hedgehog induces capillary morphogenesis by endothelial cells through phosphoinositide 3-kinase. J Biol Chem. 2003;278:8244–9. doi: 10.1074/jbc.M210635200. [DOI] [PubMed] [Google Scholar]
- 25.Seternes T, Sorensen K, Smedsrod B. Scavenger endothelial cells of vertebrates: a nonperipheral leukocyte system for high-capacity elimination of waste macromolecules. Proc Natl Acad Sci U S A. 2002;99:7594–7. doi: 10.1073/pnas.102173299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long F, Zhang XM, Karp S, et al. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development. 2001;128:5099–108. doi: 10.1242/dev.128.24.5099. [DOI] [PubMed] [Google Scholar]
- 27.Philips GM, Chan IS, Swiderska M, et al. Hedgehog signaling antagonist promotes regression of both liver fibrosis and hepatocellular carcinoma in a murine model of primary liver cancer. PLoS One. 2011;6:e23943. doi: 10.1371/journal.pone.0023943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeLeve LD, Wang X, McCuskey MK, et al. Rat liver endothelial cells isolated by anti-CD31 immunomagnetic separation lack fenestrae and sieve plates. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1187–9. doi: 10.1152/ajpgi.00229.2006. [DOI] [PubMed] [Google Scholar]
- 29.Omenetti A, Syn WK, Jung Y, et al. Repair-related activation of hedgehog signaling promotes cholangiocyte chemokine production. Hepatology. 2009;50:518–27. doi: 10.1002/hep.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Syn WK, Choi SS, Liaskou E, et al. Osteopontin is induced by hedgehog pathway activation and promotes fibrosis progression in nonalcoholic steatohepatitis. Hepatology. 2011;53:106–15. doi: 10.1002/hep.23998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung Y, Witek RP, Syn WK, et al. Signals from dying hepatocytes trigger growth of liver progenitors. Gut. 2010;59:655–65. doi: 10.1136/gut.2009.204354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie G, Wang L, Wang X, et al. Isolation of periportal, midlobular, and centrilobular rat liver sinusoidal endothelial cells enables study of zonated drug toxicity. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1204–10. doi: 10.1152/ajpgi.00302.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onoe T, Ohdan H, Tokita D, et al. Liver sinusoidal endothelial cells tolerize T cells across MHC barriers in mice. J Immunol. 2005;175:139–46. doi: 10.4049/jimmunol.175.1.139. [DOI] [PubMed] [Google Scholar]
- 34.Follenzi A, Benten D, Novikoff P, et al. Transplanted endothelial cells repopulate the liver endothelium and correct the phenotype of hemophilia A mice. J Clin Invest. 2008;118:935–45. doi: 10.1172/JCI32748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tokairin T, Nishikawa Y, Doi Y, et al. A highly specific isolation of rat sinusoidal endothelial cells by the immunomagnetic bead method using SE-1 monoclonal antibody. J Hepatol. 2002;36:725–33. doi: 10.1016/s0168-8278(02)00048-x. [DOI] [PubMed] [Google Scholar]
- 36.Sellaro TL, Ravindra AK, Stolz DB, et al. Maintenance of hepatic sinusoidal endothelial cell phenotype in vitro using organ-specific extracellular matrix scaffolds. Tissue Eng. 2007;13:2301–10. doi: 10.1089/ten.2006.0437. [DOI] [PubMed] [Google Scholar]
- 37.Rockey DC, Chung JJ. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: endothelial dysfunction in portal hypertension. Gastroenterology. 1998;114:344–51. doi: 10.1016/s0016-5085(98)70487-1. [DOI] [PubMed] [Google Scholar]
- 38.Geraud C, Schledzewski K, Demory A, et al. Liver sinusoidal endothelium: a microenvironment-dependent differentiation program in rat including the novel junctional protein liver endothelial differentiation-associated protein-1. Hepatology. 2010;52:313–26. doi: 10.1002/hep.23618. [DOI] [PubMed] [Google Scholar]
- 39.Rockey DC, Fouassier L, Chung JJ, et al. Cellular localization of endothelin-1 and increased production in liver injury in the rat: potential for autocrine and paracrine effects on stellate cells. Hepatology. 1998;27:472–80. doi: 10.1002/hep.510270222. [DOI] [PubMed] [Google Scholar]
- 40.March S, Hui EE, Underhill GH, et al. Microenvironmental regulation of the sinusoidal endothelial cell phenotype in vitro. Hepatology. 2009;50:920–8. doi: 10.1002/hep.23085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katoh Y, Katoh M. Hedgehog target genes: mechanisms of carcinogenesis induced by aberrant hedgehog signaling activation. Curr Mol Med. 2009;9:873–86. doi: 10.2174/156652409789105570. [DOI] [PubMed] [Google Scholar]
- 42.Scales SJ, de Sauvage FJ. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends in Pharmacological Sciences. 2009;30:303–12. doi: 10.1016/j.tips.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Asai J, Takenaka H, Kusano KF, et al. Topical sonic hedgehog gene therapy accelerates wound healing in diabetes by enhancing endothelial progenitor cell-mediated microvascular remodeling. Circulation. 2006;113:2413–24. doi: 10.1161/CIRCULATIONAHA.105.603167. [DOI] [PubMed] [Google Scholar]
- 44.Renault MA, Roncalli J, Tongers J, et al. The Hedgehog transcription factor Gli3 modulates angiogenesis. Circ Res. 2009;105:818–26. doi: 10.1161/CIRCRESAHA.109.206706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renault MA, Roncalli J, Tongers J, et al. Sonic hedgehog induces angiogenesis via Rho kinase-dependent signaling in endothelial cells. J Mol Cell Cardiol. 2010;49:490–8. doi: 10.1016/j.yjmcc.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Syn WK, Witek RP, Curbishley SM, et al. Role for hedgehog pathway in regulating growth and function of invariant NKT cells. Eur J Immunol. 2009;39:1879–92. doi: 10.1002/eji.200838890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spek CA, Bijlsma MF, Queiroz KC. Canonical Hedgehog signaling drives proangiogenic responses in endothelial cells. Cell Cycle. 2010;9:1683. [PubMed] [Google Scholar]
- 48.Chinchilla P, Xiao L, Kazanietz MG, et al. Hedgehog proteins activate pro-angiogenic responses in endothelial cells through non-canonical signaling pathways. Cell Cycle. 2010;9:570–79. doi: 10.4161/cc.9.3.10591. [DOI] [PubMed] [Google Scholar]
- 49.Corbit KC, Aanstad P, Singla V, et al. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–21. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 50.Kim J, Kato M, Beachy PA. Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus. Proc Natl Acad Sci U S A. 2009;106:21666–71. doi: 10.1073/pnas.0912180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen W, Tang T, Eastham-Anderson J, et al. Canonical hedgehog signaling augments tumor angiogenesis by induction of VEGF-A in stromal perivascular cells. Proc Natl Acad Sci U S A. 2011;108:9589–94. doi: 10.1073/pnas.1017945108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hillman RT, Feng BY, Ni J, et al. Neuropilins are positive regulators of Hedgehog signal transduction. Genes Dev. 2011;25:2333–46. doi: 10.1101/gad.173054.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeLeve LD, Wang XD, Guo YM. Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology. 2008;48:920–930. doi: 10.1002/hep.22351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie G, Wang X, Wang L, et al. Role of Differentiation of Liver Sinusoidal Endothelial Cells in Progression and Regression of Hepatic Fibrosis in Rats. Gastroenterology. 2012 Apr;142:918–927.e6. doi: 10.1053/j.gastro.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fraser R, Dobbs BR, Rogers GW. Lipoproteins and the liver sieve: the role of the fenestrated sinusoidal endothelium in lipoprotein metabolism, atherosclerosis, and cirrhosis. Hepatology. 1995;21:863–74. [PubMed] [Google Scholar]
- 56.Le Couteur DG, Fraser R, Cogger VC, et al. Hepatic pseudocapillarisation and atherosclerosis in ageing. Lancet. 2002;359:1612–5. doi: 10.1016/S0140-6736(02)08524-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.